

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (10): 2085.doi: 10.7503/cjcu20140562
• Analytical Chemistry • Previous Articles Next Articles
WU Shangrong1,2, JIN Bing2, ZHANG Nan2, LIU Ying2, LIU Xiangjun2, LI Songqing1,*( ), SHANGGUAN Dihua2,*(
), SHANGGUAN Dihua2,*( )
)
Received:2014-06-20
Online:2014-10-10
Published:2014-09-19
Contact:
LI Songqing,SHANGGUAN Dihua
E-mail:lisq65@126.com;sgdh@iccas.ac.cn
Supported by:CLC Number:
TrendMD:
WU Shangrong, JIN Bing, ZHANG Nan, LIU Ying, LIU Xiangjun, LI Songqing, SHANGGUAN Dihua. New Monomethine Cyanine Dye and Its Interaction with Different DNA Forms†[J]. Chem. J. Chinese Universities, 2014, 35(10): 2085.
| DNA | Sequence | n | Kd/(μmol·L-1) |
|---|---|---|---|
| 22AGK+ | AGGGTTAGGGTTAGGGTTAGGG | 2 | 4.5±0.1 |
| c-myc | TGAGGGTGGGGAGGGTGGGGAA | 2 | 4.3±0.2 |
| 22AGNa+ | AGGGTTAGGGTTAGGGTTAGGG | 1 | 12.3±0.4 |
| TBA | GGTTGGTGTGGTTGG | 1 | 16.7±1.4 |
| ds26 | CAATCGGATCGAATTCGATCCGATTG | 2 | 8.3±0.2 |
| ss2 | GGGTTACTACGAACTGG | 2 | 6.6±0.7 |
Table 1 Binding stoichiometry [putative number of binding sites on a given DNA(n)] and apparent dissociation constants(Kd) of the DNA used in this work
| DNA | Sequence | n | Kd/(μmol·L-1) |
|---|---|---|---|
| 22AGK+ | AGGGTTAGGGTTAGGGTTAGGG | 2 | 4.5±0.1 |
| c-myc | TGAGGGTGGGGAGGGTGGGGAA | 2 | 4.3±0.2 |
| 22AGNa+ | AGGGTTAGGGTTAGGGTTAGGG | 1 | 12.3±0.4 |
| TBA | GGTTGGTGTGGTTGG | 1 | 16.7±1.4 |
| ds26 | CAATCGGATCGAATTCGATCCGATTG | 2 | 8.3±0.2 |
| ss2 | GGGTTACTACGAACTGG | 2 | 6.6±0.7 |
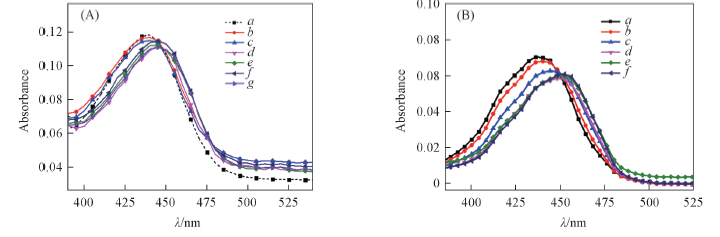
Fig.1 Absorption spectra of 5 μmol/L MTP in the presence of 5 μmol/L different kinds of DNA(A) and 3 μmol/L MTP in the presence of increasing amounts of c-myc(B) (A) a. Without DNA; b. TBA; c. 22AGNa+; d. 22AGK+; e. c-myc; f. ds26; g. ss2.(B) Concentration of c-myc(μmol·L-1) from a to f: 0, 1.0, 3.0, 6.0, 9.0, 12.
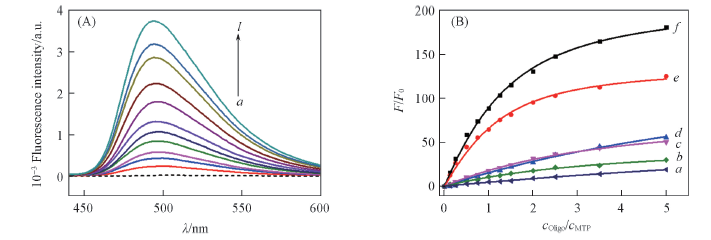
Fig.2 Fluorometric titration of MTP(2 μmol/L) with different concentrations of c-myc(A) and fluorometric titration curves of MTP(2 μmol/L) with different DNA(B) (A) Concentration of c-myc(μmol·L-1) from a to l: 0, 0.25, 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 4.0, 5.0, 7.0, 10; λex=430 nm. (B) a. TBA; b. 22AGNa+; c. ss2; d. ds26; e. 22AGK+; f. c-myc; λex=430 nm, λem=503 nm.
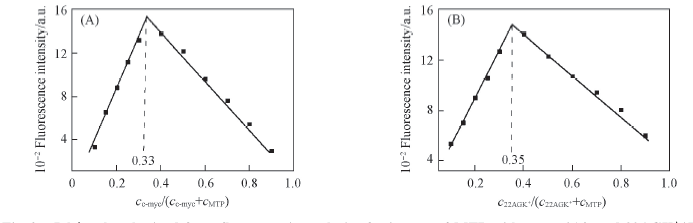
Fig.3 Job’s plot obtained from fluorometric analysis of mixtures of MTP with c-myc(A) and 22AGK+(B) cMTP+cDNA=4 μmol/L; Xaxis=molar fraction of quadruplex DNA; λex=430 nm, λem=503 nm.
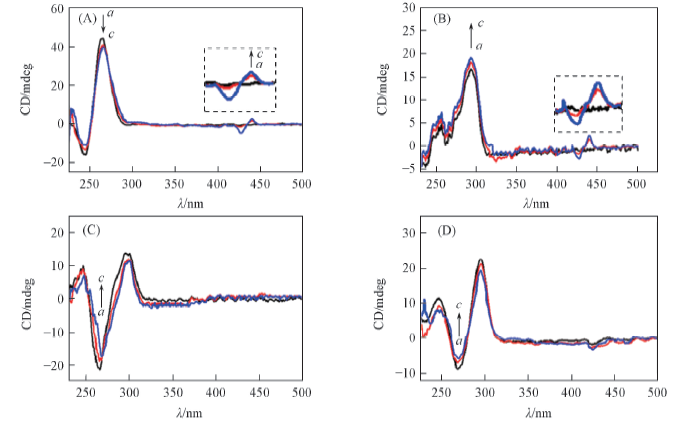
Fig.4 CD spectra of different quadruplex DNA(5 μmol/L) in the absence(a) and presence of MTP(b,c) (A) c-myc; (B) 22AGK+; (C) 22AGNa+; (D) TBA. a. DNA only; b. n(DNA)∶n(MTP)=1∶1; c. n(DNA)∶n(MTP)=1∶2.
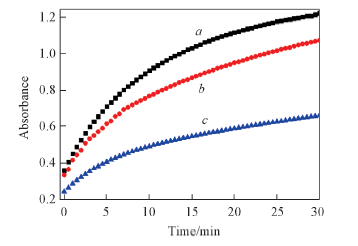
Fig.5 Inhibition activity of MTP on the peroxidase activity of G-quadruplex/hemin complex The curves represent the change of the product concentration(absorbance at 415 nm) of G-quadruplex/hemin peroxidase within 30 min. The G-quadruplex/hemin peroxidase reaction in the absence of MTP was used as control. c(ABTS)=0.4 mmol/L; c(H2O2)=0.4 mmol/L. a. 1 μmol/L c-myc+1 μmol/L Hemin; b. 1 μmol/L c-myc+2 μmol/L MTP+1 μmol/L Hemin; c. 1 μmol/L c-myc+10 μmol/L MTP+1 μmol/L Hemin.
| [1] | Chen X. Y., Peng X. J., Chemistry,2004, 67(9), 1—9 |
| (陈秀英, 彭孝军. 化学通报, 2004, 67(9), 1—9) | |
| [2] | Ma D. L., He H. Z., Leung K. H., Zhong H. J., Chan D. S. H., Leung C. H., Chem. Soc. Rev., 2013, 42, 3427—3440 |
| [3] | Peng X. J., Wu T., Fan J. L., Wang J. Y., Zhang S., Song F. L., Sun S. G., Angew. Chem. Int. Ed., 2011, 50(18), 4180—4183 |
| [4] | Yang Q.F., Xiang J. F., Yang S., Zhou Q. J., Li Q., Tang Y. L., Xu G. Z.,Chem. Commun., 2009, (9), 1103—1105 |
| [5] | Yang Q. F., Xiang J. F., Yang S., Li Q., Zhou Q. J., Guan A. J., Zhang X. F., Zhang H., Tang Y. L., Xu G. Z., Nucleic Acids Res., 2010, 38(3), 1022—1033 |
| [6] | Lubitz I., Zikich D., Kotlyar A., Biochemistry,2010, 49(17), 3567—3574 |
| [7] | Zheng A. H., Zhu Q., Xiang D. S., He Z. K., Chin. J. Anal. Chem., 2013, 41(3), 325—329 |
| (郑爱华, 朱庆, 向东山, 何治轲. 分析化学, 2013, 41(3), 325—329) | |
| [8] | Fei X. N., Liu L. J., Zhang B. L., Shi B. J., Yao K. D., Prog. Chem., 2006, 18(6), 801—807 |
| (费学宁, 刘丽娟, 张宝莲, 石博杰, 姚康德. 化学进展, 2006, 18(6), 801—807) | |
| [9] | Chen X. Y., Guo L., Zheng C. G., Gao H. Y., Wang H. J., Zhang D., Chin. J. Org. Chem., 2012, 32(8), 1445—1449 |
| (陈秀英, 郭琳, 郑昌戈, 高海燕, 王海军, 张丹. 有机化学, 2012, 32(8), 1445—1449) | |
| [10] | Jin B., Zhang X., Zheng W., Liu X. J., Qi C., Wang F. Y., Shangguan D. H., Anal. Chem., 2014, 86(1), 943—952 |
| [11] | Rye H. S., Quesada M. A., Peck K., Mathies R. A., GIazer A. N., Nucleic Acids Res., 1991, 19(2), 327—333 |
| [12] | Largy E., Hamon F., Teulade-Fichou M. P., Anal. Bioanal. Chem., 2011, 400, 3419 |
| [13] | Monchaud D., Allain C., Teulade-Fichou M. P., Nucleosides Nucleotides Nucleic Acids,2007, 26(10), 1585—1588 |
| [14] | Monchaud D., Allain C., Bertrand H., Smargiasso N., Rosu F., Gabelica V., De C. A., Mergny J. L., Teulade-Fichou M. P., Biochimie,2008. 90(8), 1207—1223 |
| [15] | Largy E., Saettel N., Hamon F., Dubruille S., Teulade-Fichou M. P., Curr. Pharm. Des., 2012. 18(14), 1992—2001 |
| [16] | Allain C., Monchaud D., Teulade-Fichou M. P., J. Am. Chem. Soc., 2006, 128(36), 11890—11893 |
| [17] | Yanga F., Xu X. L., Gong Y. H., Qiu W. W., Sun Z. R., Zhou J. W., Audebert P., Tang J., Tetrahedron., 2007, 63(37), 9188—9194 |
| [18] | Coetzee J., Cronje S., Dobrzanska L., Raubenheimer H. G., Joone G., Nell M. J., Hoppe H. C., Dalton Trans. ,2011, 40(7), 1471—1483 |
| [19] | Karlsson H. J., Lincoln P., Westman G., Bioorganic & Medicinal Chemistry,2003, 11(6), 1035—1040 |
| [20] | Cian A. D., Guittat L., Kaiser M., Sacca B., Amrane S., Bourdoncle A., Alberti P., Teulade F. M. P., Lacroix L., Mergny J. L., Methods,2007, 42(2), 183—195 |
| [21] | Cheng X., Liu X., Bing T., Zhao R., Xiong S., Shangguan D. H., Biopolymers,2009, 91(10), 874—883 |
| [22] | Martino L., Virno A., Randazzo A., Virgilio A., Esposito V., Giancola C., Bucci M., Cirino G., Mayol L., Nucleic Acids Res., 2006, 34(22), 6653—6662 |
| [23] | He Y. J., Neumann R. D., Panyutin I. G., Nucleic Acids Res., 2004, 32(18), 5359—5367 |
| [24] | Ambrus A., Chen D., Dai J. X., Bialis T., Jones R. A., Yang D. Z., Nucleic Acids Res., 2006, 34(9), 2723—2735 |
| [25] | Dai J. X., Carver M., Hurley L. H., Yang D. Z., J. Am. Chem. Soc., 2011, 133(44), 17673—17680 |
| [26] | Su J. Q., Chi B. R., Li X., Liu L., Liu L. M., Qi Y. X., Wang Z. Y., Jin N. Y., Chem. Res. Chinese Universities,2012, 28(3), 465—471 |
| [27] | Ma Y., Huang Z. S., Chem. J. Chinese Universities,2012, 33(10), 2217—2222 |
| (马彦, 黄志纾. 高等学校化学学报, 2012, 33(10), 2217—2222) | |
| [28] | Burge S., Parkinson G. N., Hazel P., Todd A. K., Neidle S., Nucleic Acids Res., 2006, 34(19), 5402—5415 |
| [29] | Yang X. J., Fang C. L., Mei H. C., Chang T. J., Cao Z. H., Shangguan D. H., Chem. Eur. J., 2011, 51(17), 14475—14484 |
| [30] | Gao N., Wang Y. B., Wei C. Y., Chem. Res. Chinese Universities,2014, 30(3), 495—499 |
| [31] | Boer D. R., Canals A., Coll M., Dalton Trans,2009, 21(3) 399—414 |
| [32] | Cheng X. H., Liu X. J., Bing T., Cao Z. H., Biochem. ,2009, 48(33), 7817—7823 |
| [1] | LIU Suyu, DING Fei, LI Qian, FAN Chunhai, FENG Jing. Azobenzene-integrated DNA Nanomachine [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220122. |
| [2] | WU Yushuai, SHANG Yingxu, JIANG Qiao, DING Baoquan. Research Progress of Controllable Self-assembled DNA Origami Structure as Drug Carrier [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220179. |
| [3] | WANG Junyang, LIU Zheng, ZHANG Qian, SUN Chunyan, LI Hongxia. Application of DNA Silver Nanoclusters in the Fluorescence Biosensors based on Functional Nucleic Acids [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220010. |
| [4] | ZHAO Yongmei, MU Yeshu, HONG Chen, LUO Wen, TIAN Zhiyong. Bis-naphthalimide Derivatives for Picronitric Acid Detection in Aqueous Solution [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210765. |
| [5] | TANG Qian, DAN Feijun, GUO Tao, LAN Haichuang. Synthesis and Application of Quinolinone-coumarin-based Colorimetric Fluorescent Probe for Recognition of Hg2+ [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210660. |
| [6] | WANG Di, ZHONG Keli, TANG Lijun, HOU Shuhua, LYU Chunxin. Synthesis of Schiff-based Covalent Organic Framework and Its Recognition of I ‒ [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220115. |
| [7] | HUANG Shan, YAO Jiandong, NING Gan, XIAO Qi, LIU Yi. Efficient Determination of Alkaline Phosphatase Activity Based on Graphene Quantum Dots Fluorescent Probes [J]. Chem. J. Chinese Universities, 2021, 42(8): 2412. |
| [8] | LI Anran, ZHAO Bing, KAN Wei, SONG Tianshu, KONG Xiangdong, BU Fanqiang, SUN Li, YIN Guangming, WANG Liyan. ON-OFF-ON Double Colorimetric and Fluorescent Probes Based on Phenanthro[9,10-d]imidazole Derivatives and Their Living Cells Imaging [J]. Chem. J. Chinese Universities, 2021, 42(8): 2403. |
| [9] | WU Yangyi, CHEN Jianping, Ai Yijing, WANG Qingxiang, GAO Fei, GAO Feng. Synthesis of 2-(2-Hydroxy-3-methoxyphenyl)-C60 and Its Application for Sensing of Cauliflower Mosaic Virus 35S Promotor [J]. Chem. J. Chinese Universities, 2021, 42(6): 1754. |
| [10] | YANG Xinjie, LAI Yanqiong, LI Qiuyang, ZHANG Yanli, WANG Hongbin, PANG Pengfei, YANG Wenrong. An Enzyme-free and Label-free Fluorescent Probe for Detection of Microcystin-LR Based on Circular DNA-Silver Nanoclusters [J]. Chem. J. Chinese Universities, 2021, 42(12): 3600. |
| [11] | HU Ling, YIN Yao, KE Guoliang, ZHANG Xiaobing. Regulation of Cell-cell Interactions Based on DNA Nanostructures [J]. Chem. J. Chinese Universities, 2021, 42(11): 3284. |
| [12] | LIU Xuejiao, YANG Fan, LIU Shuang, ZHANG Chunjuan, LIU Qiaoling. Progress in Aptamer-targeted Membrane Protein Recognition and Functional Regulation [J]. Chem. J. Chinese Universities, 2021, 42(11): 3277. |
| [13] | MAO Yu, QU Hao, ZHENG Lei. Research Progress on RNA⁃cleaving DNAzyme for the Detection of Pathogenic Bacteria [J]. Chem. J. Chinese Universities, 2021, 42(11): 3445. |
| [14] | CHEN Weiju, CHEN Shiya, XUE Caoye, LIU Bo, ZHENG Jing. Fluorescent Probe for Hypoxia-triggered Imaging and Cancer Therapy [J]. Chem. J. Chinese Universities, 2021, 42(11): 3433. |
| [15] | REN Yushuang, GUO Yuanyuan, LIU Xueyi, SONG Jie, ZHANG Chuan. Platinum(Ⅳ) Prodrug-grafted Phosphorothioate DNA and Its Self-assembled Nanostructure for Targeted Drug Delivery [J]. Chem. J. Chinese Universities, 2020, 41(8): 1721. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||