

高等学校化学学报 ›› 2025, Vol. 46 ›› Issue (5): 20250012.doi: 10.7503/cjcu20250012
收稿日期:2025-01-10
出版日期:2025-05-10
发布日期:2025-03-14
通讯作者:
韩媛媛,崔杰
E-mail:hanyuanyuan@lnpu.edu.cn;cuijie@lnpu.edu.cn
基金资助:
HUANG Huachen, XU Guanghai, HAN Yuanyuan( ), CUI Jie(
), CUI Jie( )
)
Received:2025-01-10
Online:2025-05-10
Published:2025-03-14
Contact:
HAN Yuanyuan, CUI Jie
E-mail:hanyuanyuan@lnpu.edu.cn;cuijie@lnpu.edu.cn
Supported by:摘要:
采用Monte Carlo模拟方法研究了环形A4B6C6三嵌段共聚物在A嵌段选择性溶剂中的自组装行为, 并与线形A4B6C6和A4C6B6三嵌段共聚物的自组装行为进行对比. 模拟结果表明, 通过调节C嵌段的疏水性以及B嵌段与C嵌段之间的疏水性差异, 环形A4B6C6三嵌段共聚物能够自组装形成节状蠕虫、 节状片层、 单室以及多室节状囊泡等多种形貌各异的聚合物胶束. 由于环形嵌段共聚物特殊的拓扑结构, 即使B嵌段与C嵌段之间存在疏水性差异, 这些胶束的疏水核心均倾向于呈B嵌段和C嵌段交替排列的节状结构. 相对于环形体系, 线形A4B6C6和A4C6B6三嵌段共聚物在相同参数条件下的自组装行为较单一, 体系中大多形成了球状胶束, 而B嵌段和C嵌段在球状胶束疏水核心中的排布强烈依赖于嵌段共聚物的链结构. 上述模拟结果有利于理解链结构对嵌段共聚物胶束形貌的影响机制, 为制备具有特定疏水核心结构的聚合物胶束提供了理论依据.
中图分类号:
TrendMD:
黄华琛, 徐广海, 韩媛媛, 崔杰. 环形ABC三嵌段共聚物在A嵌段选择性溶剂中自组装行为的Monte Carlo模拟. 高等学校化学学报, 2025, 46(5): 20250012.
HUANG Huachen, XU Guanghai, HAN Yuanyuan, CUI Jie. Monte Carlo Simulation of the Self-assembly Behavior of Cyclic ABC Triblock Copolymers in Block A Selective Solvents. Chem. J. Chinese Universities, 2025, 46(5): 20250012.
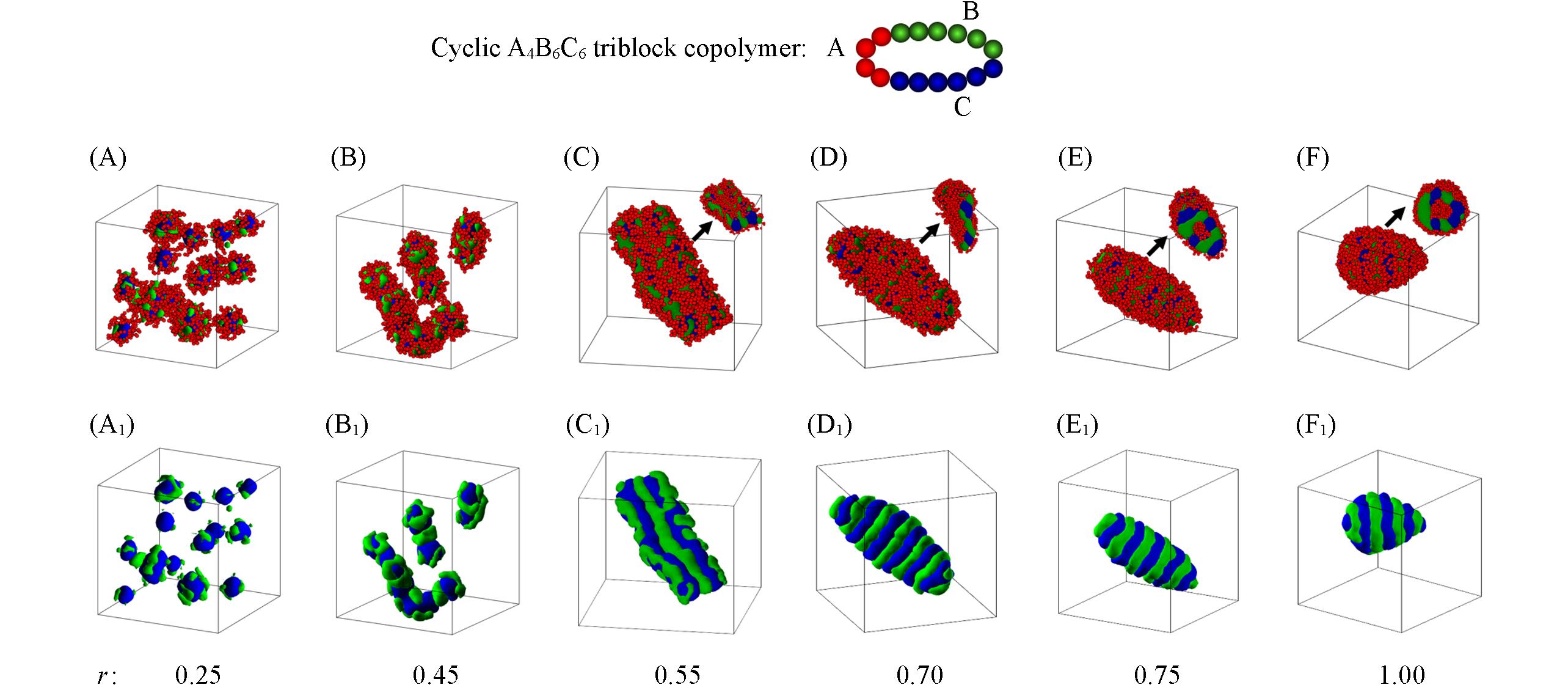
Fig.1 Snapshots of the micelle morphologies formed by cyclic A4B6C6 triblock copolymers in block A selective solvents with different r(r=εBS/εCS) when εCS=3.0(A—F) Overall micelle morphologies; (A1—F1) the hydrophobic parts of Figs.1(A—F); the cross-sections of corresponding micelles are also given in Figs.1(C—F) for clarity. The hydrophobic blocks B and C are drawn in green and blue, and the hydrophilic block A is drawn in red in the images.
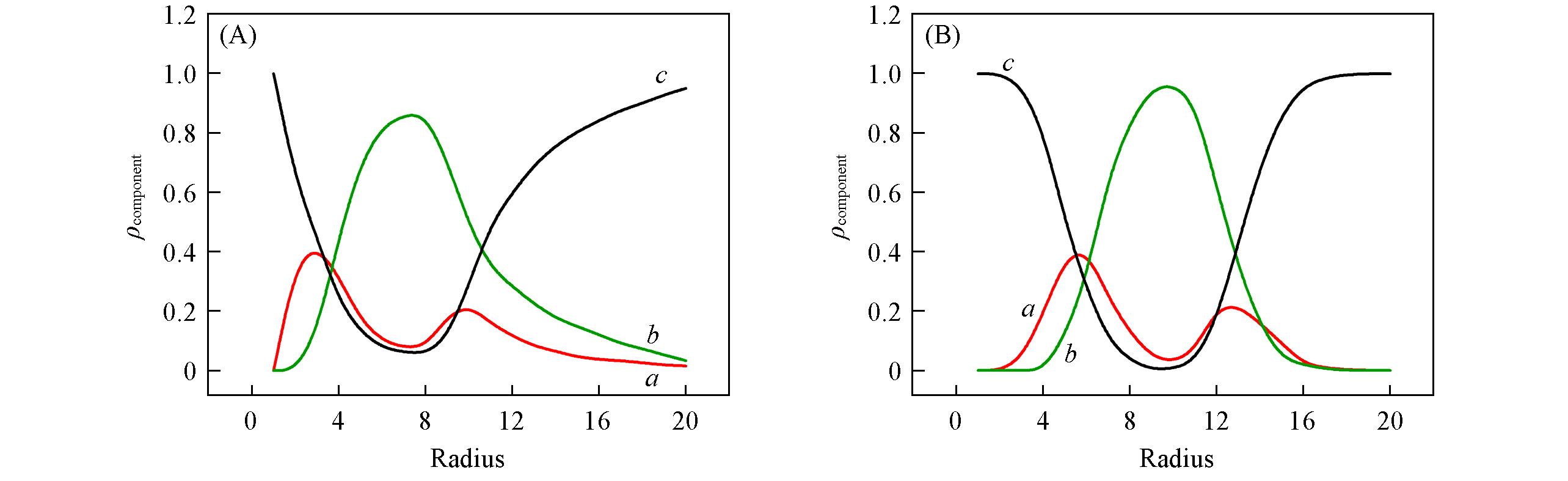
Fig.2 Variations of densities of hydrophilic component(block A, a), hydrophobic component(blocks B+C, b) and solvent(c) with the radius around the mass center of the vesicle shown in Fig.1(E) and Fig.1(F), respectively(A) r=0.75; (B) r=1.0.
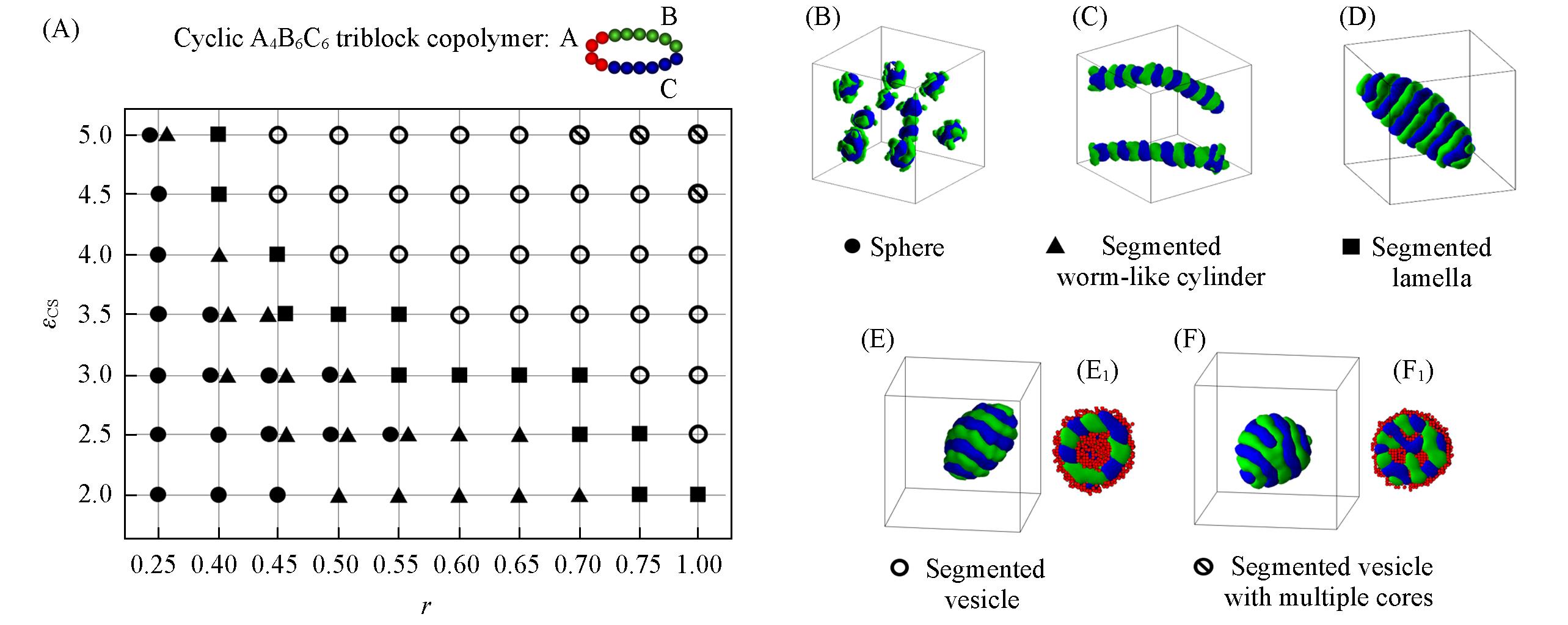
Fig.3 Morphological phase diagram of cyclic A4B6C6 triblock copolymers in block A selective solvents as a function of εCS and r(A) and typical micelle morphologies(B—F)(E1) and (F1) are cross-sections of Figs.(E) and (F), respectively. The hydrophobic blocks B and C are drawn in green and blue, and the hydrophilic block A is drawn in red in the images.
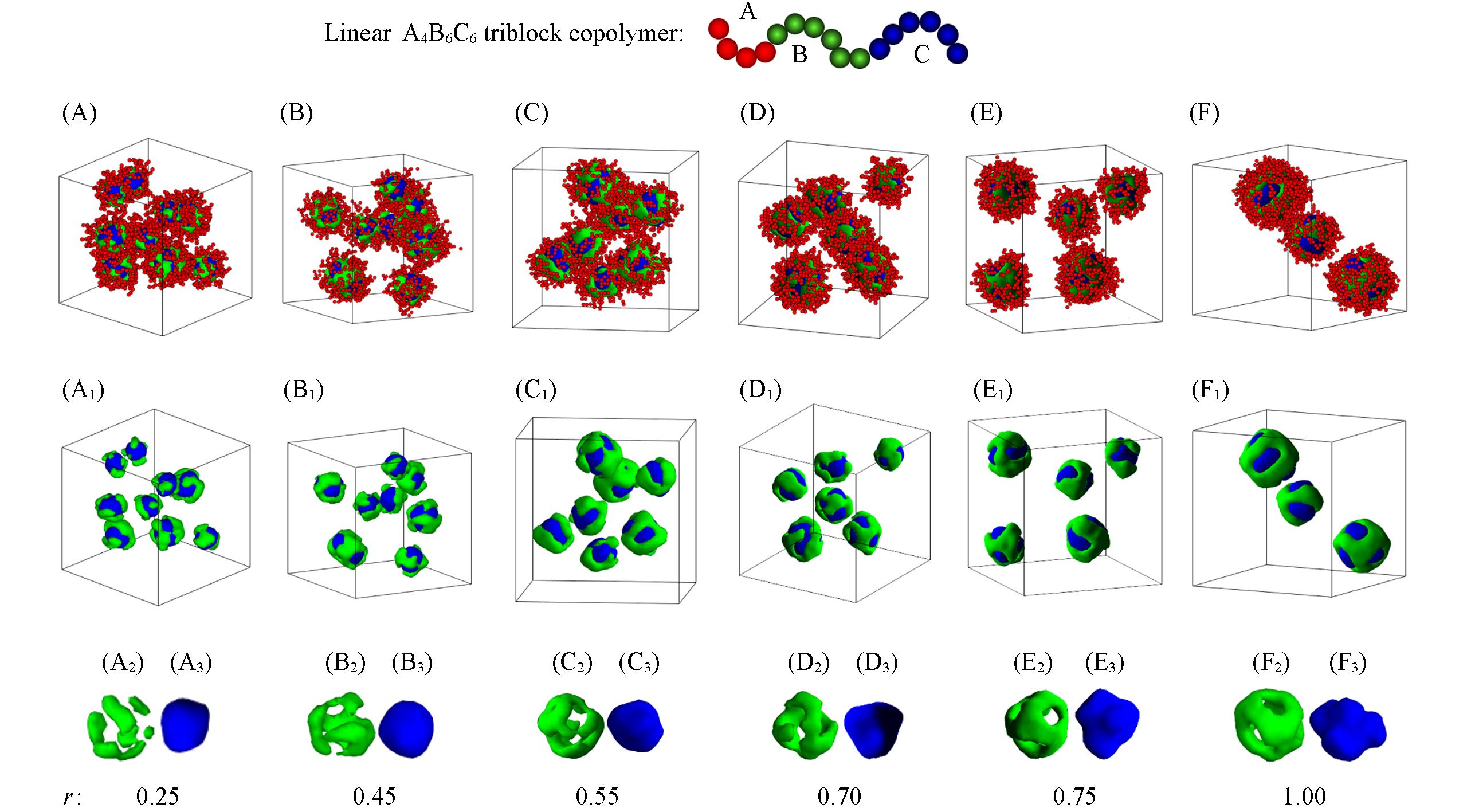
Fig.4 Snapshots of the micelle morphologies formed by linear A4B6C6 triblock copolymers in block A selective solvents with different r(r=εBS/εCS) when εCS=3.0(A—F) Overall micelle morphologies; (A1—F1) the hydrophobic parts of Figs.(A—F); (A2—F2) the morphologies of block B; (A3—F3) the morphologies of block C. The hydrophobic blocks B and C are drawn in green and blue, respectively and the hydrophilic block A is drawn in red in the images.
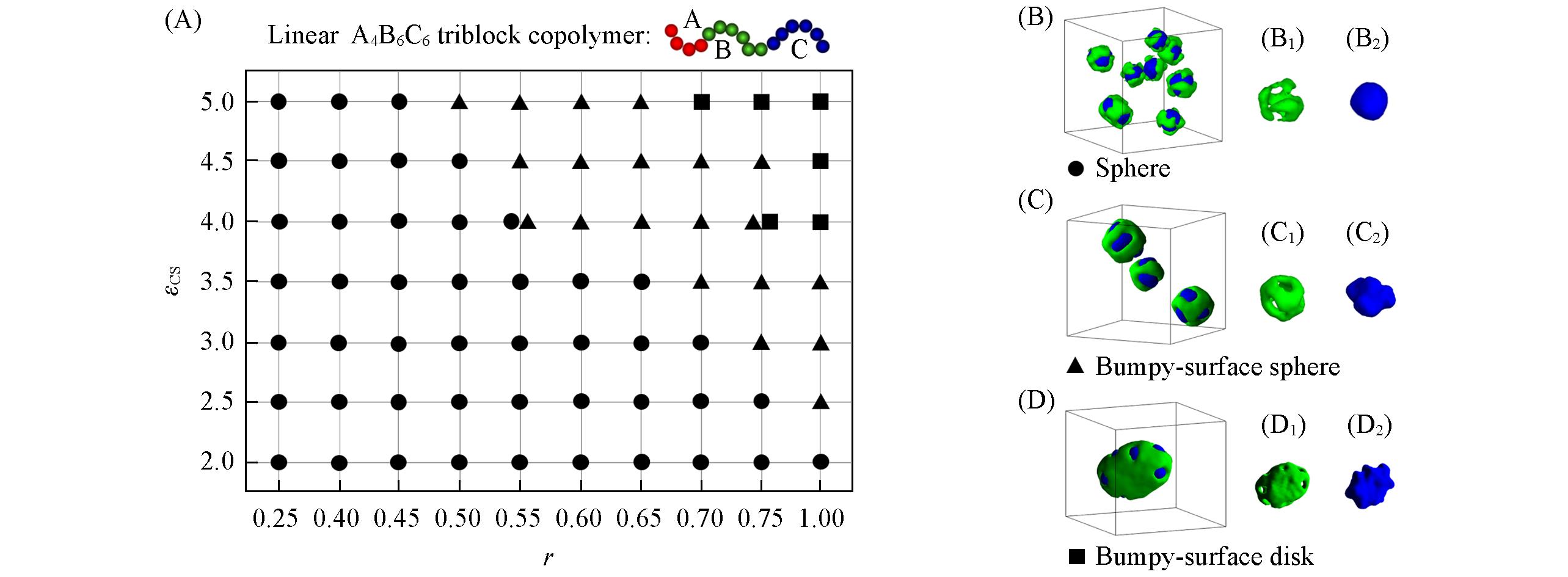
Fig.5 Morphological phase diagram of linear A4B6C6 triblock copolymers in block A selective solventsas a function of εCS and r(A) and typical morphologies(B—D)(B1—D1) The morphologies of block B; (B2—D2) the morphologies of block C. The hydrophobic blocks B and C are drawn in green and blue, respectively.
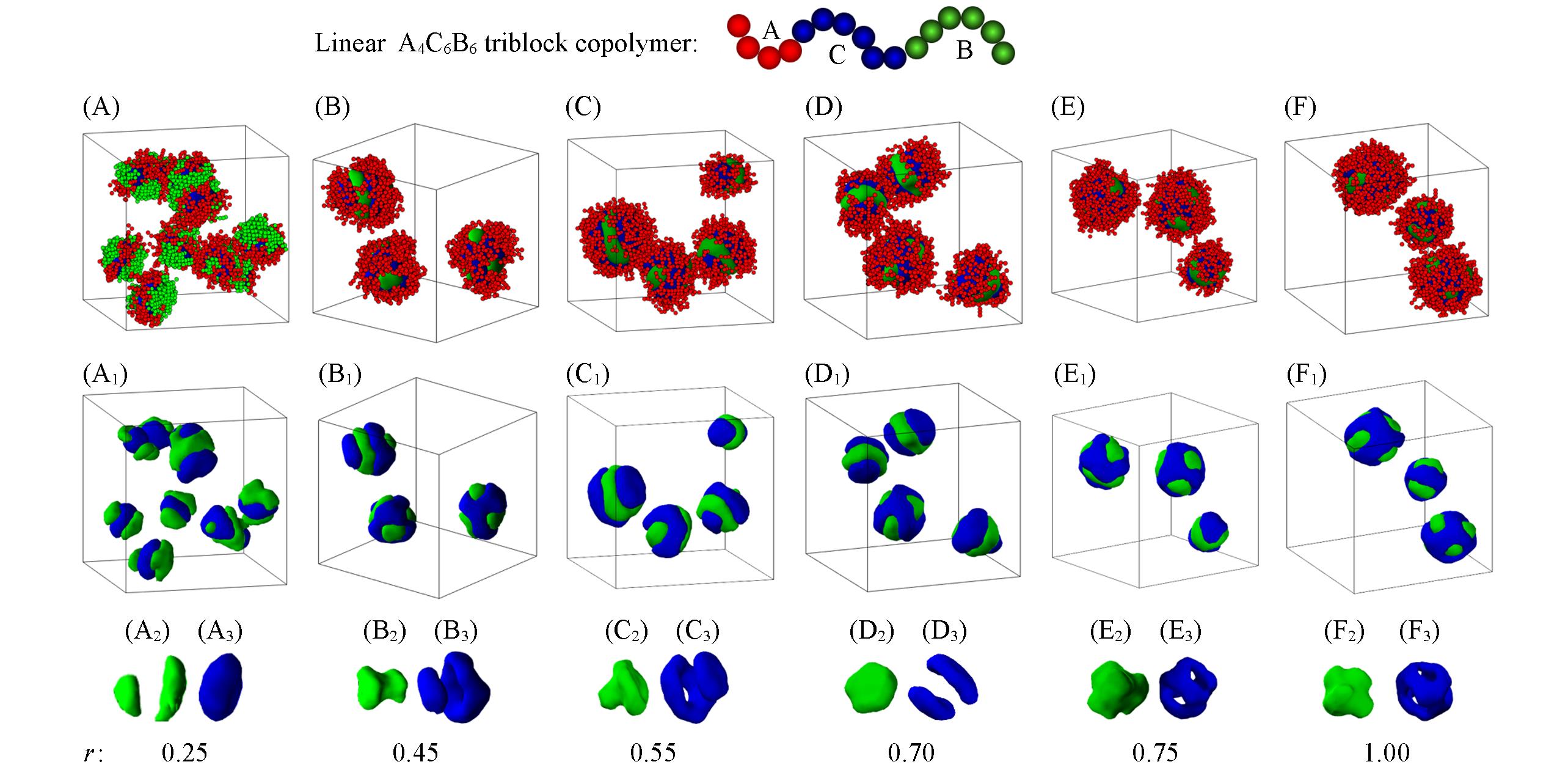
Fig.6 Snapshots of the micelle morphologies formed by linear A4C6B6 triblock copolymers in block A selective solvents with different r(r=εBS/εCS) when εCS=3.0(A—F) Overall micelle morphologies; (A1—F1) the hydrophobic parts of Figs.(A—F); (A2—F2) the morphologies of block B; (A3—F3) the morphologies of block C. The hydrophobic blocks B and C are drawn in green and blue, respectively and the hydrophilic block A is drawn in red in the images.
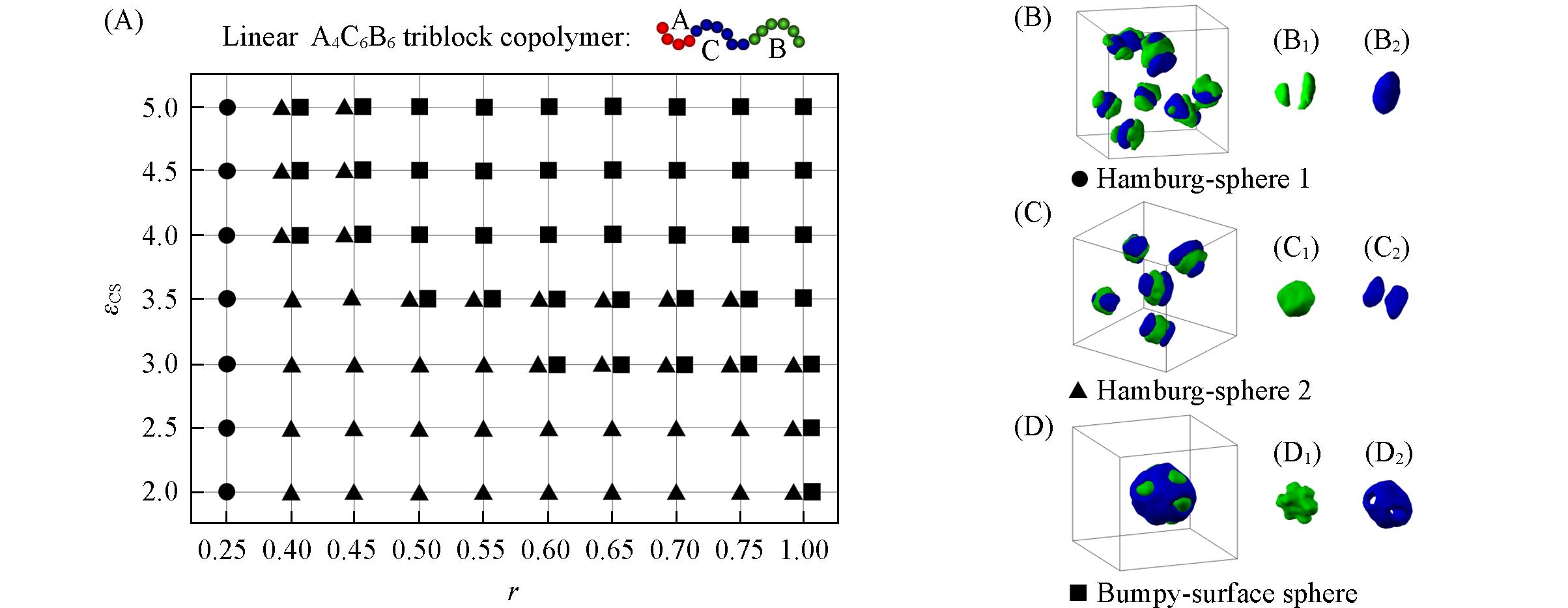
Fig.7 Morphological phase diagram of linear A4C6B6 triblock copolymers in block A selective solvents as a function of εCS andr(A) and typical morphologies(B—D)(B1—D1) The morphologies of block B; (B2—D2) the morphologies of block C. The hydrophobic blocks B and C are drawn in green and blue in the images.
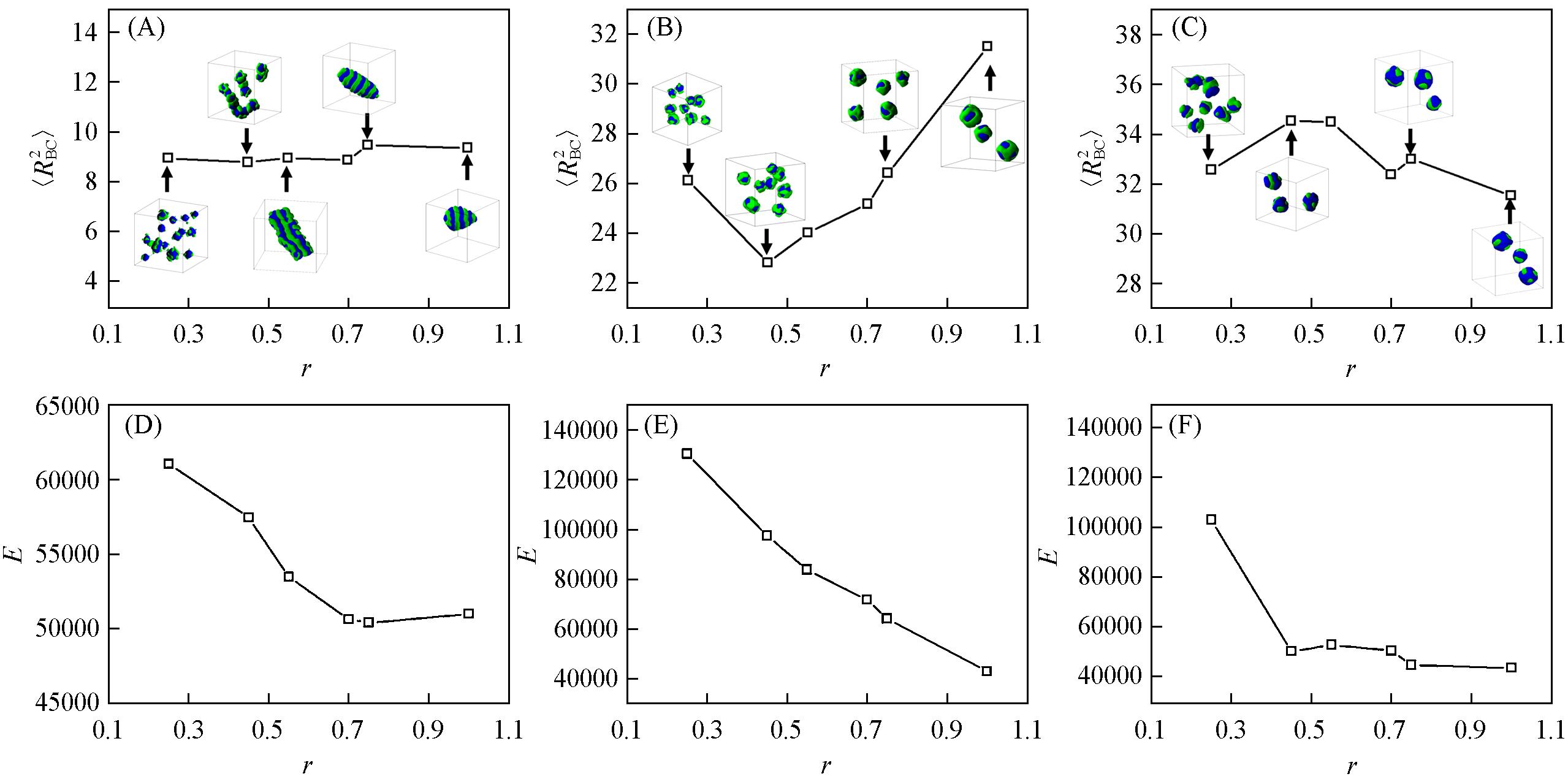
Fig.8 Variations of the total mean square end⁃to⁃end distances(〈RBC2〉) of the hydrophobic blocks(A—C) and the interaction energy(E)(D—F) of cyclic A4B6C6(A, D), linear A4B6C6(B, E) and linear A4C6B6(C, F) with different r(r=εBS/εCS) when εCS=3.0Insets: the corresponding typical micelle morphologies are given in Figs.(A—C) for clarity, and the hydrophobic blocks B and C are drawn in green and blue, respectively.
| 1 | Bates C. M., Bates F. S., Macromolecules, 2017, 50(1), 3—22 |
| 2 | Zhang Y., Liu X. J., Yan N., Hu Y. X., Li H. Y., Zhu Y. T., Prog. Chem., 2018, 30(2), 166—178 |
| 张艳, 刘雪杰, 闫南, 胡跃鑫, 李海英, 朱雨田. 化学进展, 2018, 30(2), 166—178 | |
| 3 | Li B., Zhu Y. L., Xu D., Pei H. W., Liu H., Chem. J. Chinese Unversities, 2013, 34(7), 1667—1672 |
| 李斌, 朱有亮, 徐丹, 裴汉文, 刘鸿. 高等学校化学学报, 2013, 34(7), 1667—1672 | |
| 4 | Rong Q. Q., Ma L., Zhu Y. L., Huang Y. N., Sun Z. Y., Chem. J. Chinese Unversities, 2018, 39(12), 2805—2810 |
| 容婧婧, 马兰, 朱有亮, 黄以能, 孙昭艳, 高等学校化学学报, 2018, 39(12), 2805—2810 | |
| 5 | Zhang H. F., Zhang G. X., Hu Y. X., Han X. Y., Han Y. Y., Zhao G. Y., J. Liaoning Petrochem. Univ., 2024, 44(1), 9—14 |
| 张海峰, 张桂鑫, 胡跃鑫, 韩向艳, 韩媛媛, 赵桂艳. 辽宁石油化工大学学报, 2024, 44(1), 9—14 | |
| 6 | Blanazs A., Armes S. P., Ryan A. J., Macromol. Rapid Commun., 2009, 30(4/5), 267—277 |
| 7 | Wang M. Y., Huang G., You Z. Q., Jia R. X., Zhong Y., Bai F., Chem. Res. Chinese Universities, 2023, 39(4), 612—623 |
| 8 | Huang X. J., Wang Z. R., Wang T., Wang W. He P. L., Chem. Res. Chinese Universities, 2023, 39(4), 660—665 |
| 9 | Aqib R. M., Yang C. P., Wu X. H., Wang Y., Fan J. Shang Y. X., Liu J. B., Ding B. Q., Chem. Res. Chinese Universities, 2023, 39(6), 884—890 |
| 10 | Zhang L. F., Eisenberg A., J. Am. Chem. Soc., 1996, 118(13), 3168—3181 |
| 11 | Zhang L. F., Eisenberg A., Polym. Adv. Technol., 1998, 9(10/11), 677—699 |
| 12 | Shen H., Eisenberg A., J. Phys. Chem. B, 1999, 103(44), 9473—9487 |
| 13 | Zhulina E. B., Borisov O. V., ACS Macro Lett., 2013, 2(4), 292—295 |
| 14 | Guan L., Chen Y. X., Wang Y., Wang X., Chemistryselect, 2020, 5(1), 407—414 |
| 15 | Romio M., Trachsel L., Morgese G., Ramakrishna S. N., Spencer N. D., Benetti E. M., ACS Macro Lett., 2020, 9(7), 1024—1033 |
| 16 | Hadziioannou G., Cotts P. M., ten Brinke G., Han C. C., Lutz P., Strazielle C., Rempp P., Kovacs A. J., Macromolecules, 1987, 20(3), 493—497 |
| 17 | Jang S. S., Çagin T., Goddard W. A. III, J. Chem. Phys., 2003, 119(3), 1843—1854 |
| 18 | Ye J., Xu J., Hu J., Wang X., Zhang G., Liu S., Wu C., Macromolecules, 2008, 41(12), 4416—4422 |
| 19 | Yu G. E., Yang Z., Attwood D., Price C., Booth C., Macromolecules, 1996, 29(26), 8479—8486 |
| 20 | Yu G. E., Zhou Z. K., Attwood D., Price C., Booth C., Griffiths P. C., Stilbs P., J. Chem. Soc., Faraday Trans., 1996, 92(24), 5021—5028 |
| 21 | Yu G. E., Garrett C. A., Mai S. M., Altinok H., Attwood D., Price C., Booth C., Langmuir, 1998, 14(9), 2278—2285 |
| 22 | Honda S., Yamamoto T., Tezuka Y., J. Am. Chem. Soc., 2010, 132(30), 10251—10253 |
| 23 | Honda S., Yamamoto T., Tezuka Y., Nat. Commun., 2013, 4, 2585 |
| 24 | Zhang B., Zhang H., Li Y., Hoskins J. N., Grayson S. M., ACS Macro Lett., 2013, 2(10), 845—848 |
| 25 | Baba E., Yatsunami T., Tezuka Y., Yamamoto T., Langmuir, 2016, 32(40), 10344—10349 |
| 26 | Song Y., Jiang R., Wang Z., Wang L., Yin Y., Li B., Shi A. C., Macromol. Theory Simul., 2016, 25(6), 559—570 |
| 27 | Song Y., Xie T., Jiang R., Wang Z., Yin Y., Li B., Shi A. C., Langmuir, 2018, 34(13), 4013—4023 |
| 28 | He L., Chen Z., Zhang R., Zhang L., Jiang Z., J. Chem. Phys., 2013, 138(9), 094907 |
| 29 | Li L. Y., Li Z. W., Fu C. L., Sun Z. Y., An L. J., Chem. J. Chinese Unversities, 2014, 35(1), 168—174 |
| 李良一, 李占伟, 付翠柳, 孙昭艳, 安立佳. 高等学校化学学报, 2014, 35(1), 168—174 | |
| 30 | Zhang W. P., Wang X. H., He L. L., Chinese J. Polym. Sci., 2016, 34(4), 420—430 |
| 31 | Han Y. Y., Lu Y. N., Song T. J., Cui J., Fan J. J., Langmuir, 2024, 40(31), 16103—16112 |
| 32 | Qiang Y., Li W., Macromolecules, 2021, 54(19), 9071—9078 |
| 33 | Xu G. H., Sun Y. H., Cui J., Han Y. Y., Fan J. J., Polymer Bulletin, 2024, 37(4), 532—542 |
| 徐广海, 孙雨涵, 崔杰, 韩媛媛, 樊娟娟. 高分子通报, 2024, 37(4), 532—542 | |
| 34 | Kong W., Li B., Jin Q., Ding D., Shi A. C., Langmuir, 2010, 26(6), 4226—4232 |
| 35 | Kong W., Jiang W., Zhu Y., Li B., Langmuir, 2012, 28(32), 11714—11724 |
| 36 | Larson R. G., J. Chem. Phys., 1989, 91(4), 2479—2488 |
| 37 | Ji S. C., Ding J. D., Langmuir, 2006, 22(2), 553—559 |
| 38 | Metropolis N., Rosenbluth A. W., Rosenbluth M. N., Teller A. H., Teller E., J. Chem. Phys., 1953, 21(6), 1087—1092 |
| 39 | Han Y., Yu H., Du H., Jiang W., J. Am. Chem. Soc., 2010, 132(3), 1144—1150 |
| 40 | Rubinstein M., Colby R. H., Polymer Physics, Oxford University Press, Oxford, 2003 |
| 41 | Fan J. J., Han Y. Y., Cui J., Chem. J. Chinese Unversities, 2021, 42(3), 857—865 |
| 樊娟娟, 韩媛媛, 崔杰. 高等学校化学学报, 2021, 42(3), 857—865 | |
| 42 | Hakobyan K., McErlean C. S. P., Müllner M., Macromolecules, 2021, 54(17), 7732—7742 |
| 43 | Polymeropoulos G., Bilalis P., Hadjichristidis N., ACS Macro Lett., 2016, 5(11), 1242—1246 |
| [1] | 李嘉明, 王丹, 冯玉军, 殷鸿尧. 油酰二乙醇胺在丙三醇/水混合溶剂中的自组装及流变性能[J]. 高等学校化学学报, 2025, 46(4): 20240528. |
| [2] | 陈晓萍, 黄士, 郭千千, 刘宁, 倪建聪, 杨伟强, 林振宇. 镓铟共晶-自组装单分子层的功能性分子结研究进展[J]. 高等学校化学学报, 2025, 46(2): 20240451. |
| [3] | 白乙艳, 王晨阳, 高阳, 李佳敏, 杨海英. 基于单颗粒碰撞电化学的烷硫醇在纳米金表面的自组装行为[J]. 高等学校化学学报, 2025, 46(2): 20240402. |
| [4] | 陈俊年, 张海峰, 王海兵, 杨火诚, 罗忠. 基于超分子纳米递送系统的精准医疗研究进展[J]. 高等学校化学学报, 2025, 46(1): 20240267. |
| [5] | 雍海洋, 李智立, 郭蕊, 周德重. 内环化聚(β-氨基酯)的制备与生物物理性能[J]. 高等学校化学学报, 2024, 45(2): 20230466. |
| [6] | 段一雄, 杨柏, 李云峰. 纤维素纳米晶的空间受限自组装: 从胶体液晶到功能材料[J]. 高等学校化学学报, 2023, 44(2): 20220474. |
| [7] | 雷娜娜, 杨守业, 范黎明, 陈晓. 低共熔溶剂中多色稀土发光增强超分子凝胶的构筑及性能[J]. 高等学校化学学报, 2023, 44(12): 20230389. |
| [8] | 段小丽, 韩慧敏, 赵明坤, 任丽霞. PNDC-b-PNTPE自组装制备兼具结构色和荧光色的薄膜[J]. 高等学校化学学报, 2023, 44(11): 20230291. |
| [9] | 沈欣怡, 张森, 王树涛, 宋永杨. 聚合物中空微球的合成策略[J]. 高等学校化学学报, 2023, 44(1): 20220627. |
| [10] | 仵宇帅, 尚颖旭, 蒋乔, 丁宝全. 可控自组装DNA折纸结构作为药物载体的研究进展[J]. 高等学校化学学报, 2022, 43(8): 20220179. |
| [11] | 李琳, 齐丰莲, 邱丽莉, 孟子晖. 基于六边形磁纳米片构建动态非晶态光学结构图案[J]. 高等学校化学学报, 2022, 43(8): 20220123. |
| [12] | 俞彬, 谌小燕, 赵越, 陈卫昌, 肖新颜, 刘海洋. 氧化石墨烯基钴卟啉复合材料的电催化析氢反应[J]. 高等学校化学学报, 2022, 43(2): 20210549. |
| [13] | 徐海东, 王睿, 梁高林. 自组装多肽探针在磁共振成像中的应用[J]. 高等学校化学学报, 2022, 43(12): 20220440. |
| [14] | 李波, 孟禹汐, 王雯雯, 臧宏瑛. 多核多氧硫钼酸盐化合物的合成及质子传导性能[J]. 高等学校化学学报, 2022, 43(1): 20210657. |
| [15] | 杜顺福, 王文经, EL-SAYED El-Sayed M., 苏孔钊, 袁大强, 洪茂椿. 一种具有化学发光性能的锆基金属有机四面体[J]. 高等学校化学学报, 2022, 43(1): 20210628. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||