

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (9): 1859.doi: 10.7503/cjcu20140529
• Articles: Inorganic Chemistry • Previous Articles Next Articles
HE Yongke, YAN Yan, YANG Fen, WU Junbiao, SONG Xiaowei*( )
)
Received:2014-06-11
Online:2014-09-10
Published:2014-07-28
Contact:
SONG Xiaowei
E-mail:xiaoweisong@jlu.edu.cn
Supported by:CLC Number:
TrendMD:
HE Yongke, YAN Yan, YANG Fen, WU Junbiao, SONG Xiaowei. Ionothermal Syntheses and Characterization of Open-framework Manganese Phosphites (NH4)2Mn3(HPO3)4 and Mn(HPO3)†[J]. Chem. J. Chinese Universities, 2014, 35(9): 1859.
| Empirical formula | H12Mn3N2O12P4 | Z | 3 |
|---|---|---|---|
| Mr | 520.81 | Dc/(g·cm-3) | 2.673 |
| T/K | 296(2) | μ/mm-1 | 3.509 |
| λ/nm | 0.071073 | F(000) | 741 |
| Crystal system | Trigonal | θ range/(°) | 1.65—28.37 |
| Space group | R | Goodness-of-fit on F2 | 1.021 |
| a/nm | 0.54415(3) | Limiting indices | -7≤h≤5, -7≤k≤7, -39≤l≤49 |
| b/nm | 0.54415(3) | Reflections collected/unique | 2364/358 |
| c/nm | 3.7114(4) | Completeness to 28.37° | 100.0% |
| α/(°) | 90 | Data/restraints/parameters | 358/6/31 |
| β/(°) | 90 | R1, wR2[I>2σ(I)] | R1=0.0326, wR2=0.0883 |
| γ/(°) | 120 | R1, wR2(all data) | R1=0.0337, wR2=0.0889 |
| V/nm3 | 0.95171(13) |
Table 1 Crystallographic data for compound 1
| Empirical formula | H12Mn3N2O12P4 | Z | 3 |
|---|---|---|---|
| Mr | 520.81 | Dc/(g·cm-3) | 2.673 |
| T/K | 296(2) | μ/mm-1 | 3.509 |
| λ/nm | 0.071073 | F(000) | 741 |
| Crystal system | Trigonal | θ range/(°) | 1.65—28.37 |
| Space group | R | Goodness-of-fit on F2 | 1.021 |
| a/nm | 0.54415(3) | Limiting indices | -7≤h≤5, -7≤k≤7, -39≤l≤49 |
| b/nm | 0.54415(3) | Reflections collected/unique | 2364/358 |
| c/nm | 3.7114(4) | Completeness to 28.37° | 100.0% |
| α/(°) | 90 | Data/restraints/parameters | 358/6/31 |
| β/(°) | 90 | R1, wR2[I>2σ(I)] | R1=0.0326, wR2=0.0883 |
| γ/(°) | 120 | R1, wR2(all data) | R1=0.0337, wR2=0.0889 |
| V/nm3 | 0.95171(13) |
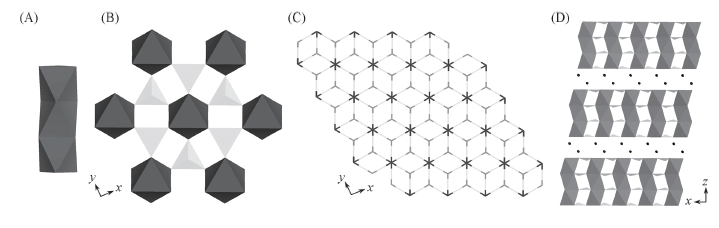
Fig.4 Views of Mn3 cluster formed by MnO6 octahedra(A), Mn3 cluster linking to six adjacent Mn3 clusters by [HPO3] units along the [001] direction(B), the layer consisted of MnO6 and [HPO3] along the [001] direction(C) and NH4+ ions residing between the layers along the [010] direction(D)
| [1] | Wang Z. P., Yu J. H., Xu R. R., Chem. Soc. Rev., 2012, 41, 1729—1741 |
| [2] | Li Y., Yu J. H., Chem. Rev., 2014, 114, 7268—7316 |
| [3] | Wang Y. Y., Li Y., Yan Y., Xu J., Guan B. Y., Wang Q., Li J. Y., Yu J. H., Chem. Commun., 2013, 49, 9006—9008 |
| [4] | Yu J. H., Xu R. R., Acc. Chem. Res., 2010, 43, 1195—1204 |
| [5] | Yu J. H., Xu R. R., Chem. Soc. Rev., 2006, 35, 593—604 |
| [6] | Cundy C. S., Cox P. A., Chem. Rev., 2003, 103, 663—701 |
| [7] | Cheetham A. K., Ferey G., Loiseau T., Angew. Chem. Int. Ed., 1999, 38, 3268—3292 |
| [8] | Li Y., Yu J. H., Xu R. R., Angew. Chem. Int. Ed., 2013, 52, 1673—1677 |
| [9] | Li Y., Yu J. H., Xu R. R., Angew. Chem. Int. Ed., 2008, 47, 4401—4405 |
| [10] | Yang Y., Zhao Y. N., Yu J. G., Wu S. Z., Wang R. J., Inorg. Chem., 2008, 47, 769—771 |
| [11] | Zhao L., Li J. Y., Chen P., Li G. H., Yu J. H., Xu R. R., Chem. Mater., 2008, 20, 17—19 |
| [12] | Xing H. Z., Yang W. T., Su T., Li Y., Xu J., Nakano T., Yu J. H., Xu R. R., Angew. Chem. Int. Ed., 2010, 49, 2328—2331 |
| [13] | Li J. Y., Li L., Liang J., Chen P., Yu J. H., Xu R. R., Crystal Growth & Design,2008, 8, 2318—2323 |
| [14] | Wang X., Han Y. D., Hao S. Q., Yu J. H., Xu R. R., Acta Chim. Sinica,2012, 70, 1496—1500 |
| (王曦, 韩义德, 郝素琴, 于吉红, 徐如人. 化学学报, 2012, 70, 1496—1500) | |
| [15] | Wang X. L., Yan Y., Wu J. B., Zhang C. Q., Li J. Y., CrystEngComm,2014, 16, 2266—2272 |
| [16] | Wang X. L., Yan Y., Wu J. B., Zou Y. C., Zhang C. Q., Li J. Y., Chem. J. Chinese Universities,2014, 35(6), 1142—1146 |
| (王学雷, 颜岩, 吴俊标, 邹永存, 张传奇, 李激扬. 高等学校化学学报, 2014, 35(6), 1142—1146 ) | |
| [17] | Dong Z. J., Zhao L., Liang Z. Q., Chen P., Yan Y., Li J. Y., Yu J. H., Xu R. R., Dalton Trans., 2010, 39, 5439—5445 |
| [18] | Chung U. C., Mesa J. L., Pizarro J. L., Jubera V., Lezama L., Arriortua M. I., Rojo T., J. Solid State Chem., 2005, 178, 2913—2921 |
| [19] | Jin L. N., Hong J. M., Ni Y. H., Mater. Chem. Phys., 2010, 123, 337—342 |
| [20] | Fernandez S., Mesa J.L., Pizarro J. L., Lezama L., Arriortua M. I., Olazcuaga R., Rojo T., Chem. Mater., 2000, 12, 2092—2098 |
| [21] | Cooper E. R., Andrews C. D., Wheatley P. S., Webb P. B., Wormald P., Morris R. E., Nature,2004, 430, 1012—1016 |
| [22] | Parnham E. R., Morris R. E., Acc. Chem. Res., 2007, 40, 1005—1013 |
| [23] | Ding Y., Chen H. L., Guo L. Q., Fu H., Chen W. L., Wang E. B., Chem. J. Chinese Universities,2012, 33(12), 2623—2627 |
| (丁艳, 陈鸿利, 郭立泉, 傅海, 陈维林, 王恩波. 高等学校化学学报, 2012, 33(12), 2623—2627 | |
| [24] | Ma Z., Yu J. H., Dai S., Adv. Mater., 2010, 22, 261—285 |
| [25] | Morris R.E.,Chem. Commun., 2009, 2990—2998 |
| [26] | He Y. K., Yan Y., Yang F., Wu J. B., Song X. W., Inorg. Chem. Commun., 2014, 44, 151—154 |
| [27] | Xing H. Z., Li J. Y., Yan W. F., Chen P., Jin Z., Yu J. H., Dai S., Xu R. R., Chem. Mater., 2008, 20, 4179—4181 |
| [28] | Su T., Xing H. Z., Xu J., Yu J. H., Xu R. R., Inorg. Chem., 2010, 50, 1073—1078 |
| [29] | Elaiwi A., Hitchcock P.B., Seddon K. R., Srinivasan N., Tan Y. M., Welton T., Zora J. A., J. Chem. Soc.,Dalton Trans., 1995, 3467—3472 |
| [30] | Bruker AXS Inc. SHELXTL, Madison, WI, 2000 |
| [31] | Wang W., Li Y., Liu L., Dong J. X., Dalton Trans., 2012, 41, 10511—10513 |
| [1] | LIU Qingqing, WANG Pu, WANG Yongshuai, ZHAO Man, DONG Huanli. Synthesis and Topochemical Polymerization Study of Naphthalene/perylene Imides Substituted Diacetylene Derivatives [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220091. |
| [2] | SHI Naike, ZHANG Ya, SANSON Andrea, WANG Lei, CHEN Jun. Uniaxial Negative Thermal Expansion and Mechanism in Zn(NCN) [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220124. |
| [3] | HONG Yangyu, XING Hongzhu, BING Qiming, GAO Xuwen, QI Bin, CHEN Yakun, SU Tan, ZOU Bo. Synthesis and Fluorescence Properties of Novel Ce3+-doped Manganese Phosphite Open-framework Materials [J]. Chem. J. Chinese Universities, 2021, 42(9): 2725. |
| [4] | YUE Shengli, WU Guangbao, LI Xing, LI Kang, HUANG Gaosheng, TANG Yi, ZHOU Huiqiong. Research Progress of Quasi-two-dimensional Perovskite Solar Cells [J]. Chem. J. Chinese Universities, 2021, 42(6): 1648. |
| [5] | TIAN Xia,YANG Fuqun,YUAN Wei,ZHAO Lei,YAO Lei,ZHEN Xiaoli,HAN Jianrong,LIU Shouxin. Synthesis, Structure and Recognition Properties of Macrocyclic Crown Ethers with Oxadiazole † [J]. Chem. J. Chinese Universities, 2020, 41(3): 490. |
| [6] | LIU Dongmei,SU Yajing,LI Shanshan,XU Qiwei,LI Xia. Transition Metal Coordination Polymers Constructed by 4-(4-Carboxyphenoxy)isophthalic Acid: Synthesis, Crystal Structure, Fluorescence Sensing and Photocatalysis † [J]. Chem. J. Chinese Universities, 2020, 41(2): 253. |
| [7] | QIN Liulei,LIU Yang,GUAN Xiaoqin,ZHENG Xiaoyuan,ZHANG Ziyu,LIU Zunqi. Synthesis and Switchable Dielectric Properties of an Inorganic-organic Hybrid Complex [H2(DABCO)CuCl4]·H2O † [J]. Chem. J. Chinese Universities, 2020, 41(1): 70. |
| [8] | LI Bing,WANG Xuemin,BAI Fengying,LIU Shuqing. Synthesises, Structures and Antibacterial Activities of a Series of Rare Earth Nitrogen Heterocyclic Complexes† [J]. Chem. J. Chinese Universities, 2019, 40(4): 632. |
| [9] | Baozhen SHI,Shan LI,Dianpeng WANG,Yunzhi ZHOU,Jinyu SUN. Synthesis and Physical Properties of Cobalt-Zinc Hybrid Porous Metal-organic Frameworks † [J]. Chem. J. Chinese Universities, 2019, 40(12): 2443. |
| [10] |
JIANG Xianming,WANG Huaiqian,CAO Yu,SUN Zhihui,CAO Yufang,WU Weibin.
Structure Prediction and Photoelectron Spectroscopy Study of Rare Earth-doped Silicon-based Clusters of MS |
| [11] | WANG Dongmei,LIU Zihua,LI Guanghua,LIU Yunling,LI Chunxia. Synthesis, Structure and Fluorescent Property of Indium-based Bimetallic Metal-organic Frameworks† [J]. Chem. J. Chinese Universities, 2018, 39(9): 1886. |
| [12] | WANG Zhixiu, MU Ying, WANG Yilin, SUN Xiaoyuan, SU Tan, LIU Jingyao. Proton Conduction Property of a Manganese Phosphite Open Framework Compound† [J]. Chem. J. Chinese Universities, 2018, 39(6): 1138. |
| [13] |
TIAN Huan, ZHANG Menglong, WANG Lisha, TONG Bihai, ZHAO Zhuo.
Synthesis of 4,13-Dithio Benzene and-18-Crown-6 and Its Selective Extractability on A |
| [14] | LIU Jingwei,YU Guangtao,SHEN Xiaopeng,HUANG Xuri,CHEN Wei. Theoretical Studies on the Structures, Electronic and Magnetic Properties of Fully and Partically Fluorizated Germanene Nanoribbons† [J]. Chem. J. Chinese Universities, 2018, 39(5): 977. |
| [15] | CONG Rimin, YU Huaiqing, LUO Yunjun, LI Jiao, WANG Weiwei, LI Qiuhong, SUN Wuzhu, SI Weimeng, ZHANG Hua. Synthesis and Properties of Bi25FeO40/α-Fe2O3 Composite Nanoparticle Photocatalysts† [J]. Chem. J. Chinese Universities, 2018, 39(4): 629. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||