

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (6): 1164.doi: 10.7503/cjcu20170810
• Analytical Chemistry • Previous Articles Next Articles
ZHOU Min1,2, XU Xiaoying1, LONG Yuande1,*( )
)
Received:2017-12-22
Online:2018-06-10
Published:2018-05-22
Contact:
LONG Yuande
E-mail:ydlong@cioc.ac.cn
CLC Number:
TrendMD:
ZHOU Min, XU Xiaoying, LONG Yuande. Enantioseparation of Seventeen Kinds of β-Lactams on Carboxymethyl-β-cyclodextrin Chiral Stationary Phase and Research on Enantioseparation Mechanism[J]. Chem. J. Chinese Universities, 2018, 39(6): 1164.
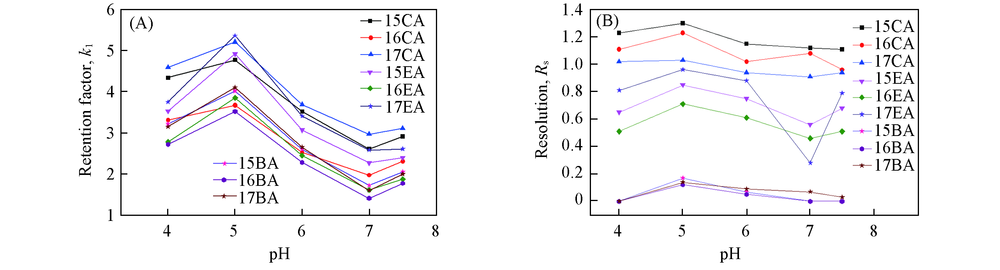
Fig.2 Effects of pH values of mobile phase on retention factor(A) and resolution of several compouds(B)Mobile phase: (0.50% NH4Ac+acid or base)-MeOH(50∶50, volume ratio); flow rate: 1.0 mL/min; detection: UV at 254 nm; temperature: 37 ℃.
| pH | Additive | 15CA | 15EA | 15BA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| k1 | α | Rs | k1 | α | Rs | k1 | α | Rs | ||
| 7.5 | NH3·H2O | 2.91 | 1.16 | 1.11 | 2.40 | 1.11 | 0.68 | 2.05 | 1.00 | 0.00 |
| TEA | 2.67 | 1.15 | 1.05 | 2.16 | 1.11 | 0.61 | 1.91 | 1.00 | 0.00 | |
| DEA | 2.49 | 1.16 | 1.04 | 2.12 | 1.11 | 0.61 | 1.86 | 1.00 | 0.00 | |
| 6.0 | HAc | 3.90 | 1.15 | 1.16 | 3.17 | 1.11 | 0.72 | 2.59 | 1.05 | 0.06 |
| TFA | 4.17 | 1.15 | 1.20 | 3.51 | 1.11 | 0.77 | 2.84 | 1.05 | 0.09 | |
| FA | 3.52 | 1.16 | 1.15 | 3.07 | 1.11 | 0.75 | 2.60 | 1.05 | 0.07 | |
| 5.0 | HAc | 5.49 | 1.15 | 1.27 | 4.73 | 1.10 | 0.81 | 3.78 | 1.05 | 0.15 |
| TFA | 5.89 | 1.15 | 1.29 | 5.12 | 1.10 | 0.80 | 4.19 | 1.00 | 0.00 | |
| FA | 4.77 | 1.18 | 1.30 | 4.92 | 1.10 | 0.85 | 4.02 | 1.06 | 0.17 | |
| 4.0 | HAc | 4.37 | 1.13 | 1.12 | 3.70 | 1.09 | 0.68 | 3.23 | 1.00 | 0.00 |
| TFA | 5.31 | 1.13 | 1.10 | 4.64 | 1.08 | 0.52 | 3.22 | 1.00 | 0.00 | |
| FA | 4.34 | 1.15 | 1.23 | 3.53 | 1.10 | 0.65 | 3.22 | 1.00 | 0.00 | |
Table 1 Effects of pH values of mobile phase on retention factor(k1), separation factor(α) and resolution(Rs) of compounds 15CA, 15EA and 15BA*
| pH | Additive | 15CA | 15EA | 15BA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| k1 | α | Rs | k1 | α | Rs | k1 | α | Rs | ||
| 7.5 | NH3·H2O | 2.91 | 1.16 | 1.11 | 2.40 | 1.11 | 0.68 | 2.05 | 1.00 | 0.00 |
| TEA | 2.67 | 1.15 | 1.05 | 2.16 | 1.11 | 0.61 | 1.91 | 1.00 | 0.00 | |
| DEA | 2.49 | 1.16 | 1.04 | 2.12 | 1.11 | 0.61 | 1.86 | 1.00 | 0.00 | |
| 6.0 | HAc | 3.90 | 1.15 | 1.16 | 3.17 | 1.11 | 0.72 | 2.59 | 1.05 | 0.06 |
| TFA | 4.17 | 1.15 | 1.20 | 3.51 | 1.11 | 0.77 | 2.84 | 1.05 | 0.09 | |
| FA | 3.52 | 1.16 | 1.15 | 3.07 | 1.11 | 0.75 | 2.60 | 1.05 | 0.07 | |
| 5.0 | HAc | 5.49 | 1.15 | 1.27 | 4.73 | 1.10 | 0.81 | 3.78 | 1.05 | 0.15 |
| TFA | 5.89 | 1.15 | 1.29 | 5.12 | 1.10 | 0.80 | 4.19 | 1.00 | 0.00 | |
| FA | 4.77 | 1.18 | 1.30 | 4.92 | 1.10 | 0.85 | 4.02 | 1.06 | 0.17 | |
| 4.0 | HAc | 4.37 | 1.13 | 1.12 | 3.70 | 1.09 | 0.68 | 3.23 | 1.00 | 0.00 |
| TFA | 5.31 | 1.13 | 1.10 | 4.64 | 1.08 | 0.52 | 3.22 | 1.00 | 0.00 | |
| FA | 4.34 | 1.15 | 1.23 | 3.53 | 1.10 | 0.65 | 3.22 | 1.00 | 0.00 | |
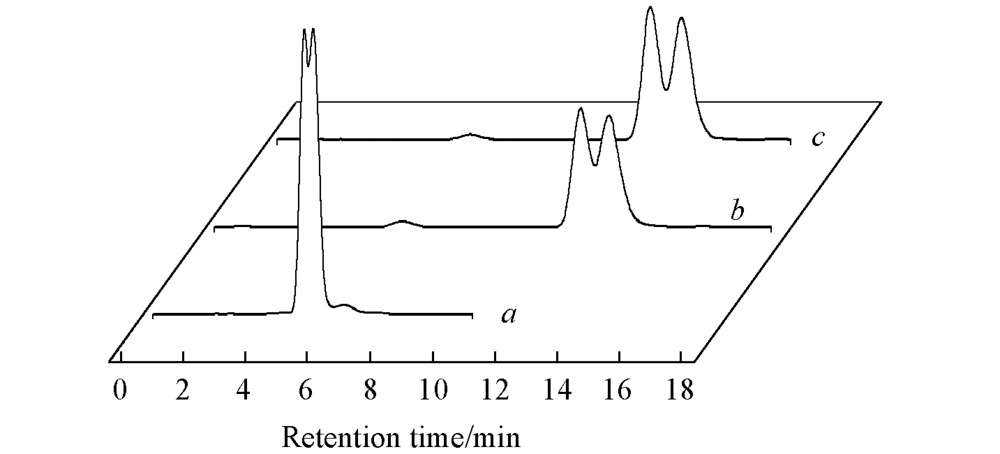
Fig.3 Effect of salt type on resolution of compound 15EAa. NH4H2PO4; b. NH4Cl; c. NH4Ac. Mobile phase: (salt+FA)(pH=5.0)-MeOH(50∶50, volume ratio); flow rate: 1.0 mL/min; detection: UV at 254 nm; temperature: 37 ℃.
| Analyte | CSP | k1 | α | Rs | Pb |
|---|---|---|---|---|---|
| 15CA | CM-β-CD | 13.64 | 1.15 | 1.71 | 1.00 |
| Native β-CD | 6.12 | 1.20 | — | 0.98 | |
| 16CA | CM-β-CD | 10.07 | 1.14 | 1.66 | 1.00 |
| Native β-CD | 5.43 | 1.18 | — | 0.92 | |
| 17CA | CM-β-CD | 13.41 | 1.13 | 1.57 | 0.97 |
| Native β-CD | 7.11 | 1.17 | — | 0.93 | |
| 18CA | CM-β-CD | 13.06 | 1.06 | 0.54 | 0.28 |
| Native β-CD | 7.14 | 1.10 | — | 0.52 | |
| 20CA | CM-β-CD | 12.08 | 1.14 | 1.60 | 1.00 |
| Native β-CD | 5.90 | 1.19 | — | 0.90 | |
| 21CA | CM-β-CD | 14.29 | 1.16 | 1.74 | 1.00 |
| Native β-CD | 6.76 | 1.22 | — | 0.95 | |
| 22CA | CM-β-CD | 6.49 | 1.14 | 1.57 | 1.00 |
| Native β-CD | 4.88 | 1.15 | — | 0.88 | |
| 15EA | CM-β-CD | 10.98 | 1.12 | 1.38 | 0.94 |
| Native β-CD | 5.44 | 1.13 | — | 0.72 | |
| 16EA | CM-β-CD | 8.13 | 1.12 | 1.34 | 0.93 |
| Native β-CD | 4.67 | 1.12 | — | 0.72 | |
| 17EA | CM-β-CD | 11.28 | 1.13 | 1.51 | 0.96 |
| Native β-CD | 6.61 | 1.17 | — | 0.91 | |
| Analyte | CSP | k1 | α | Rs | Pb |
| 18EA | CM-β-CD | 12.27 | 1.06 | 0.54 | 0.31 |
| Native β-CD | 6.88 | 1.09 | — | 0.42 | |
| 20EA | CM-β-CD | 9.52 | 1.12 | 1.38 | 0.94 |
| Native β-CD | 5.12 | 1.15 | — | 0.80 | |
| 21EA | CM-β-CD | 13.88 | 1.11 | 1.23 | 0.85 |
| Native β-CD | 5.91 | 1.11 | — | 0.59 | |
| 22EA | CM-β-CD | 5.43 | 1.08 | 0.74 | 0.48 |
| Native β-CD | 3.79 | 1.04 | — | 0.05 | |
| 15BA | CM-β-CD | 10.43 | 1.08 | 0.82 | 0.55 |
| Native β-CD | 4.29 | 1.11 | — | 0.59 | |
| 16BA | CM-β-CD | 8.51 | 1.08 | 0.92 | 0.67 |
| Native β-CD | 4.09 | 1.10 | — | 0.45 | |
| 17BA | CM-β-CD | 9.73 | 1.08 | 0.91 | 0.64 |
| Native β-CD | 4.73 | 1.11 | — | 0.57 |
Table 3 Enantioseparation of chiral compounds on CM-β-CD CSP in the reversed-phase modea
| Analyte | CSP | k1 | α | Rs | Pb |
|---|---|---|---|---|---|
| 15CA | CM-β-CD | 13.64 | 1.15 | 1.71 | 1.00 |
| Native β-CD | 6.12 | 1.20 | — | 0.98 | |
| 16CA | CM-β-CD | 10.07 | 1.14 | 1.66 | 1.00 |
| Native β-CD | 5.43 | 1.18 | — | 0.92 | |
| 17CA | CM-β-CD | 13.41 | 1.13 | 1.57 | 0.97 |
| Native β-CD | 7.11 | 1.17 | — | 0.93 | |
| 18CA | CM-β-CD | 13.06 | 1.06 | 0.54 | 0.28 |
| Native β-CD | 7.14 | 1.10 | — | 0.52 | |
| 20CA | CM-β-CD | 12.08 | 1.14 | 1.60 | 1.00 |
| Native β-CD | 5.90 | 1.19 | — | 0.90 | |
| 21CA | CM-β-CD | 14.29 | 1.16 | 1.74 | 1.00 |
| Native β-CD | 6.76 | 1.22 | — | 0.95 | |
| 22CA | CM-β-CD | 6.49 | 1.14 | 1.57 | 1.00 |
| Native β-CD | 4.88 | 1.15 | — | 0.88 | |
| 15EA | CM-β-CD | 10.98 | 1.12 | 1.38 | 0.94 |
| Native β-CD | 5.44 | 1.13 | — | 0.72 | |
| 16EA | CM-β-CD | 8.13 | 1.12 | 1.34 | 0.93 |
| Native β-CD | 4.67 | 1.12 | — | 0.72 | |
| 17EA | CM-β-CD | 11.28 | 1.13 | 1.51 | 0.96 |
| Native β-CD | 6.61 | 1.17 | — | 0.91 | |
| Analyte | CSP | k1 | α | Rs | Pb |
| 18EA | CM-β-CD | 12.27 | 1.06 | 0.54 | 0.31 |
| Native β-CD | 6.88 | 1.09 | — | 0.42 | |
| 20EA | CM-β-CD | 9.52 | 1.12 | 1.38 | 0.94 |
| Native β-CD | 5.12 | 1.15 | — | 0.80 | |
| 21EA | CM-β-CD | 13.88 | 1.11 | 1.23 | 0.85 |
| Native β-CD | 5.91 | 1.11 | — | 0.59 | |
| 22EA | CM-β-CD | 5.43 | 1.08 | 0.74 | 0.48 |
| Native β-CD | 3.79 | 1.04 | — | 0.05 | |
| 15BA | CM-β-CD | 10.43 | 1.08 | 0.82 | 0.55 |
| Native β-CD | 4.29 | 1.11 | — | 0.59 | |
| 16BA | CM-β-CD | 8.51 | 1.08 | 0.92 | 0.67 |
| Native β-CD | 4.09 | 1.10 | — | 0.45 | |
| 17BA | CM-β-CD | 9.73 | 1.08 | 0.91 | 0.64 |
| Native β-CD | 4.73 | 1.11 | — | 0.57 |
| [1] | Lamotte J., Dive G., Ghuysen J. M., Eur. J. Med. Chem., 1991, 26(1), 43—50 |
| [2] | Adlington R. M., Baldwin J. E., Becker G. W., Chen B. N., Cheng L. F., Cooper S. L., Hermann R. B., Howe T. J., Mccoull W., Mcnulty A. M., Neubauer B. L., Pritchard G. J., J. Med. Chem., 2001, 44(10), 1491—1508 |
| [3] | Burnett A .D., Curr. Med. Chem., 2004, 11(14), 1873—1887 |
| [4] | Palomo C., Aizpurua J. M., Ganboa I., Oiarbide M., Synlett, 2001, 12, 1813—1826 |
| [5] | Fülöp F., Chem. Rev., 2001, 101(7), 2181—2204 |
| [6] | Wasserman H. H., Matsuyama H., Robinson R. P., Tetrahedron, 2002, 58, 7177—7190 |
| [7] | Palomo C., Ganboa I., Oiarbide M., Sciano G. T.,Miranda J. L.,Arkivoc, 2002, 5, 8—16 |
| [8] | Fülöp F., Forró E., Tóth G .K., Org. Lett., 2004, 6(23), 4239—4241 |
| [9] | Dražić T., MolČanov K., Sachdev V., Malnar M., Hećimović S., Patankar J. V., Obrowsky S., Levak-Frank S., Habuš I., Kratky D., Eur. J. Med. Chem., 2014, 7, 722—734 |
| [10] | Dražić T., Sachdev V., Leopold C., Patankar J. V., Malnar M., Hećimović S., Levak-Frank S., Habuš I., Kratky D., Bioorg. Med. Chem., 2015, 23(10), 2353—2359 |
| [11] | Nuzzi A., Fiasella A., Ortega J. A., Pagliuca C., Ponzano S., Pizzirani D., Bertozzi S. M., Ottonello G., Tarozzo G., Reggiani A., Bandiera T., Bertozzi F., Piomelli D., Eur. J. Med. Chem., 2016, 111, 138—159 |
| [12] | Boyd D. B., J. Med. Chem., 1983, 26(7), 1010—1013 |
| [13] | Pirkle W. H., Spence P. L., Chirality, 1998, 10(5), 430—433 |
| [14] | Cirilli R., Del Giudice M. R., Ferretti R., La Torre F., J. Chromatogr. A, 2001, 923(1/2), 27—36 |
| [15] | Pataj Z., Ilisz I., Berkecz R., Forró E., Fülöp F., Péter A., Chirality, 2010, 22(1), 120—128 |
| [16] | Péter A., rki A., Forró E., Fülöp F., Armstrong D. W., Chirality, 2005, 17(4), 193—200 |
| [17] | Dražić T., Roje M., Jurin M., Pescitelli G., Eur. J. Org.Chem., 2016, 2016(24), 4189—4199 |
| [18] | Huang T. B., Kuang C. Y., Zhou J. X., Gou D. M., Chinese J. Anal. Chem., 1991, 19(6), 687—689 |
| (黄天宝, 旷昌渝, 周竞先, 苟大明. 分析化学, 1991, 19(6), 687—689) | |
| [19] | Sun P., Wang C., Armstrong D., Péter A., Forró E.,J. Liq. Chromatogr. R. T., 2006, 29(13), 1847—1860 |
| [20] | Berkecz R., Török R., Ilisz I., Forró E., Fülöp F., Armstrong D. W., Péter A., Chromatographia., 2006, 63(S13), S37—S43 |
| [21] | Fodor G., Ilisz I., Szemán J., Iványi R., Szente L., Varga G., Forró E., Fülöp F., Péter A.,Chromatographia., 2010, 71(S1), 29—34 |
| [22] | Zhou M., Zhi Y.G., Xu X. Y., Long Y. D., Chin. Chem. Lett., https://doi.org/10.1016/j.cclet.2017.10.039 |
| [23] | Machida Y., Nishi H., Nakamura K., J. Chromatogr. A, 1999, 830, 311—320 |
| [24] | Lin C., Liu W. N., Fan J., Wang Y. K., Zheng S. R., Lin R., Zhang H., Zhang W., J. Chromatogr. A, 2013, 1283, 68—74 |
| [25] | Sardella R., Ianni F., Lisanti A., Marinozzi M., Scorzoni S., Natalini B., Biomed. Chromatogr., 2014, 28(1), 159—167 |
| [26] | Wang Y., Ong T. T., Li L. S., Tan T. T. Y., Ng S. C., J. Chromatogr. A, 2009, 1216(12), 2388—2393 |
| [27] | Glajch J. L., Kirkland J. J., Squire K. M., J. Chromatogr., 1980, 199, 57—59 |
| [1] | WANG Mingfang, FU Hua, FU Zhibo, WANG Yuerong, ZHANG Hongyang, ZHANG Min, HU Ping. Separation and Characterization of Polymer Blends Using Online Ultra-high Performance Liquid Chromatography-Size Exclusion Chromatography [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210865. |
| [2] | LI Jing, SU Wei, WANG Xueyuan, FU Peng, SUN Yan. Synthesis and Characterization of Antihypertensive Drug Aranidipine and Its Related Impurities [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210663. |
| [3] | WANG Tianqi,YU Qiongwei,FENG Yuqi. Analysis of Imidazole Propionic Acid in Serum of Patients with Type 2 Diabetes Based on NiO@SiO2 Solid-phase Extraction Coupled with Liquid Chromatography-Mass Spectrometry † [J]. Chem. J. Chinese Universities, 2020, 41(2): 262. |
| [4] | ZHAO Mengxin, MENG Zhe, LI Heping, MA Zongqin, ZHAN Haijuan, LIU Wanyi. Photodegradation of Antibiotic in Environmental Water by Graphene Oxide Modulation Bismuth Molybdate Under Visible Light Irradiation [J]. Chem. J. Chinese Universities, 2020, 41(11): 2479. |
| [5] | ZHANG Hui, ZHANG Chenjie, XU Minmin, YUAN Yaxian, YAO Jianlin. Investigation on the Reaction of o-Aminothiophenol and 2-Iodobenzoyl Chloride Monitored by SERS-HPLC Technique [J]. Chem. J. Chinese Universities, 2020, 41(11): 2496. |
| [6] | ZHAO Huanxi,WANG Qiuying,SUN Xiuli,LI Xue,MIAO Rui,WU Dongxue,LIU Shuying,XIU Yang. Discrimination of Ginseng Origins and Identification of Ginsenoside Markers Based on HPLC-MS Combined with Multivariate Statistical Analysis† [J]. Chem. J. Chinese Universities, 2019, 40(2): 246. |
| [7] | HE Yangyang,LI Yi,LI Baozong,YANG Yonggang. Biomimetic Mineralization at a Dilute Concentration and Application in Enantioseparation† [J]. Chem. J. Chinese Universities, 2019, 40(2): 393. |
| [8] | QIU Xiuzhen, HUA Yongbiao, GUO Huishi, LU Wenguan. Preparation of a Molecularly Imprinted Polymer Nanotubes Membrane and Its Application in the Determination of Catecholamines in Urine Samples† [J]. Chem. J. Chinese Universities, 2018, 39(4): 653. |
| [9] | MIAO Rui,WU Dongxue,WANG Qiuying,ZHAO Huanxi,LI Xue,XIU Yang,LIU Shuying. Rapid Separation of Ginsenosides Based on Multi-walled Carbon Nanotubes† [J]. Chem. J. Chinese Universities, 2018, 39(10): 2178. |
| [10] | LI Nan, ZHAO Huanxi, LI Jing, WANG Nan, YU Bohao, YUE Hao, YU Shanshan. Transformation of Total Notoginsenosides by Recombinant Endocellulase Fpendo5A† [J]. Chem. J. Chinese Universities, 2017, 38(12): 2185. |
| [11] | YUAN Liming, SU Yingqiu, DUAN Aihong, ZHENG Ying, AI Ping, CHEN Xuexian. Optical Resolution of D,L-Phenylglycine and Chiral Separation Mechanism Using an Enantioselective Membrane of Vancomycin† [J]. Chem. J. Chinese Universities, 2016, 37(11): 1960. |
| [12] | NONG Ruiyu, KONG Jiao, ZHANG Junhui, CHEN Ling, TANG Bo, XIE Shengming, YUAN Liming. Chiral Metal-organic Framework{[Co(L-trp)(bpe)(H2O)]·H2O·NO3}n Used for High Performance Liquid Chromatographic Separation† [J]. Chem. J. Chinese Universities, 2016, 37(1): 19. |
| [13] | CHENG Biaoping, LI Laisheng, ZHOU Rendan, LI Liang, ZHANG Hongfu. Enantioseparations of Triazole Chiral Pesticides on Two β-Cyclodextrin-bonded Stationary Phases with Different Linkages by HPLC† [J]. Chem. J. Chinese Universities, 2015, 36(5): 872. |
| [14] | TURSON Mamat, DAWUT Gulbahar, EMIN Risalat, CHU Ganghui, JELIL Mahmutjan, TURHON Muhetar. Preparation of Quercetin Imprinted Polymer by Living Radical Polymerization and Its Application in the Composition Analysis of Zukamu Granules for Uighur Medicine† [J]. Chem. J. Chinese Universities, 2015, 36(12): 2402. |
| [15] | WEI Yu, CHEN Yufu, ZHOU Qiang, YUAN Qiuyue, TAN Fengyu, XIE Tianyao. Enantioseparation of Underivatized D,L-Serine in Biological Matrices by Capillary Electrophoresis with Contactless Conductivity Detection† [J]. Chem. J. Chinese Universities, 2014, 35(7): 1409. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||