

高等学校化学学报 ›› 2018, Vol. 39 ›› Issue (10): 2206.doi: 10.7503/cjcu20180182
李阳1, 李志文1, 朱俊飞1, 刘世会1( ), 何军林2(
), 何军林2( )
)
收稿日期:2018-03-08
出版日期:2018-10-10
发布日期:2018-09-29
作者简介:联系人简介: 何军林, 女, 博士, 副研究员, 主要从事核酸化学方面的研究. E-mail: 基金资助:
LI Yang1, LI Zhiwen1, ZHU Junfei1, LIU Shihui1,*( ), HE Junlin2,*(
), HE Junlin2,*( )
)
Received:2018-03-08
Online:2018-10-10
Published:2018-09-29
Contact:
LIU Shihui,HE Junlin
E-mail:liush05@163.com;hejunlin@bmi.ac.cn
Supported by:摘要:
通过7-芘乙炔基-8-氮-7-去氮-2'-脱氧腺苷(2)和7-芘乙基-8-氮-7-去氮-2'-脱氧腺苷(3)2个脱氧腺苷类似物在双螺旋DNA(dsDNA)中构建芘基对, 同时保持2'-脱氧腺苷的碱基配对专一性, 探讨了芘基对的多种组合在dsDNA中的荧光光谱变化及其与dsDNA形成和解离的关系. DNA双螺旋的热稳定性和圆二色光谱表征结果反映了连接臂与芘基在dsDNA大沟区的相互作用. 芘基荧光光谱表明, 化合物2在dsDNA中的荧光光谱反映了双螺旋的变化, PY02+D02在形成双螺旋前后发生了荧光猝灭, 而PY03+PY05在形成双螺旋前后呈现荧光, 这2个芘基对组合在含双螺旋结构域的功能核酸和生物传感器中具有潜在应用价值.
中图分类号:
TrendMD:
李阳, 李志文, 朱俊飞, 刘世会, 何军林. 芘基对在dsDNA中的构建:基于8-氮-7-去氮-2'-脱氧腺苷的7位取代及连接臂对荧光性质的影响. 高等学校化学学报, 2018, 39(10): 2206.
LI Yang, LI Zhiwen, ZHU Junfei, LIU Shihui, HE Junlin. Construction of Pyrenyl Pairs in dsDNA: Fluorescent Properties of Multiple Pyrenyl-attached dsDNAs Through 7-Substituted 8-Aza-7-deaza-2'-deoxyadenosine Analogues†. Chem. J. Chinese Universities, 2018, 39(10): 2206.
| Name | Sequence | MS(calcd.), m/z | Name | Sequence | MS(calcd.), m/z |
|---|---|---|---|---|---|
| PY01 | 5'-d(TAGGTC22TACT)-3' | 4092.2(4090.8) | PY06 | 5'-d(TAGGTC33TACT)-3' | 4100.4(4101.0) |
| PY02 | 5'-d(TAGGTC2AT2CT)-3' | 4092.7(4090.8) | PY07 | 5'-d(TAGGTC3AT3CT)-3' | 4100.1(41010) |
| PY03 | 5'-d(TAGGTC2ATACT)-3' | 3867.9(3868.7) | PY08 | 5'-d(TAGGTC3ATACT)-3' | 3871.7(3872.7) |
| PY04 | 5'-d(TAGGTCA2T2CT)-3' | 4092.0(4090.8) | PY09 | 5'-d(TAGGTCA3T3CT)-3' | 4100.2(41010) |
| PY05 | 5'-d(AGT2TTGACCTA)-3' | 3868.1(3868.7) | PY10 | 5'-d(AGT3TTGACCTA)-3' | 3872.1(3872.7) |
Table 1 MS characterization of the DNA sequences
| Name | Sequence | MS(calcd.), m/z | Name | Sequence | MS(calcd.), m/z |
|---|---|---|---|---|---|
| PY01 | 5'-d(TAGGTC22TACT)-3' | 4092.2(4090.8) | PY06 | 5'-d(TAGGTC33TACT)-3' | 4100.4(4101.0) |
| PY02 | 5'-d(TAGGTC2AT2CT)-3' | 4092.7(4090.8) | PY07 | 5'-d(TAGGTC3AT3CT)-3' | 4100.1(41010) |
| PY03 | 5'-d(TAGGTC2ATACT)-3' | 3867.9(3868.7) | PY08 | 5'-d(TAGGTC3ATACT)-3' | 3871.7(3872.7) |
| PY04 | 5'-d(TAGGTCA2T2CT)-3' | 4092.0(4090.8) | PY09 | 5'-d(TAGGTCA3T3CT)-3' | 4100.2(41010) |
| PY05 | 5'-d(AGT2TTGACCTA)-3' | 3868.1(3868.7) | PY10 | 5'-d(AGT3TTGACCTA)-3' | 3872.1(3872.7) |
| Name | Sequence | Tm/℃ | ΔTm/℃ | Name | Sequence | Tm/℃ | ΔTm/℃ |
|---|---|---|---|---|---|---|---|
| D01+D02 | 5'-d(TAGGTCAATACT)-3' | 49.8 | PY06+D02 | 5'-d(TAGGTC33TACT)-3' | 45.9 | -3.9 | |
| 3'-d(ATCCAGTTATGA)-5' | 3'-d(ATCCAGTTATGA)-5' | ||||||
| PY03+D02 | 5'-d(TAGGTC2ATACT)-3' | 39.4 | -10.4 | PY07+D02 | 5'-d(TAGGTC3AT3CT)-3' | 61.7 | 11.9 |
| 3'-d(ATCCAGTTATGA)-5' | 3'-d(ATCCAGTTATGA)-5' | ||||||
| PY08+D02 | 5'-d(TAGGTC3ATACT)-3' | 54.0 | 4.2 | PY09+D02 | 5'-d(TAGGTCA3T3CT)-3' | 59.5 | 9.7 |
| 3'-d(ATCCAGTTATGA)-5' | 3'-d(ATCCAGTTATGA)-5' | ||||||
| D01+PY05 | 5'-d(TAGGTCAATACT)-3' | 41.0 | -8.8 | PY01+PY05 | 5'-d(TAGGTC22TACT)-3' | 48.8 | -1.0 |
| 3'-d(ATCCAGTT2TGA)-5' | 3'-d(ATCCAGTT2TGA)-5' | ||||||
| D01+PY10 | 5'-d(TAGGTCAATACT)-3' | 57.0 | 7.2 | PY02+PY05 | 5'-d(TAGGTC2AT2CT)-3' | 54.0 | 4.2 |
| 3'-d(ATCCAGTT3TGA)-5' | 3'-d(ATCCAGTT2TGA)-5' | ||||||
| PY03+PY05 | 5'-d(TAGGTC2ATACT)-3' | 49.8 | 0 | PY04+PY05 | 5'-d(TAGGTCA2T2CT)-3' | 60.0 | 10.2 |
| 3'-d(ATCCAGTT2TGA)-5' | 3'-d(ATCCAGTT2TGA)-5' | ||||||
| PY01+D02 | 5'-d(TAGGTC22TACT)-3' | 44.3 | -4.5 | PY06+PY10 | 5'-d(TAGGTC33TACT)-3' | 55.2 | 5.4 |
| 3'-d(ATCCAGTTATGA)-5' | 3'-d(ATCCAGTT3TGA)-5' | ||||||
| PY02+D02 | 5'-d(TAGGTC2AT2CT)-3' | 36.5 | -13.3 | PY09+PY10 | 5'-d(TAGGTCA3T3CT)-3' | 59.4 | 9.6 |
| 3'-d(ATCCAGTTATGA)-5' | 3'-d(ATCCAGTT3TGA)-5' | ||||||
| PY04+D02 | 5'-d(TAGGTCA2T2CT)-3' | 52.1 | 2.3 | PY07+PY10 | 5'-d(TAGGTC3AT3CT)-3' | 58.6 | 8.8 |
| 3'-d(ATCCAGTTATGA)-5' | 3'-d(ATCCAGTT3TGA)-5' | ||||||
| PY08+PY10 | 5'-d(TAGGTC3ATACT)-3' | 64.2 | 14.4 | ||||
| 3'-d(ATCCAGTT3TGA)-5' | |||||||
Table 2 Thermal stability(Tm) of dsDNAs containing pyrenyl group
| Name | Sequence | Tm/℃ | ΔTm/℃ | Name | Sequence | Tm/℃ | ΔTm/℃ |
|---|---|---|---|---|---|---|---|
| D01+D02 | 5'-d(TAGGTCAATACT)-3' | 49.8 | PY06+D02 | 5'-d(TAGGTC33TACT)-3' | 45.9 | -3.9 | |
| 3'-d(ATCCAGTTATGA)-5' | 3'-d(ATCCAGTTATGA)-5' | ||||||
| PY03+D02 | 5'-d(TAGGTC2ATACT)-3' | 39.4 | -10.4 | PY07+D02 | 5'-d(TAGGTC3AT3CT)-3' | 61.7 | 11.9 |
| 3'-d(ATCCAGTTATGA)-5' | 3'-d(ATCCAGTTATGA)-5' | ||||||
| PY08+D02 | 5'-d(TAGGTC3ATACT)-3' | 54.0 | 4.2 | PY09+D02 | 5'-d(TAGGTCA3T3CT)-3' | 59.5 | 9.7 |
| 3'-d(ATCCAGTTATGA)-5' | 3'-d(ATCCAGTTATGA)-5' | ||||||
| D01+PY05 | 5'-d(TAGGTCAATACT)-3' | 41.0 | -8.8 | PY01+PY05 | 5'-d(TAGGTC22TACT)-3' | 48.8 | -1.0 |
| 3'-d(ATCCAGTT2TGA)-5' | 3'-d(ATCCAGTT2TGA)-5' | ||||||
| D01+PY10 | 5'-d(TAGGTCAATACT)-3' | 57.0 | 7.2 | PY02+PY05 | 5'-d(TAGGTC2AT2CT)-3' | 54.0 | 4.2 |
| 3'-d(ATCCAGTT3TGA)-5' | 3'-d(ATCCAGTT2TGA)-5' | ||||||
| PY03+PY05 | 5'-d(TAGGTC2ATACT)-3' | 49.8 | 0 | PY04+PY05 | 5'-d(TAGGTCA2T2CT)-3' | 60.0 | 10.2 |
| 3'-d(ATCCAGTT2TGA)-5' | 3'-d(ATCCAGTT2TGA)-5' | ||||||
| PY01+D02 | 5'-d(TAGGTC22TACT)-3' | 44.3 | -4.5 | PY06+PY10 | 5'-d(TAGGTC33TACT)-3' | 55.2 | 5.4 |
| 3'-d(ATCCAGTTATGA)-5' | 3'-d(ATCCAGTT3TGA)-5' | ||||||
| PY02+D02 | 5'-d(TAGGTC2AT2CT)-3' | 36.5 | -13.3 | PY09+PY10 | 5'-d(TAGGTCA3T3CT)-3' | 59.4 | 9.6 |
| 3'-d(ATCCAGTTATGA)-5' | 3'-d(ATCCAGTT3TGA)-5' | ||||||
| PY04+D02 | 5'-d(TAGGTCA2T2CT)-3' | 52.1 | 2.3 | PY07+PY10 | 5'-d(TAGGTC3AT3CT)-3' | 58.6 | 8.8 |
| 3'-d(ATCCAGTTATGA)-5' | 3'-d(ATCCAGTT3TGA)-5' | ||||||
| PY08+PY10 | 5'-d(TAGGTC3ATACT)-3' | 64.2 | 14.4 | ||||
| 3'-d(ATCCAGTT3TGA)-5' | |||||||
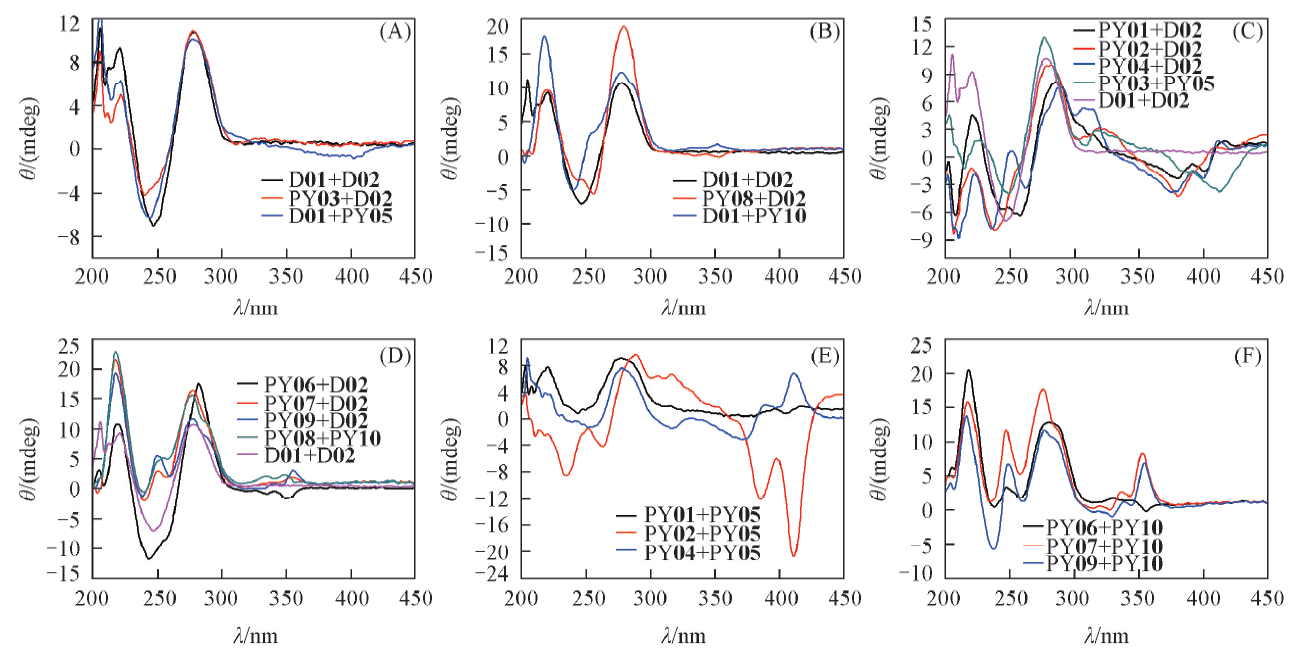
Fig.2 CD spectra of the pyrenyl-containing dsDNAsThe dsDNA in the buffer(50 mmol/L Tris-HCl, 20 mmol/L Mg2+, pH=7.5) with 1 μmol/L of pyrenyl group was measured on a MOS-450 spectropolarimeter(Biologic, France), in the quartz cuvette of 1 cm optical path length, with a speed of 100 nm/min.
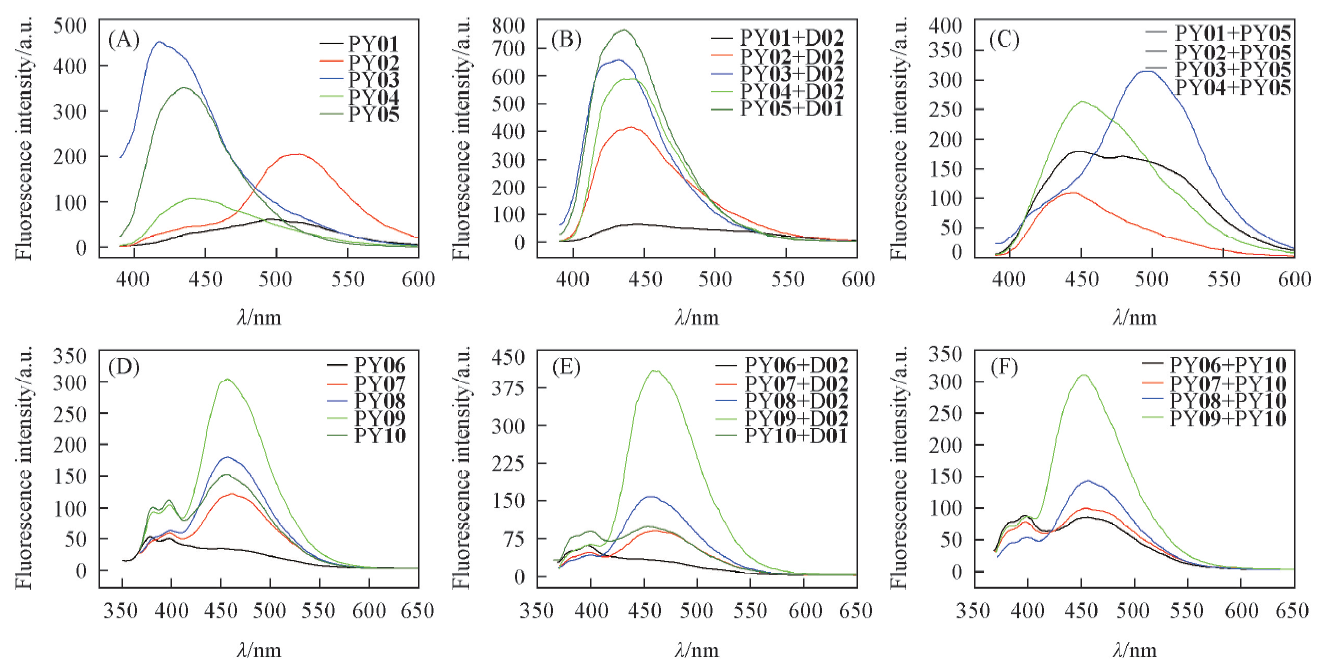
Fig.3 Fluorescence spectra of the single sequences and dsDNAs containing residues 2 and 3The single sequences and dsDNA, 1.0 μmol/L of pyrenyl group in the buffer(50 mmol/L Tris-HCl, 20 mmol/L Mg2+, pH=7.5) was measured, with λex=377 or 340 nm for the samples containing residue 2 or 3, respectively.
| [1] | Guo J., Xu N., Li Z., Zhang S., Wu J., Kim D.H., Marma M. S., Meng Q., Cao H., Li X., Shi S., Yu L., Kalachikov S., Russo J. J., Turro N. J., Ju J., Proc. Natl. Acad. Sci., 2008, 105, 9145—9150 |
| [2] | Astakhova I.V., Korshun V. A., Jahn K., Kjems J., Wengel J., Bioconjugate Chem., 2008, 19, 1995—2007 |
| [3] | Manna S., Panse C.H., Sontakke V. A., Sangamesh S., Srivatsan S. G., ChemBioChem, 2017, 18, 1604—1615 |
| [4] | Sarder P., Maji D., Achilefu S., Bioconjugate Chem., 2015, 26, 963—974 |
| [5] | Iida K., Nakamura T., Yoshida W., Tera M., Nakabayashi K., Hata K., Ikebukuro K., Nagasawa K., Angew. Chem. Int. Ed., 2013, 52, 12052—12055 |
| [6] | Wang Y.F., Zhang X., Liu C. X., Zhou X., Acta Chim.Sinica, 2017, 75, 692—698 |
| (王雅芬, 张雄, 刘朝兴, 周翔. 化学学报, 2017, 75, 692—698) | |
| [7] | Ying Z.M., Wu Z., Tu B., Tan W., Jiang J. H., J. Am. Chem. Soc., 2017, 139, 9779—9782 |
| [8] | McManus S. A., Li Y., J. Am. Chem. Soc., 2013, 135, 7181—7186 |
| [9] | Lu H., Zheng Y., Zhao X., Wang L., Ma S., Han X., Xu B., Tian W., Gao H., Angew. Chem. Int. Ed., 2016, 55, 155—159 |
| [10] | Park S., Otomo H., Zheng L., Sugiyama H., Chem. Commun., 2014, 50, 1573—1575 |
| [11] | Davies M.J., Shah A., Bruce I. J., Chem. Soc. Rev., 2000, 29, 97—107 |
| [12] | Yamauchi T., Takeda T., Yanagi M., Takahashi N., Suzuki A., Saito Y., Tetrahedron Lett., 2017, 58, 117—120 |
| [13] | Wranne M.S., Füchtbauer A. F., Dumat B., Bood M., El-Sagheer A. H., Brown T., Gradén H., Grøtli M., Wilhelmsson L. M., J. Am. Chem. Soc., 2017, 139, 9271—9280 |
| [14] | Xu W., Chan K.M., Kool E. T., Nat. Chem., 2017, 9, 1043—1055 |
| [15] | Winnik F.M., Chem. Rev., 1993, 93, 587—614 |
| [16] | Nagatoishi S., Nojima T., Juskowiak B., Takenaka S., Angew. Chem. Int. Ed., 2005, 44, 5067—5070 |
| [17] | Sahoo D., Narayanaswami V., Kay C.M., Ryan R. O., Biochem., 2000, 39, 6594—6601 |
| [18] | Chen S., Wang L., Fahmi N.E., Benkovic S. J., Hecht S. M., J. Am. Chem. Soc., 2012, 134, 18883—18885 |
| [19] | Okamoto A., Kanatani K., Saito I., J. Am. Chem. Soc., 2004, 126, 4820—4827 |
| [20] | Ueda T., Kobori A., Yamayoshi A., Yoshida H., Yamaguchi M., Murakami A., Bioorg. Med. Chem., 2012, 20, 6034—6039 |
| [21] | Kostenko E., Dobrikov M., Pyshnyi D., Petyuk V., Komarova N., Vlassov V., Zenkova M., Nucleic Acids Res., 2001, 29, 3611—3620 |
| [22] | Smalley M.K., Silverman S. K., Nucleic Acids Res., 2006, 34, 152—166 |
| [23] | Martí A., Li X., Jockusch S., Li Z., Raveendra B., Kalachikov S., Russo J.J., Morozova I., Puthanveettil S. V., Ju J., Turro N. J., Nucleic Acids Res., 2006, 34, 3161—3168 |
| [24] | Yamana K., Ohtani Y., Nakanoa H., Saitob I., Bioorg. Med. Chem. Lett., 2003, 13, 3429—3431 |
| [25] | Huang P.J. J., Lin J., Cao J., Vazin M., Liu J., Anal. Chem., 2014, 86, 1816—1821 |
| [26] | Huang J., Wu Y., Chen Y., Zhu Z., Yang X., Yang C.J., Wang K., Tan W., Angew. Chem. Int. Ed., 2011, 50, 401—404 |
| [27] | Karuppannan S., Chambron J. C., Chem. Asian J., 2011, 6, 964—984 |
| [28] | Hrdlicka P.J., Karmakar S., Org. Biomol. Chem., 2017, 15, 9760—9774 |
| [29] | Zhu H., Lewis F.D., Bioconjugate Chem., 2007, 18, 1213—1217 |
| [30] | Malakhov A.D., Skorobogatyi M. V., Prokhorenko I. A., Gontarev S. V., Kozhich D. T., Stetsenko D. A., Stepanova I. A., Shenkarev Z. O., Berlin Y. A., Korshun V. A., Eur. J. Org. Chem., 2004, 2004(6), 1298—1307 |
| [31] | Ren R.X. F., Chaudhuri N. C., Paris P. L., Rumney IV S., Kool E. T., J. Am. Chem. Soc., 1996, 118, 7671—7678 |
| [32] | Hwang G.T., Seo Y. J., Kim B. H., Tetrahedron Lett., 2005, 46, 1475—1477 |
| [33] | Mayer E., Valis L., Wagner C., Rist M., Amann N., Wagenknecht H.A., ChemBioChem, 2004, 5, 865—868 |
| [34] | Wang G., Bobkov G.V., Mikhailov S. N., Schepers G., Van Aerschot A., Rozenski J., Van der Auweraer M., Herdewijn P., Feyter S. D., ChemBioChem, 2009, 10, 1175—1185 |
| [35] | Okamoto A., Ochia Y., Saito I., Chem. Commun., 2005, 1128—1130 |
| [36] | Rist M., Amann N., Wagenknecht H.A., Eur. J. Org. Chem., 2003, 2003(13), 2498—2504 |
| [37] | Hwang G.T., Seo Y. J., Kim S. J., Kim B. H., Tetrahedron Lett., 2004, 45, 3543—3546 |
| [38] | Hrdlicka P.J., Babu B. R., Sørensen M. D., Harrit N., Wengel J., J. Am. Chem. Soc., 2005, 127, 13293—13299 |
| [39] | Østergaard M.E., Cheguru P., Papasani M. R., Hill R. A., Hrdlicka P. J., J. Am. Chem. Soc., 2010, 132, 14221—14230 |
| [40] | Imincan G., Pei F., Yu L., Jin H., Zhang L., Yang X., Zhang L., Tang X., Anal. Chem., 2016, 88, 4448—4455 |
| [41] | Ingale S.A., Pujari S. S., Sirivolu V. R., Ding P., Xiong H., Mei H., Seela F., J. Org. Chem., 2012, 77, 188—199 |
| [42] | Honcharenko D., Zhou C., Chattopadhyaya J., J. Org. Chem., 2008, 73, 2829—2842 |
| [43] | Karlsen K.K., Pasternak A., Jensen T. B., Wengel J., ChemBioChem, 2012, 13, 590—601 |
| [44] | Seo Y.J., Hwang G. T., Kim B. H., Tetrahedron Lett., 2006, 47, 4037—4039 |
| [45] | Grünewald C., Kwon T., Piton N., Förster U., Wachtveitl J., Engels J.W., Bioorg. Med. Chem., 2008, 16, 19—26 |
| [46] | Dioubankova N.N., Malakhov A. D., Stetsenko D. A., Gait M. J., Volynsky P. E., Efremov R. G., Korshun V. A., Chem. Bio. Chem., 2003, 4, 841—847 |
| [47] | Li Z., Zhu J., He J., Org. Biomol. Chem., 2016, 14, 9846—9858 |
| [48] | He J., Seela F., Nucleic Acids Res., 2002, 30, 5485—5496 |
| [49] | Doluca O., Withers J.M., Loo T. S., Edwards P. J. B., González C., Filichev V. V., Org. Biomol. Chem., 2015, 13, 3742—3748 |
| [50] | Wojciechowski F., Lietard J., Leumann C.J., Org. Lett., 2012, 14, 5176—5179 |
| [51] | Fukuda M., Nakamura M., Takada T., Yamana K., Tetrahedron Lett., 2010, 51, 1732—1735 |
| [52] | Anderson B.A., Karmakar S., Hrdlicka P. J., Molecules, 2015, 20, 13780—13793 |
| [1] | 常斯惠, 陈涛, 赵黎明, 邱勇隽. 离子液体增塑生物基聚丁内酰胺的热分解机理[J]. 高等学校化学学报, 2022, 43(11): 20220353. |
| [2] | 张俊, 王彬, 潘莉, 马哲, 李悦生. 含咪唑离子聚乙烯离聚体的合成与性能[J]. 高等学校化学学报, 2020, 41(9): 2070. |
| [3] | 田霞,杨福群,袁伟,赵雷,姚雷,甄小丽,韩建荣,刘守信. 含噁二唑大环冠醚的合成、 结构及金属离子识别性能[J]. 高等学校化学学报, 2020, 41(3): 490. |
| [4] | 王梦雨, 曹思敏, 李昊阳, 张梦婕, 李栋, 赵泽楠, 徐建华. 辅酶NADH与色氨酸共振能量转移的荧光动力学研究[J]. 高等学校化学学报, 2020, 41(11): 2473. |
| [5] | 马祥英, 廖艳娟, 覃方红, 尹源浩, 黄在银, 陈其锋. 碳自掺杂g-C3N4光催化性能的原位光微量热-荧光光谱研究[J]. 高等学校化学学报, 2020, 41(11): 2526. |
| [6] | 魏馨, 邓要亮, 郑旭明, 赵彦英. 2-氨基苯并噻唑的结构及激发态质子转移动力学[J]. 高等学校化学学报, 2019, 40(8): 1679. |
| [7] | 冉诗雅, 沈海峰, 李晓楠, 王子路, 郭正虹, 方征平. 三氟甲烷磺酸稀土盐对聚丙烯热稳定性的影响及机理[J]. 高等学校化学学报, 2019, 40(6): 1333. |
| [8] | 房夕杰, 刘瑞云, 林森, 石磊, 王润伟, 李乙, 李俊英. 蒸汽辅助合成STW结构硅锗酸盐分子筛[J]. 高等学校化学学报, 2019, 40(5): 867. |
| [9] | 刘韬, 李文静, 张恩爽, 钟锦洋, 张凡, 刘圆圆, 赵英民. 柔性交联型聚酰亚胺气凝胶的制备及性能[J]. 高等学校化学学报, 2019, 40(2): 403. |
| [10] | 尹萌欣,刘东升,赵东越,丁彤,田野,李新刚. Cu掺杂对Pt/Ba/CuxMg1-xAl2O4催化剂高温稀燃 NOx消除性能的影响[J]. 高等学校化学学报, 2019, 40(10): 2170. |
| [11] | 刘仪, 许晓洲, 莫松, 翟磊, 何民辉, 范琳. 含硅氧烷结构聚酰亚胺树脂的耐热稳定性及高温结构演变[J]. 高等学校化学学报, 2019, 40(1): 187. |
| [12] | 白蕾, 霍淑慧, 陈晶, 卢小泉. 用于手性识别α-氨基酸的方酰胺荧光探针分子[J]. 高等学校化学学报, 2019, 40(1): 41. |
| [13] | 孟嘉锋, 倪旭峰, 郑豪, 沈之荃. 苯氧基亚胺钛催化降冰片烯与1-辛烯共聚合[J]. 高等学校化学学报, 2018, 39(8): 1853. |
| [14] | 崔胜峰, 万敬伟, 周成合. 地西泮与乙醇协同作用机制的荧光光谱分析[J]. 高等学校化学学报, 2018, 39(6): 1178. |
| [15] | 史鹏辉, 边六交. 头孢西丁与金属β-内酰胺酶BcⅡ相互作用的光谱分析及分子动力学模拟[J]. 高等学校化学学报, 2018, 39(5): 971. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||