

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (9): 1912.doi: 10.7503/cjcu20131050
• Organic Chemistry • Previous Articles Next Articles
BU Huijuan, ZHANG Jing, MU Boshuai, LI Yuan*( )
)
Received:2013-10-28
Online:2014-09-10
Published:2019-08-01
Contact:
LI Yuan
E-mail:liyuanhbsd@163.com
Supported by:CLC Number:
TrendMD:
BU Huijuan, ZHANG Jing, MU Boshuai, LI Yuan. Mannich Reaction of Aromatic Ketones Based on Acetic Acid as Solvent and Catalyst†[J]. Chem. J. Chinese Universities, 2014, 35(9): 1912.
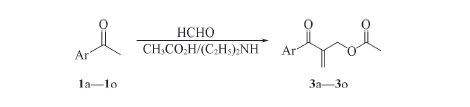
Scheme 2 Reaction of aromatic ketones 1 with formaldehyde and diethylamine in acetic acid Ar: a.Ph; b. 4-F-C6H4; c. 4-Cl-C6H4; d. 4-Br-C6H4; e. 4-CH3-C6H4; f. 4-OCH3-C6H4; g. 4-NO2-C6H4; h. 2-F-C6H4; i. 3-F-C6H4; j. 2,4-F2-C6H3; k. 3,4-F2-C6H3; l. 3-Cl-C6H4; m. 3-NO2-C6H4; n. 2-naphthyl; o. 4-C6H5C6H4.
| Ketone | n(Ketone 1)∶n(HCHO) | Amine | n(Ketone 1)∶n(Amine) | Time/h | Product | Yieldb(%) |
|---|---|---|---|---|---|---|
| 1a | 1∶1.2 | Et2NH | 1∶1.0 | 2 | 3a | 30 |
| 1a | 1∶1.2 | Et2NH | 1∶1.0 | 4 | 3a | 31 |
| 1a | 1∶1.5 | Et2NH | 1∶1.0 | 5 | 3a | 43 |
| 1a | 1∶3.0 | Et2NH | 1∶2.0 | 3 | 3a | 69 |
| 1a | 1∶6.0 | Et2NH | 1∶0.5 | 4 | 3a | 67 |
| 1a | 1∶3.0 | Morpholine | 1∶2.0 | 4 | 3a | 38 |
| 1a | 1∶3.0 | (i-Pr)2NH | 1∶1.0 | 4 | 3a | 45 |
| 1b | 1∶1.3 | Et2NH | 1∶1.0 | 5 | 3b | 35 |
| 1b | 1∶1.5 | Et2NH | 1∶1.5 | 5 | 3b | 40 |
| 1b | 1∶3.0 | Et2NH | 1∶2.0 | 3 | 3b | 72 |
Table 1 Reaction of aromatic ketones 1a and 1b in acetic acida
| Ketone | n(Ketone 1)∶n(HCHO) | Amine | n(Ketone 1)∶n(Amine) | Time/h | Product | Yieldb(%) |
|---|---|---|---|---|---|---|
| 1a | 1∶1.2 | Et2NH | 1∶1.0 | 2 | 3a | 30 |
| 1a | 1∶1.2 | Et2NH | 1∶1.0 | 4 | 3a | 31 |
| 1a | 1∶1.5 | Et2NH | 1∶1.0 | 5 | 3a | 43 |
| 1a | 1∶3.0 | Et2NH | 1∶2.0 | 3 | 3a | 69 |
| 1a | 1∶6.0 | Et2NH | 1∶0.5 | 4 | 3a | 67 |
| 1a | 1∶3.0 | Morpholine | 1∶2.0 | 4 | 3a | 38 |
| 1a | 1∶3.0 | (i-Pr)2NH | 1∶1.0 | 4 | 3a | 45 |
| 1b | 1∶1.3 | Et2NH | 1∶1.0 | 5 | 3b | 35 |
| 1b | 1∶1.5 | Et2NH | 1∶1.5 | 5 | 3b | 40 |
| 1b | 1∶3.0 | Et2NH | 1∶2.0 | 3 | 3b | 72 |
| Ketone | Solvent | Amine | Catalyst | Time/h | Product | Yieldb(%) |
|---|---|---|---|---|---|---|
| 1a | HOAc | Et2NH | HOAc | 3 | 3a | 69 |
| 1a | EtOH | Et2NH | HOAcc | 5 | ||
| 1a | TFA | Et2NH | TFA | 15 | ||
| 1a | THF | Et2NH | HOAcc | 12 | ||
| 1b | HOAc | Et2NH | HOAc | 3 | 3b | 72 |
| 1b | EtOH | Et2NH | HOAcc | 12 | ||
| 1b | TFA | Et2NH | TFA | 15 | ||
| 1b | THF | (i-Pr)2NH | TFAd | 12 | ||
| 1b | THF | Et2NH | HOAcc | 10 |
Table 2 Effects of solvents on the reaction of aromatic ketones 1a and 1b with formaldehyde and diethylamine a
| Ketone | Solvent | Amine | Catalyst | Time/h | Product | Yieldb(%) |
|---|---|---|---|---|---|---|
| 1a | HOAc | Et2NH | HOAc | 3 | 3a | 69 |
| 1a | EtOH | Et2NH | HOAcc | 5 | ||
| 1a | TFA | Et2NH | TFA | 15 | ||
| 1a | THF | Et2NH | HOAcc | 12 | ||
| 1b | HOAc | Et2NH | HOAc | 3 | 3b | 72 |
| 1b | EtOH | Et2NH | HOAcc | 12 | ||
| 1b | TFA | Et2NH | TFA | 15 | ||
| 1b | THF | (i-Pr)2NH | TFAd | 12 | ||
| 1b | THF | Et2NH | HOAcc | 10 |
| Entry | Ar | Product | Yieldb(%) | Entry | Ar | Product | Yieldb(%) |
|---|---|---|---|---|---|---|---|
| 1 | C6H5 | 3a | 69 | 9 | 3-F-C6H4 | 3i | 71 |
| 2 | 4-F-C6H4 | 3b | 72 | 10 | 2,4-F2-C6H3 | 3j | 70 |
| 3 | 4-Cl-C6H4 | 3c | 70 | 11 | 3,4-F2-C6H3 | 3k | 65 |
| 4 | 4-Br-C6H4 | 3d | 73 | 12 | 3-Cl-C6H4 | 3l | 68 |
| 5 | 4-CH3-C6H4 | 3e | 68 | 13 | 3-NO2-C6H4 | 3m | 73 |
| 6 | 4-OCH3-C6H4 | 3f | 68 | 14 | 2-Naphthyl | 3n | 69 |
| 7 | 4-NO2-C6H4 | 3g | 72 | 15 | 4-C6H5C6H4 | 3o | 71 |
| 8 | 2-F-C6H4 | 3h | 72 |
Table 3 Reaction of aromatic ketones with formaldehyde and diethylamine in acetic acida
| Entry | Ar | Product | Yieldb(%) | Entry | Ar | Product | Yieldb(%) |
|---|---|---|---|---|---|---|---|
| 1 | C6H5 | 3a | 69 | 9 | 3-F-C6H4 | 3i | 71 |
| 2 | 4-F-C6H4 | 3b | 72 | 10 | 2,4-F2-C6H3 | 3j | 70 |
| 3 | 4-Cl-C6H4 | 3c | 70 | 11 | 3,4-F2-C6H3 | 3k | 65 |
| 4 | 4-Br-C6H4 | 3d | 73 | 12 | 3-Cl-C6H4 | 3l | 68 |
| 5 | 4-CH3-C6H4 | 3e | 68 | 13 | 3-NO2-C6H4 | 3m | 73 |
| 6 | 4-OCH3-C6H4 | 3f | 68 | 14 | 2-Naphthyl | 3n | 69 |
| 7 | 4-NO2-C6H4 | 3g | 72 | 15 | 4-C6H5C6H4 | 3o | 71 |
| 8 | 2-F-C6H4 | 3h | 72 |
| [1] | Guo Q. S., Zhao C. G., Arman. H., Tetrahedron Lett., 2012, 53(36), 4866—4869 |
| [2] | Wang H. G., Liu D. H., Zhang Z. Q., Li Y. X., Yang Q. B., Du J. S., Chem. J. Chinese Universities,2012, 33(5), 1074—1077 |
| (王恒国, 刘大海, 张朝群, 李耀先, 杨清彪, 杜建时. 高等学校化学学报, 2012, 33(5), 1074—1077) | |
| [3] | Song Z. C., Wang S. S., Song Q. L., Shu T., Zhao W. J., Chem. J. Chinese Universities,2012, 33(4), 744—749 |
| (宋志成, 王世盛, 宋其玲, 舒涛, 赵伟杰. 高等学校化学学报, 2012, 33(4), 744—749) | |
| [4] | Jacobine A. M., Puchlopek A. L. A., Zercher C. K., Briggs J. B., Jasinski J. P., Butcher R. J., Tetrahedron,2012, 68, 7799—7805 |
| [5] | Li N. G., Song S. L., Shen M. Z., Tang Y. P., Shi Z. H., Tang H., Shi Q. P., Fu Y. F., Duan J. A., Bioorg. Med. Chem., 2012, 20, 6919—6923 |
| [6] | Zhong W. H., Wu Y. T., Zhang X. X., Su W. K., Chin. J. Med. Chem., 2008, 18(6), 426—428 |
| (钟为慧, 吴窈窕, 张兴贤, 苏为科. 中国药物化学杂志, 2008, 18(6), 426—428) | |
| [7] | Shi M., Cui S. H., Liu Y. H., Tetrahedron,2005, 61(21), 4965—4970 |
| [8] | Yue C. B., Yi T. F., Zhu C. B., Liu L., J. Ind. Eng. Chem., 2009, 15(5), 653—656 |
| [9] | He M., Pan Z. X., Bai S., Li P., Zhang Y. P., Jin L. H., Hu D. Y., Yang S., Song B. A., Sci. China Chem., 2013, 56(3), 321—328 |
| [10] | Wang L. M., Han J. W., Sheng J., Fan Z. Y., Tian H., Chin. J. Org. Chem., 2005, 25(5), 591—594 |
| (王利民, 韩建伟, 盛佳, 樊兆玉, 田禾. 有机化学, 2005, 25(5), 591—594) | |
| [11] | Tang Z. L., Xia Z. W., Ma H. W., Liu H. W., Ou X. M., Chin. J. Appl. Chem., 2013, 30(9), 993—998 |
| (唐子龙, 夏赞稳, 马红伟, 刘汉文, 欧晓明. 应用化学, 2013, 30(9), 993—998) | |
| [12] | Yuan J., Su X., Zhang X., Cong L., Guo C., Chem. Res. Chinese Universities,2011, 27(6), 955—957 |
| [13] | Chen J. L., Xie Z. F., Xing Y., Chin. J. Org. Chem., 2011, 31(10), 1714—1718 |
| (陈君丽, 解正峰, 邢烨. 有机化学, 2011, 31(10), 1714—1718) | |
| [14] | Wang L. M., Han J. W., Sheng J., Tian H., Fan Z. Y., Catal. Commun., 2005, 6(3), 201—204 |
| [15] | Zhang P., Huang J. H., Wang L. Z., Liu W., Li Y., Chin. J. Org. Chem., 2010, 30(12), 1939—1943 |
| (张萍, 黄金华, 王兰芝, 刘薇, 李媛. 有机化学, 2010, 30(12), 1939—1943) | |
| [16] | Bugarin A., Jones K. D., Connell B. T., Chem. Commun., 2010, 46, 1715—1717 |
| [17] | de Mancilha M., de Conti R., Moran P. J. S., Rodrigues J. A. R.,Arkivoc, 2001, (vi), 85—93 |
| [18] | Zhang P., Xuan Y., Li W., Li Y., Chin. J. Org. Chem., 2011, 31(8), 1240—1244 |
| (张萍, 宣烨, 刘薇, 李媛. 有机化学, 2011, 31(8), 1240—1244) | |
| [19] | Arend M., Westermann B., Risch N., Angew. Chem. Int. Ed., 1998, 37, 1044—1070 |
| [20] | Takahashi H., Kashiwa N., Hashimoto Y., Nagasawa K., Tetrahedron Lett., 2002, 43, 2935—2938 |
| [1] | ZHANG Juanrong,YOU Huimei,JING Yuxing,ZHAO Jiaowen,WANG Wei,LIU Wenxing,ZHOU Min,JIANG Zhiyong. Three New Phenolic Compounds from Salacia cochinchinensis Lour and Their α-Glucosidase Inhibitory Activities† [J]. Chem. J. Chinese Universities, 2019, 40(3): 456. |
| [2] | TANG Lin,JIANG Zhenqi,LI Juan,WU Aiguo. Anti-glioma Active Compounds and Their Nanomicelles Preparation of the Root of Actinidiaeriantha Benth† [J]. Chem. J. Chinese Universities, 2019, 40(3): 468. |
| [3] | BAI Lei,HUO Shuhui,CHEN Jing,LU Xiaoquan. Squaramide Fluorescence Probe for Chiral Recognition of α-Amino Acids† [J]. Chem. J. Chinese Universities, 2019, 40(1): 41. |
| [4] | TIAN Yao,ZHANG Chunquan,WANG Wenzhe,ZHOU Yingfang,LU Yitong,ZHANG Peng,JIA Zhenfu,ZHOU Chengyu,CHEN Shilan. Preparation of Polyrotaxane Cross-linking Agent with “Pulley” Effect and Its Potential Application in Swelling Grain Used as Profile Control and Water Plugging Agent† [J]. Chem. J. Chinese Universities, 2018, 39(9): 2098. |
| [5] | HUANG Weiwei, REN Jiawang, FANG Qianrong, Valentin VALTCHEV. Synthesis of Zeolite β@IISERP-COF2 Core-shell Hybrid Materials† [J]. Chem. J. Chinese Universities, 2018, 39(6): 1127. |
| [6] | ZHOU Min, XU Xiaoying, LONG Yuande. Enantioseparation of Seventeen Kinds of β-Lactams on Carboxymethyl-β-cyclodextrin Chiral Stationary Phase and Research on Enantioseparation Mechanism [J]. Chem. J. Chinese Universities, 2018, 39(6): 1164. |
| [7] | XIA Kun,WANG Yi,ZHOU Dan,HUANG Zhe,WU Zhonghan,XIA Qinghua. Rapid Synthesis of CoSAPO-5 Zeolite and Efficiently Catalytic Epoxidation of α-Pinene with Air† [J]. Chem. J. Chinese Universities, 2018, 39(5): 941. |
| [8] | SHI Penghui,BIAN Liujiao. Mechanism Study on the Interaction Between Cefoxitin and Metal β-Lactamase BcⅡ Based on Spectroscopic Methods and Computional Simulations† [J]. Chem. J. Chinese Universities, 2018, 39(5): 971. |
| [9] | LIU Li, MA Yangyang, WANG Kuan, JIA Yunjing, LI Wan, ZHU Huajie. Anti-tumor and Antimicrobial Activities of β-Carbolines† [J]. Chem. J. Chinese Universities, 2018, 39(4): 674. |
| [10] | YANG Qinghua, WANG Longgang, LIU Jie, LU Yong, CHEN Tianyun. Preparation and Characterization of Star-shaped β-Cyclodextrin Based Polymer† [J]. Chem. J. Chinese Universities, 2018, 39(4): 793. |
| [11] | CONG Rimin, YU Huaiqing, LUO Yunjun, LI Jiao, WANG Weiwei, LI Qiuhong, SUN Wuzhu, SI Weimeng, ZHANG Hua. Synthesis and Properties of Bi25FeO40/α-Fe2O3 Composite Nanoparticle Photocatalysts† [J]. Chem. J. Chinese Universities, 2018, 39(4): 629. |
| [12] | HUA Chenghe, MA Hongchao, DONG Xiaoli, ZHANG Xiufang. Synthesis and Photocatalytic Activity of α-Bi2O3 Nanotubes/Nitrogen Doped Carbon Quantum Dots Hybrid Material† [J]. Chem. J. Chinese Universities, 2018, 39(2): 200. |
| [13] | ZHANG Huan, DONG Xiaoyan. Effects of EGCG on Amyloid β-Protein Fibrillogenesis and Cytotoxicity at Different pH Values† [J]. Chem. J. Chinese Universities, 2018, 39(11): 2534. |
| [14] | LIN Musong, PENG Lei, FU Qiang, QIAN Yihua, CHEN Tiansheng, ZHANG Sheng, MA Xiaoqian. Research on Self-healing Insulating Material Based on Host-guest Cooperation† [J]. Chem. J. Chinese Universities, 2018, 39(11): 2572. |
| [15] | ZHANG Jin, SHI Tiancai, LUO Liwen, LIU Jia, LIU Rong, LIU Le, LIANG Ming, MA Yangmin. One-pot Synthesis of β-Carboline Derivatives Catalyzed by CuO Nanoparticles† [J]. Chem. J. Chinese Universities, 2018, 39(11): 2411. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||