

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (3): 538.doi: 10.7503/cjcu20130646
• Organic Chemistry • Previous Articles Next Articles
LIU Qian1, LI Wenhong2, QIU Zhaolai1, LI Yuan1,*( )
)
Received:2013-07-09
Online:2014-03-10
Published:2014-01-20
Contact:
LI Yuan
E-mail:liyuanhbsd@163.com
Supported by:CLC Number:
TrendMD:
LIU Qian, LI Wenhong, QIU Zhaolai, LI Yuan. Michael Addition of Aminothiophenols to α,β-Unsaturated Ketones with High Steric Hindrance Catalyzed by CeCl3·7H2O-NaI†[J]. Chem. J. Chinese Universities, 2014, 35(3): 538.
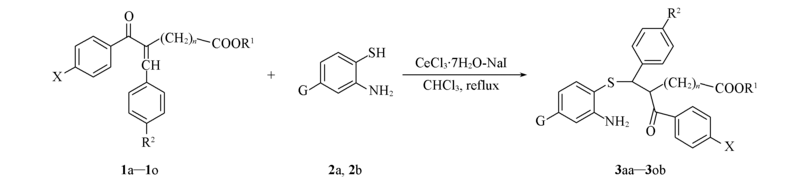
Scheme 1 Michael addition of 2-aminothiophenols to α,β-unsaturated ketones catalyzed by CeCl3·7H2O-NaI 1a—1c(n=1): R1=C2H5, X=H, R2=H(1a), CH3(1b), Br(1c); G=H(2a); G=Cl(2b); 1d—1o(n=2): R1=C2H5, X=Cl, R2=H(1d), CH3(1e), F(1f); R1=C2H5, X=H, R2=H(1g), CH3(1h), F(1i), Cl(1j), Br(1k); R1=C2H5, X=CH3, R2=H(1l), CH3(1m), F(1n); R1=CH(CH3)2, X=H, R2=H(1o)
| Entry | Catalyst | Molar ratio of catalyst to 1d | Solvent | Time/h | Yield(%) |
|---|---|---|---|---|---|
| 1 | TsOH | 1:10 | CH3OH | 2 | 0b |
| 2 | TsOH | 1:10 | CHCl3 | 2 | Traceb |
| 3 | TsOH | 1:10 | CHCl3 | 6 | Traceb |
| 4 | FeCl3 | 1:10 | CHCl3 | 2 | 0b |
| 5 | HClO4-SiO2 | 1:10 | CH3OH | 2 | 0b |
| 6 | HClO4-SiO2 | 1:10 | CHCl3 | 2 | 0b |
| 7 | HOAc | 1:10 | CHCl3 | 2 | 0b |
| 8 | HOAc | 1:10 | CH3OH | 2 | 0b |
| 9 | CeCl3·7H2O-NaI | 1:3 | CHCl3 | 2 | 42.8c |
| 10 | CeCl3·7H2O-NaI | 1:2 | CHCl3 | 2 | 50.9c |
| 11 | CeCl3·7H2O | 1:3 | CHCl3 | 2 | Traceb |
| 12 | (CH3)3COK | 1:3 | CHCl3 | 2 | 0b |
| 13 | (CH3CH2)3N | 1:3 | CHCl3 | 2 | 0b |
| 14 | (CH3CH2)3N | 1:3 | CH3OH | 2 | 0b |
| 15 | (CH3CH2)3N | 1:3 | CH3OH | 8 | 0b |
| 16 | DIPEA | 1:3 | CHCl3 | 2 | 0b |
| 17 | EtONa | 1:10 | DMF | 2 | 0b |
| 18 | EtONa | 1:10 | CHCl3 | 2 | 0b |
| 19 | Montmorillonite K10 | 2%d | CHCl3 | 2 | 0b |
Table 1 Michael addition of compound 2a to 1d catalyzed by different catalystsa
| Entry | Catalyst | Molar ratio of catalyst to 1d | Solvent | Time/h | Yield(%) |
|---|---|---|---|---|---|
| 1 | TsOH | 1:10 | CH3OH | 2 | 0b |
| 2 | TsOH | 1:10 | CHCl3 | 2 | Traceb |
| 3 | TsOH | 1:10 | CHCl3 | 6 | Traceb |
| 4 | FeCl3 | 1:10 | CHCl3 | 2 | 0b |
| 5 | HClO4-SiO2 | 1:10 | CH3OH | 2 | 0b |
| 6 | HClO4-SiO2 | 1:10 | CHCl3 | 2 | 0b |
| 7 | HOAc | 1:10 | CHCl3 | 2 | 0b |
| 8 | HOAc | 1:10 | CH3OH | 2 | 0b |
| 9 | CeCl3·7H2O-NaI | 1:3 | CHCl3 | 2 | 42.8c |
| 10 | CeCl3·7H2O-NaI | 1:2 | CHCl3 | 2 | 50.9c |
| 11 | CeCl3·7H2O | 1:3 | CHCl3 | 2 | Traceb |
| 12 | (CH3)3COK | 1:3 | CHCl3 | 2 | 0b |
| 13 | (CH3CH2)3N | 1:3 | CHCl3 | 2 | 0b |
| 14 | (CH3CH2)3N | 1:3 | CH3OH | 2 | 0b |
| 15 | (CH3CH2)3N | 1:3 | CH3OH | 8 | 0b |
| 16 | DIPEA | 1:3 | CHCl3 | 2 | 0b |
| 17 | EtONa | 1:10 | DMF | 2 | 0b |
| 18 | EtONa | 1:10 | CHCl3 | 2 | 0b |
| 19 | Montmorillonite K10 | 2%d | CHCl3 | 2 | 0b |
| Entry | Solvent | Molar ratio of CeCl3·7H2O to 1d | Yield(%) | Entry | Solvent | Molar ratio of CeCl3·7H2O to 1d | Yield(%) |
|---|---|---|---|---|---|---|---|
| 1 | CHCl3 | 1:1 | 44.6b | 6 | DMSO | 1:1 | 0c |
| 2 | C6H6 | 1:1 | 35.6b | 7 | THF | 1:1 | Tracec |
| 3 | CH3OH | 1:1 | Tracec | 8 | CHCl3 | 1:5 | 40.5b |
| 4 | CH3CN | 1:1 | 0c | 9 | CHCl3 | 1:3 | 42.8b |
| 5 | DMF | 1:1 | 0c | 10 | CHCl3 | 1:2 | 50.9b |
Table 2 Effects of solvent and the amount of catalysts on the Michael addition of 2a to 1da
| Entry | Solvent | Molar ratio of CeCl3·7H2O to 1d | Yield(%) | Entry | Solvent | Molar ratio of CeCl3·7H2O to 1d | Yield(%) |
|---|---|---|---|---|---|---|---|
| 1 | CHCl3 | 1:1 | 44.6b | 6 | DMSO | 1:1 | 0c |
| 2 | C6H6 | 1:1 | 35.6b | 7 | THF | 1:1 | Tracec |
| 3 | CH3OH | 1:1 | Tracec | 8 | CHCl3 | 1:5 | 40.5b |
| 4 | CH3CN | 1:1 | 0c | 9 | CHCl3 | 1:3 | 42.8b |
| 5 | DMF | 1:1 | 0c | 10 | CHCl3 | 1:2 | 50.9b |
| Chalcone | Amino thiophenol | Product | Yieldb (%) | Chalcone | Amino thiophenol | Product | Yieldb (%) |
|---|---|---|---|---|---|---|---|
| 1a | 2a | 3aa | 53.6 | 1j | 2a | 3ja | 48.1 |
| 1b | 2a | 3ba | 50.9 | 1k | 2a | 3ka | 48.3 |
| 1c | 2a | 3ca | 48.7 | 1g | 2b | 3gb | 58.8 |
| 1a | 2b | 3ab | 43.5 | 1h | 2b | 3hb | 43.1 |
| 1b | 2b | 3bb | 44.3 | 1i | 2b | 3ib | 48.3 |
| 1c | 2b | 3cb | 45.7 | 1j | 2b | 3jb | 46.4 |
| 1d | 2a | 3da | 50.9 | 1k | 2b | 3kb | 45.7 |
| 1e | 2a | 3ea | 44.9 | 1l | 2a | 3la | 47.6 |
| 1f | 2a | 3fa | 47.3 | 1m | 2a | 3ma | 43.1 |
| 1d | 2b | 3db | 53.9 | 1n | 2a | 3na | 57.0 |
| 1e | 2b | 3eb | 46.6 | 1l | 2b | 3lb | 45.5 |
| 1f | 2b | 3fb | 49.5 | 1m | 2b | 3mb | 47.5 |
| 1g | 2a | 3ga | 43.2 | 1n | 2b | 3nb | 45.1 |
| 1h | 2a | 3ha | 51.2 | 1o | 2a | 3oa | 48.6 |
| 1i | 2a | 3ia | 52.8 | 1o | 2b | 3ob | 46.9 |
Table 3 Michael addition of 2-aminothiophenol(4-chloro-2-aminothiophenol) to α,β-unsaturated ketones catalyzed by CeCl3·7H2O-NaIa
| Chalcone | Amino thiophenol | Product | Yieldb (%) | Chalcone | Amino thiophenol | Product | Yieldb (%) |
|---|---|---|---|---|---|---|---|
| 1a | 2a | 3aa | 53.6 | 1j | 2a | 3ja | 48.1 |
| 1b | 2a | 3ba | 50.9 | 1k | 2a | 3ka | 48.3 |
| 1c | 2a | 3ca | 48.7 | 1g | 2b | 3gb | 58.8 |
| 1a | 2b | 3ab | 43.5 | 1h | 2b | 3hb | 43.1 |
| 1b | 2b | 3bb | 44.3 | 1i | 2b | 3ib | 48.3 |
| 1c | 2b | 3cb | 45.7 | 1j | 2b | 3jb | 46.4 |
| 1d | 2a | 3da | 50.9 | 1k | 2b | 3kb | 45.7 |
| 1e | 2a | 3ea | 44.9 | 1l | 2a | 3la | 47.6 |
| 1f | 2a | 3fa | 47.3 | 1m | 2a | 3ma | 43.1 |
| 1d | 2b | 3db | 53.9 | 1n | 2a | 3na | 57.0 |
| 1e | 2b | 3eb | 46.6 | 1l | 2b | 3lb | 45.5 |
| 1f | 2b | 3fb | 49.5 | 1m | 2b | 3mb | 47.5 |
| 1g | 2a | 3ga | 43.2 | 1n | 2b | 3nb | 45.1 |
| 1h | 2a | 3ha | 51.2 | 1o | 2a | 3oa | 48.6 |
| 1i | 2a | 3ia | 52.8 | 1o | 2b | 3ob | 46.9 |
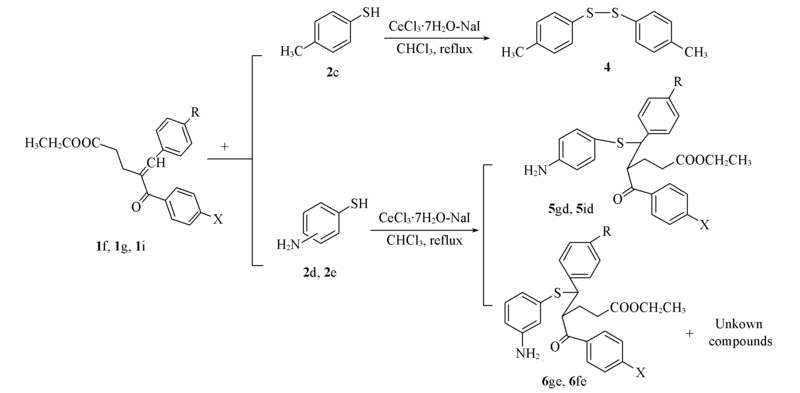
Scheme 2 Michael addition of substituted thiophenols(2c—2e) to α,β-unsaturated ketones (1f, 1g and 1i) catalyzed by CeCl3·7H2O-NaI1f: X=Cl, R=F; 1g: X=H, R=H; 1i: X=H, R=F; 2d: p-NH2-C6H4-SH; 2e: m-NH2-C6H4-SH
| Chalcone | Aminothiophenol | Molar ratiob | Time/h | Product | Yield(%) |
|---|---|---|---|---|---|
| 1g | 2d | 1:2 | 2 | 5gd | Traced |
| 1g | 2d | 1:2 | 5 | 5gd | 30.3e |
| 1i | 2d | 1:2 | 5 | 5id | 18.4e |
| 1g | 2e | 1:1 | 5 | 6gec | Traced |
| 1g | 2e | 1:5 | 5 | 6gec | Traced |
| 1g | 2e | 1:2 | 5 | 6gec | 30.6e |
| 1g | 2e | 1:2 | 8 | 6gec | 30.6e |
| 1f | 2e | 1:2 | 5 | 6fec | 8.4e |
Table 4 Michael addition of substituted thiophenols 2d and 2e to α,β-unsaturated ketones(1f, 1g and 1i) catalyzed by CeCl3·7H2O-NaIa
| Chalcone | Aminothiophenol | Molar ratiob | Time/h | Product | Yield(%) |
|---|---|---|---|---|---|
| 1g | 2d | 1:2 | 2 | 5gd | Traced |
| 1g | 2d | 1:2 | 5 | 5gd | 30.3e |
| 1i | 2d | 1:2 | 5 | 5id | 18.4e |
| 1g | 2e | 1:1 | 5 | 6gec | Traced |
| 1g | 2e | 1:5 | 5 | 6gec | Traced |
| 1g | 2e | 1:2 | 5 | 6gec | 30.6e |
| 1g | 2e | 1:2 | 8 | 6gec | 30.6e |
| 1f | 2e | 1:2 | 5 | 6fec | 8.4e |
| Reused times | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| Yieldb(%) | 50.9 | 49.5 | 46.9 | 43.3 | 41.8 |
Table 5 Reusability of CeCl3·7H2O-NaI in Michael addition of compounds 2a and 1da
| Reused times | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| Yieldb(%) | 50.9 | 49.5 | 46.9 | 43.3 | 41.8 |
| [1] | Bartoli G., Marcantoni E., Sambri L.,Synlett, 2003, (14), 2101—2116 |
| [2] | Bartoli G., Bosco M., Bellucci M.C., Marcantoni E., Sambri L., Torregiani E.,Eur. J. Org. Chem., 1999, 617—620 |
| [3] | Bartoli G., Bartolacci M., Giuliani A., Marcantoni E., Massaccesi M., Torregiani E., J. Org. Chem., 2005, 70(1), 169—174 |
| [4] | Garg S.K., Kumar R., Chakraborti A. K.,Synlett, 2005, (9), 1370—1374 |
| [5] | Garg S. K., Kumar R., Chakraborti A. K., Tetrahedron Lett., 2005, 46(10), 1721—1724 |
| [6] | Kawatsura M., Komatsu Y., Yamamoto M., Hayase S., Itoh T., Tetrahedron,2008, 64(16), 3488—3493 |
| [7] | Yadav L. D. S., Rai V. K., Tetrahedron Lett., 2008, 49(38), 5553—5556 |
| [8] | Bartoli G., Bellucci M. C., Petrini M., Marcantoni E., Sambri L., Torregiani E., Org. Lett., 2000, 2(13), 1791—1793 |
| [9] | Bartoli G., Di Antonio G., Giovannini R., Giuli S., Lanari S., Paoletti M., Marcantoni E., J. Org. Chem., 2008, 73(5), 1919—1924 |
| [10] | Bartoli G., Giovannini R., Giuliani A., Marcantoni E., Massaccesi M., Melchiorre P., Paoletti M., Sambri L.,Eur. J. Org. Chem., 2006, (6), 1476—1482 |
| [11] | Marotta E., Foresti E., Marcelli T., Peri F., Righi P., Scardovi N., Rosini G., Org. Lett., 2002, 4(25), 4451—4453 |
| [12] | Deo M. D., Marcantoni E., Torregiani E., Bartoli G., Bellucci M. C., Bosco M., Sambri L., J. Org. Chem., 2000, 65(9), 2830—2833 |
| [13] | Yadav J.S., Reddy B. V. S., Reddy K. S.,Synlett, 2002, (3), 468—470 |
| [14] | Bartoli G., Bosco M., Marcantoni E., Sambri L., Torregiani E.,Synlett, 1998, (2), 209—211 |
| [15] | Yadav L. D. S., Kapoor R., Synlett,2008, 15, 2348—2354 |
| [16] | Bartoli G., Bosco M., Bellucci M. C., Marcantoni E., Sambri L., Torregiani E., Eur. J. Org. Chem., 1999, 3, 617—620 |
| [17] | Bartoli G., Bosco M., Marcantoni E., Petrini M., Sambri L., Torregiani E., J. Org. Chem., 2001, 66(26), 9052—9055 |
| [18] | Bartoli G., Bartolacci M., Bosco M., Foglia G., Giuliani A., Marcantoni E., Sambri L., Torregiani E., J. Org. Chem., 2003, 68(11), 4594—4597 |
| [19] | Bartoli G., Bartolacci M., Giuliani A., Marcantoni E., Massaccesi M., Torregiani E., J. Org.Chem., 2005, 70(1), 169—174 |
| [20] | Qiu Z. L., Li W. H., Zhu H. F., Liu Q., Li Y., Chem. J. Chinese Universities,2013, 34(3), 579—589 |
| (邱召来, 李文红, 朱海菲, 刘倩, 李媛. 高等学校化学学报, 2013, 34(3), 579—589) | |
| [21] | Ye J. H., Xu G. J., Zhang W. C., Tian G. R., Wang S. B., Chin. J. Org. Chem., 2009, 29(10), 1664—1667 |
| (叶家海, 徐国际, 张文超, 田桂蓉, 王胜兵. 有机化学, 2009, 29(10), 1664—1667) | |
| [22] | Khatik G. L., Sharma G., Kumar R., Chakraborti A. K., Tetrahedron,2007, 63(5), 1200—1210 |
| [23] | Kawatsura M., Komatsu Y., Yamamoto M., Hayase S., Itoh T., Tetrahedron Lett., 2007, 48(37), 6480—6482 |
| [24] | Bartoli G., Fernández-Bolaños J. G., Antonio G. D., Foglia G., Giuli S., Gunnella R., Mancinelli M., Marcantoni E., Paoletti M., J. Org. Chem., 2007, 72(16), 6029—6036 |
| [25] | Bartoli G., Bosco M., Giuliani A., Marcantoni E., Palmieri A., Petrini M., Sambri L., J. Org. Chem., 2004, 69(4), 1290—1297 |
| [1] | TENG Zhenyuan, ZHANG Qitao, SU Chenliang. Charge Separation and Surface Reaction Mechanisms for Polymeric Single-atom Photocatalysts [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220325. |
| [2] | YANG Jingyi, SHI Siqi, PENG Huaitao, YANG Qihao, CHEN Liang. Integration of Atomically Dispersed Ga Sites with C3N4 Nanosheets for Efficient Photo-driven CO2 Cycloaddition [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220349. |
| [3] | WANG Ruyue, WEI Hehe, HUANG Kai, WU Hui. Freezing Synthesis for Single Atom Materials [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220428. |
| [4] | WANG Xintian, LI Pan, CAO Yue, HONG Wenhao, GENG Zhongxuan, AN Zhiyang, WANG Haoyu, WANG Hua, SUN Bin, ZHU Wenlei, ZHOU Yang. Techno-economic Analysis and Industrial Application Prospects of Single-atom Materials in CO2 Catalysis [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220347. |
| [5] | QIN Yongji, LUO Jun. Applications of Single-atom Catalysts in CO2 Conversion [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220300. |
| [6] | YAO Qing, YU Zhiyong, HUANG Xiaoqing. Progress in Synthesis and Energy-related Electrocatalysis of Single-atom Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220323. |
| [7] | LIN Zhi, PENG Zhiming, HE Weiqing, SHEN Shaohua. Single-atom and Cluster Photocatalysis: Competition and Cooperation [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220312. |
| [8] | YANG Jingyi, LI Qinghe, QIAO Botao. Synergistic Catalysis Between Ir Single Atoms and Nanoparticles for N2O Decomposition [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220388. |
| [9] | LIN Gaoxin, WANG Jiacheng. Progress and Perspective on Molybdenum Disulfide with Single-atom Doping Toward Hydrogen Evolution [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220321. |
| [10] | WANG Sicong, PANG Beibei, LIU Xiaokang, DING Tao, YAO Tao. Application of XAFS Technique in Single-atom Electrocatalysis [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220487. |
| [11] | TANG Quanjun, LIU Yingxin, MENG Rongwei, ZHANG Ruotian, LING Guowei, ZHANG Chen. Application of Single-atom Catalysis in Marine Energy [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220324. |
| [12] | HAN Fuchao, LI Fujin, CHEN Liang, HE Leiyi, JIANG Yunan, XU Shoudong, ZHANG Ding, QI Lu. Enhance of CoSe2/C Composites Modified Separator on Electrochemical Performance of Li-S Batteries at High Sulfur Loading [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220163. |
| [13] | HUANG Qiuhong, LI Wenjun, LI Xin. Organocatalytic Enantioselective Mannich-type Addition of 5H-Oxazol-4-ones to Isatin Derived Ketimines [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220131. |
| [14] | TAN Yan, YU Shen, LYU Jiamin, LIU Zhan, SUN Minghui, CHEN Lihua, SU Baolian. Efficient Preparation of Mesoporous γ-Al2O3 Microspheres and Performance of Pd-loaded Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220133. |
| [15] | WANG Ruhan, JIA Shunhan, WU Limin, SUN Xiaofu, HAN Buxing. CO2-involved Electrochemical C—N Coupling into Value-added Chemicals [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220395. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||