

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (12): 2598.doi: 10.7503/cjcu20140757
• Physical Chemistry • Previous Articles Next Articles
ZHANG Ye1, ZHOU Jiajia1, WU Guisheng1,*( ), MAO Dongsen1, LU Guanzhong1,2
), MAO Dongsen1, LU Guanzhong1,2
Received:2014-08-18
Online:2014-12-10
Published:2014-11-29
Contact:
WU Guisheng
E-mail:gswu@sit.edu.cn
Supported by:CLC Number:
TrendMD:
ZHANG Ye, ZHOU Jiajia, WU Guisheng, MAO Dongsen, LU Guanzhong. Influence of the Surface Species over Co3O4 on the Formaldehyde Catalytic Oxidation Performance†[J]. Chem. J. Chinese Universities, 2014, 35(12): 2598.
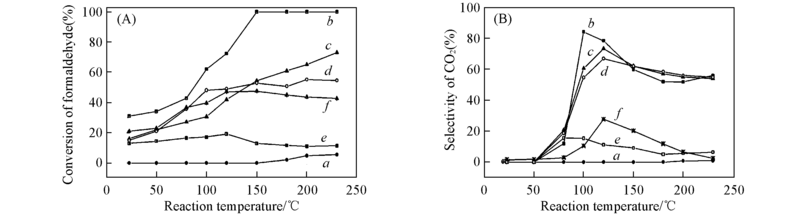
Fig.1 Catalytic activity(A) and CO2 selectivity(B) of Co3O4 pretreated in N2 or O2 at different temperatures a. Room temperature; b. N2, 200 ℃; c. N2, 300 ℃; d. N2, 400 ℃; e. O2, 200 ℃; f. O2, 400 ℃.
| [1] | Tang X. F., Li Y. G., Huang X. M., Xu Y. D., Zhu H. Q., Wang J. G., Shen W. J., Appl. Catal. B, 2006, 62(3/4), 265—273 |
| [2] | Spivey J. J., Ind. Eng. Chem. Res., 1987, 26(11), 2165—2180 |
| [3] | Guo S., Wang W. N., Jin L. X., Wang S., Wang W. L., Chem. J. Chinese Universities, 2014, 35(6), 1300—1306 |
| (郭莎, 王渭娜, 靳玲侠, 王帅, 王文亮. 高等学校化学学报, 2014, 35(6), 1300—1306) | |
| [4] | He Y. B., Ji H. B., Wang L. F., Chem. Ind. Eng. Prog., 2007, 26(8), 1104—1109 |
| (何运兵, 纪红兵, 王乐夫. 化工进展, 2007, 26(8),1104—1109) | |
| [5] | Collins J. J., Ness R., Tyl R. W., Krivanek N., Esmen N. A., Hall T. A., Regul. Toxicol. Pharm., 2001, 34, 17—34 |
| [6] | Sherman M. H., Hodgson A. T., Indoor Air, 2004, 14(1), 2—8 |
| [7] | Boonamnuayvitaya V., Sae-ung S., Tanthapanichakoon W., Sep. Purif. Technol., 2005, 42(2), 159—168 |
| [8] | Khan F. I., Ghoshal A. K., J. Loss. Prevent. Proc., 2000, 13(6), 527—545 |
| [9] | Zhang C. B., He H., Tanaka K., Appl. Catal. B: Environ., 2006, 65(1), 37—43 |
| [10] | He Y. B., Ji H. B., Chin. J. Catal., 2009, 31(2), 171—175 |
| (何运兵, 纪红兵. 催化学报, 2009, 31(2), 171—175) | |
| [11] | Shi Y. Z., Zhang N., Luo M. F., Lu J. Q., J. Chin. Soc. Rare. Earths,2011, 29(3), 271—276 |
| (石艳芝, 张娜, 罗孟飞, 鲁继青. 中国稀土学报,2011, 29(3), 271—276) | |
| [12] | Zhang J., Jin Y., Li C. Y., Shen Y. N., Han L., Hu Z. X., Di X. W., Liu Z. L., Appl. Catal. B: Environ., 2009, 91(1/2), 11—20 |
| [13] | Christoskova S. G., Danova N., Georgieva M., Argirov O. K., Mehandzhiev D., Appl. Catal. A: Gen., 1995, 128(2), 219—229 |
| [14] | Sekine Y., Nishimurab A., Atmos. Environ., 2001, 35(11), 2001—2007 |
| [15] | Ma L., Wang D. S., Li J. H., Bai B. Y., Fu L. X., Li Y. D., Appl. Catal. B: Environ., 2014, 148/149, 36—43 |
| [16] | Li C. Y., Shen Y. N., Jia M. L., Sheng S. S., Adebajo M. O., Zhu H. Y., Catal. Commun., 2008, 9(3), 355—361 |
| [17] | Ma C. Y., Wang D. H., Xue W. J., Dou B. J., Wang H. L., Hao Z. P., Environ. Sci. Technol., 2011, 45(8), 3628—3634 |
| [18] | Álvarez-Galván M. C., Pawelec B., De la Peña O’Shea V. A., Fierro J. L. G., Arias P. L., Appl. Catal. B: Environ., 2004, 51(2), 83—91 |
| [19] | Álvarez-Galván M. C., De la Peña O’Shea V. A., Fierro J. L. G., Arias P. L., Catal. Commun., 2003, 4(5), 223—228 |
| [20] | Mochida I., Iwai Y., Kamo T., Fujitsu H., J. Phys. Chem., 1985, 89(25), 5439—5442 |
| [21] | Xie X. W., Li Y., Liu Z. Q., Haruta M., Shen W. J., Nature,2009, 458(7239), 746—749 |
| [22] | Tang C. W., Kuo C. C., Kuo M. C., Wang C. B., Chien S. H., Appl. Catal. A: Gen., 2006, 309(1), 37—43 |
| [23] | Jansson J., Palmqvist A. E. C., Fridell E., Skoglundh M., Osterlund L., Thormahlen P., Langer V., J. Catal., 2002, 211(2), 387—397 |
| [24] | Wang Y. Z., Zhao Y. X., Gao C. G., Liu D. S., Catal. Lett., 2008, 125(1/2), 134—138 |
| [25] | Lou Y., Wang L., Zhao Z. Y., Zhang Y. H., Zhang Z. G., Lu G. Z., Guo Y., Guo Y. L., Appl. Catal. B: Environ., 2014, 146, 43—49 |
| [26] | Liu B., Liu Y., Li C., Hu W., Jing P., Wang Q., Zhang J., Appl. Catal. B: Environ., 2012, 127, 47—58 |
| [27] | Bai B., Arandiyan H., Li J., Appl. Catal. B: Environ., 2013, 142/143, 677—683 |
| [28] | Yu Y. B., Takei T., Ohashi H., He H., Zhang X. L., Haruta M., J. Catal., 2009, 267(2), 121—128 |
| [29] | Li J., Lu G. Z., Wu G. S., Mao D. S., Wang Y. Q., Guo Y., Catal. Sci. Technol., 2012, 2(9), 1865—1871 |
| [30] | Wang Y. Z., Zhao Y. X., Gao C. G., Liu D. S., Catal. Lett., 2007, 116(3/4), 136—142 |
| [31] | Nethravathi C., Sen S., Ravishankar N., Rajamathi M., Pietzonka C., Harbrecht B. J., Phys. Chem. B: Environ., 2005, 109(23), 11468—11472 |
| [32] | Fujita J., Martell A. E., Nakamoto K., J. Chem. Phys., 1962, 36(2), 339—345 |
| [33] | Davydov A. A., Ed.: Rochester C. H., Infrared Spectroscopy of Adsorbed Species on the Surface of Transition Metal Oxides, Wiley, New York, 1990, 6 |
| [34] | Haneda M., Kintaichi Y., Bion N., Hamada H., Appl. Catal. B: Environ., 2003, 46(3), 473—482 |
| [35] | Wang C. B., Lin H. K., Tang C. W., Catal. Lett., 2004, 1/2(1/2), 69—74 |
| [36] | Köck E. M., Kogler M., Bielz T., Klötzer B., Penner S., J. Phys. Chem. C, 2013, 117(34), 17666—17673 |
| [1] | JIN Xin, FENG Xilan, LIU Dapeng, SU Yutong, ZHANG Zheng, ZHANG Yu. Auto-redox Strategy for the Synthesis of Co3O4/CeO2 Nanocomposites and Their Structural Optimization Towards Catalytic CO Oxidation [J]. Chem. J. Chinese Universities, 2020, 41(4): 652. |
| [2] | ZHOU Hai, CHEN Hao, GUO Ya, KANG Min. Synthesis of Meso-porous Co3O4 Polyhedra and Their Electrochemical Properties† [J]. Chem. J. Chinese Universities, 2019, 40(7): 1374. |
| [3] | HU Xueyan,WANG Na,HAO Yuting,XU Zhiqing,WANG Minghui,SHI Gaiqin,YANG Huimin,LIANG Zhenhai. Preparation of Cu Doped SnO2 Cathode Material for Electroreduction of Carbon Dioxide at Low Overpotential† [J]. Chem. J. Chinese Universities, 2018, 39(10): 2265. |
| [4] | ZENG Liangpeng, HUANG Fan, ZHU Xing, ZHENG Min, LI Kongzhai. Chemical Looping Conversion of Methane over CeO2-based and Co3O4-based Co3O4-CeO2 Oxygen Carriers:Controlling of Product Selectivity† [J]. Chem. J. Chinese Universities, 2017, 38(1): 115. |
| [5] | WU Yuxuan, DING Songdong, LIU Ning, HUANG Song, HUANG Huang, SU Dongping. New Method for Synthesis of Amide Podand Extractants† [J]. Chem. J. Chinese Universities, 2014, 35(2): 257. |
| [6] | GAO Yong-Qing, ZHOU Ning, Lü Yu-Jian, SHI Wei-Guo, CHENG Mao-Sheng, .... Reduction of the Aromatic Nitro Group in Peptide by Ammonium Formate Catalytic Transfer Hydrogenation——An Indirect Introduction of p-Aminophenylalanine into Peptide Chain [J]. Chem. J. Chinese Universities, 2010, 31(4): 718. |
| [7] | XU Sha, ZHANG Ping, HUANG Lin-Juan, WANG Zhong-Fu*. Pronase E Digestion of N-Glycans in Glycoprotein and Its Fluorescent Derivatives Analysis by HPLC-ESI/MS [J]. Chem. J. Chinese Universities, 2010, 31(10): 1992. |
| [8] | JIN Xing-Mei*, MA Jian-Xin, ZHOU Wei. In situ IR Study of COS Hydrolysis over Lanthanum Oxysulfide: Effect of O2, H2S and SO2 [J]. Chem. J. Chinese Universities, 2009, 30(6): 1194. |
| [9] | ZHANG Wei-Min, ZHANG Yu, DONG Guang-Ming, SUN Zhong-Xi. Hydrothermal Synthesis of Polycrystal Co3O4 with One Dimensional Nanostructures [J]. Chem. J. Chinese Universities, 2006, 27(10): 1791. |
| [10] | JU Xue-Hai, XIAO Ji-Jun, XIAO He-Ming . DFT Study of the Intermolecular Interaction of Hydrazinium Nitroformate Ion Pair [J]. Chem. J. Chinese Universities, 2003, 24(6): 1067. |
| [11] | CHEN Shao-Yuan, YU Yong-Ping, YOU Jin-Zong, CHEN Yao-Zu. Two New Methods for Synthesis of 4β-Amino-4-deoxypodophyllotoxin and 4β-Amino-4'-demethyl-4-deoxypodophyllotoxin [J]. Chem. J. Chinese Universities, 2000, 21(7): 1064. |
| [12] | LI Ya-Dong, HE Yun-Pu, LI Long-Quan, QIAN Yi-Tai . Fabrication of Co3O4Ultrafines by a Liquid-control-precipitation Method [J]. Chem. J. Chinese Universities, 1999, 20(4): 519. |
| [13] | JIA Ming-Jun, ZHANG Wen-Xiang, TAO Yu-Guo, WANG Gui-Ying, CUI Xiang-Hao, ZHANG Chun-Lei, WU Tong-Hao, DONG Guo-Qiang, LI Xue-Mei. Preparation, Characterization and CO Oxidation of Nanometer Co3O4 [J]. Chem. J. Chinese Universities, 1999, 20(4): 637. |
| [14] | LI Hai-Yan, ZHOU Jin-Mei, LIN Guo-Dong, ZHANG Hong-Bin . Studies on A New Catalytic System for Heterogeneous Carbonylation of Methanol to Methyl Formate [J]. Chem. J. Chinese Universities, 1997, 18(8): 1364. |
| [15] | YANG Su, YE Xue-Fei, DAI Min-Guang. A New Method for Rapid Determination of Adsorption Heatby Temperature Programmed Desorption Frontal Chromatography [J]. Chem. J. Chinese Universities, 1996, 17(1): 27. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||