

高等学校化学学报 ›› 2021, Vol. 42 ›› Issue (7): 2136.doi: 10.7503/cjcu20210117
收稿日期:2021-02-26
出版日期:2021-07-10
发布日期:2021-06-03
通讯作者:
崔刚龙
E-mail:ganglong.cui@bnu.edu.cn;fangwh@bnu.edu.cn
作者简介:方维海, 男, 博士, 教授, 中国科学院院士, 主要从事非绝热动力学方法发展及其应用研究. E-mail: 基金资助:
PENG Qin, FANG Yeguang, ZHANG Tengshuo, CUI Ganglong( ), FANG Weihai(
), FANG Weihai( )
)
Received:2021-02-26
Online:2021-07-10
Published:2021-06-03
Contact:
CUI Ganglong
E-mail:ganglong.cui@bnu.edu.cn;fangwh@bnu.edu.cn
Supported by:摘要:
应用高精度的多态完全活化自洽场二级微扰理论方法, 在量子力学/分子力学组合方法的理论框架 QM(MS-CASPT2//CASSCF)/MM下, 系统研究了DNA环境中2-硒和4-硒取代胸腺嘧啶和腺嘌呤碱基对(2SeT-A和4SeT-A)的最低5个电子态(S0, S1, S2, T2和T1)的结构、 性质和光物理过程. QM(MS-CASPT2//CASSCF)/MM计算揭示了DNA环境中2SeT-A和4SeT-A碱基对激发态性质和光物理过程差异性的来源, 提出的机理将有助于理解DNA类似物的光物理过程, 在光动力学治疗中具有潜在的应用.
中图分类号:
TrendMD:
彭沁, 方业广, 张腾烁, 崔刚龙, 方维海. DNA环境中硒代胸腺嘧啶和腺嘌呤碱基对的激发态性质和光物理机理的理论研究. 高等学校化学学报, 2021, 42(7): 2136.
PENG Qin, FANG Yeguang, ZHANG Tengshuo, CUI Ganglong, FANG Weihai. Theoretical Study on the Excited State Properties and Photophysical Mechanism of Selenothymine and Adenine Base Pairs in DNA Environment. Chem. J. Chinese Universities, 2021, 42(7): 2136.
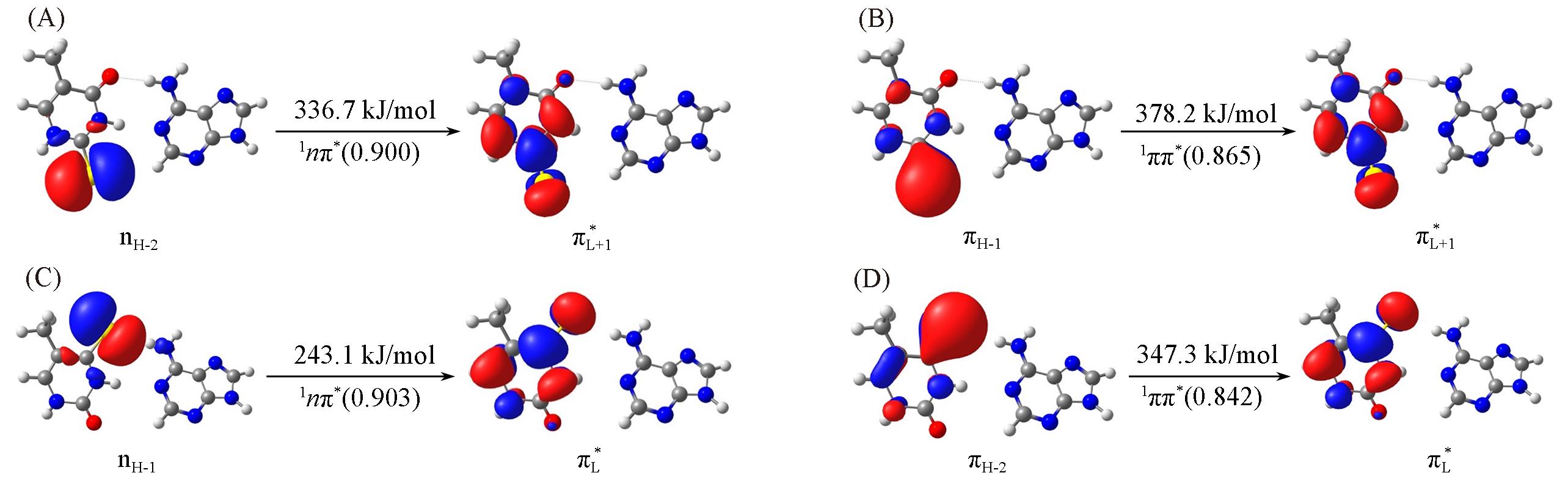
Fig.2 Molecular orbitals relevant to the lowest two excited singlet states at the Franck?Condon points of 2SeT?A and 4SeT?AAlso shown are the corresponding vertical excitation energies(in kJ/mol), excited?state characters, and weights of electronic configurations.(A) 2SeT?A, S1; (B) 2SeT?A, S2; (C) 4SeT?A, S1; (D) 4SeT?A, S2.
| System | S1(nπ*) | S2(ππ*) | T1(ππ*) | T2(nπ*) | T3(ππ*) |
|---|---|---|---|---|---|
| 2SeT?A | 336.7 | 378.2 | 272.1 | 325.2 | 422.6 |
| 4SeT?A | 243.1 | 347.3 | 244.1 | 239.3 | 391.7 |
Table 1 QM(MS-CASPT2)/MM calculated vertical excitation energies(kJ/mol) at the S0 minima of 2SeT-A and 4SeT-A
| System | S1(nπ*) | S2(ππ*) | T1(ππ*) | T2(nπ*) | T3(ππ*) |
|---|---|---|---|---|---|
| 2SeT?A | 336.7 | 378.2 | 272.1 | 325.2 | 422.6 |
| 4SeT?A | 243.1 | 347.3 | 244.1 | 239.3 | 391.7 |
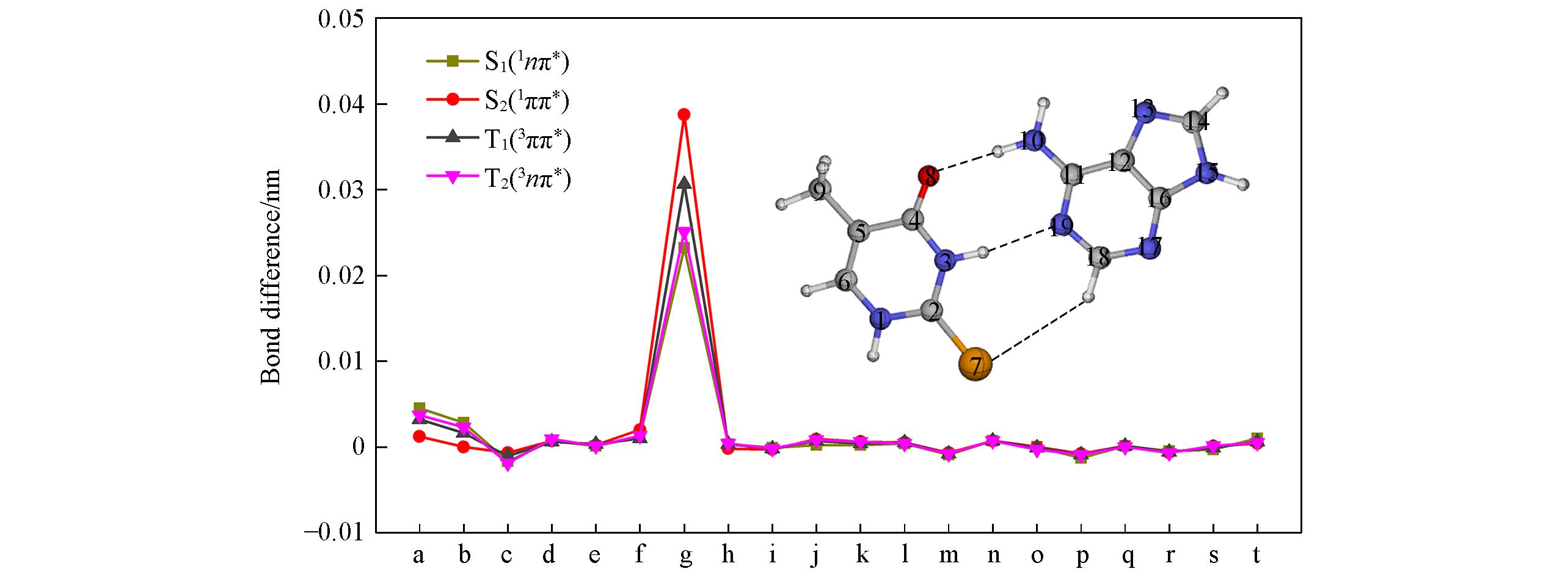
Fig.4 Bond?length changes of QM(CASSCF)/MM optimized excited?state minimum?energy structures of 2SeT?A?U relative to corresponding ground?state structuresa. N1─C2; b. C2─N3; c. N3─C4; d. C4─N5; e. C5─C6; f. C6─N1; g. C2─Se7; h. C4─O8; i. C5─C9; j. N10─C11; k. C11─C12; l. C12─N13; m. C12─C16; n. N13─C14; o. C14─N15; p. N15─C16; q. C16─N17; r. N17─C18; s. C18─N19; t. N19─C11.
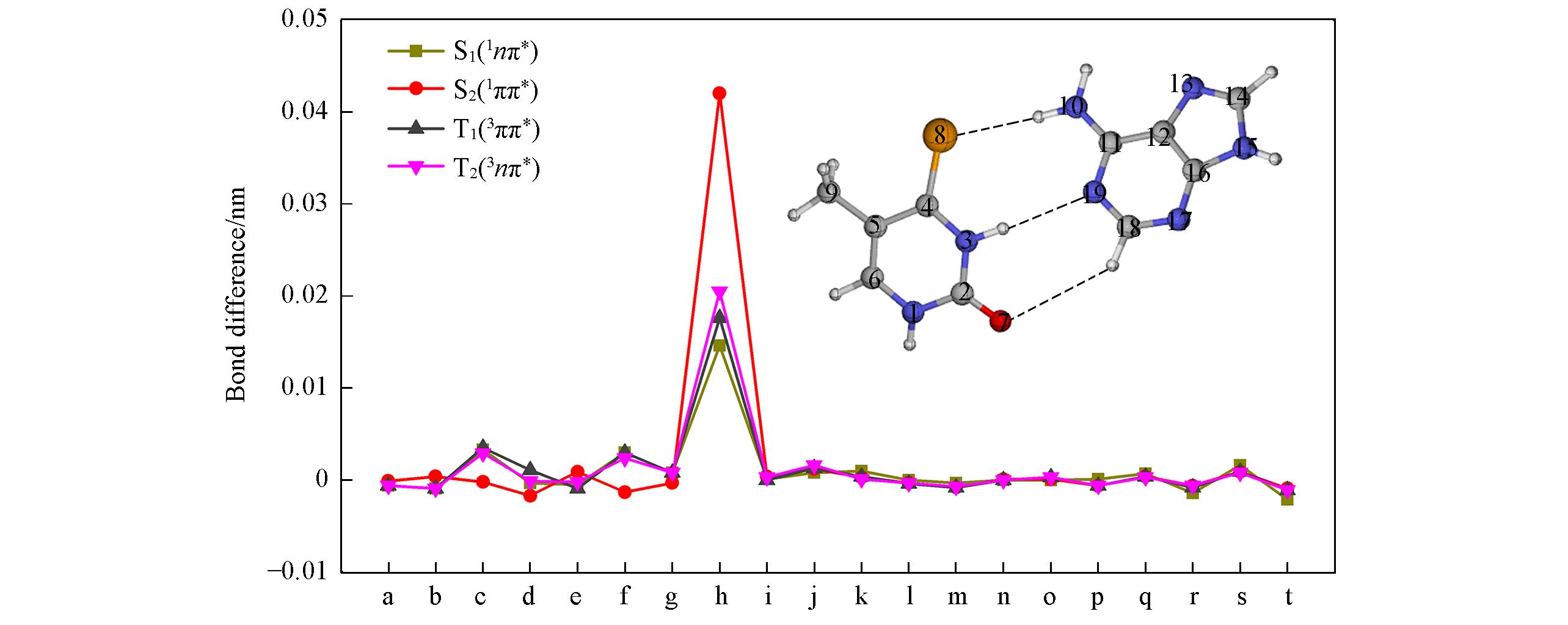
Fig.6 Bond?length changes of QM(CASSCF)/MM optimized excited?state minimum?energy structures of 4SeT?A?U relative to corresponding ground?state structuresa. N1─C2; b. C2─N3; c. N3─C4; d. C4─N5; e. C5─C6; f. C6─N1; g. C2─O7; h. C4─Se8; i. C5─C9; j. N10─C11; k. C11─C12; l. C12─N13; m. C12─C16; n. N13─C14; o. C14─N15; p. N15─C16; q. C16─N17; r. N17─C18; s. C18─N19; t. N19─C11.
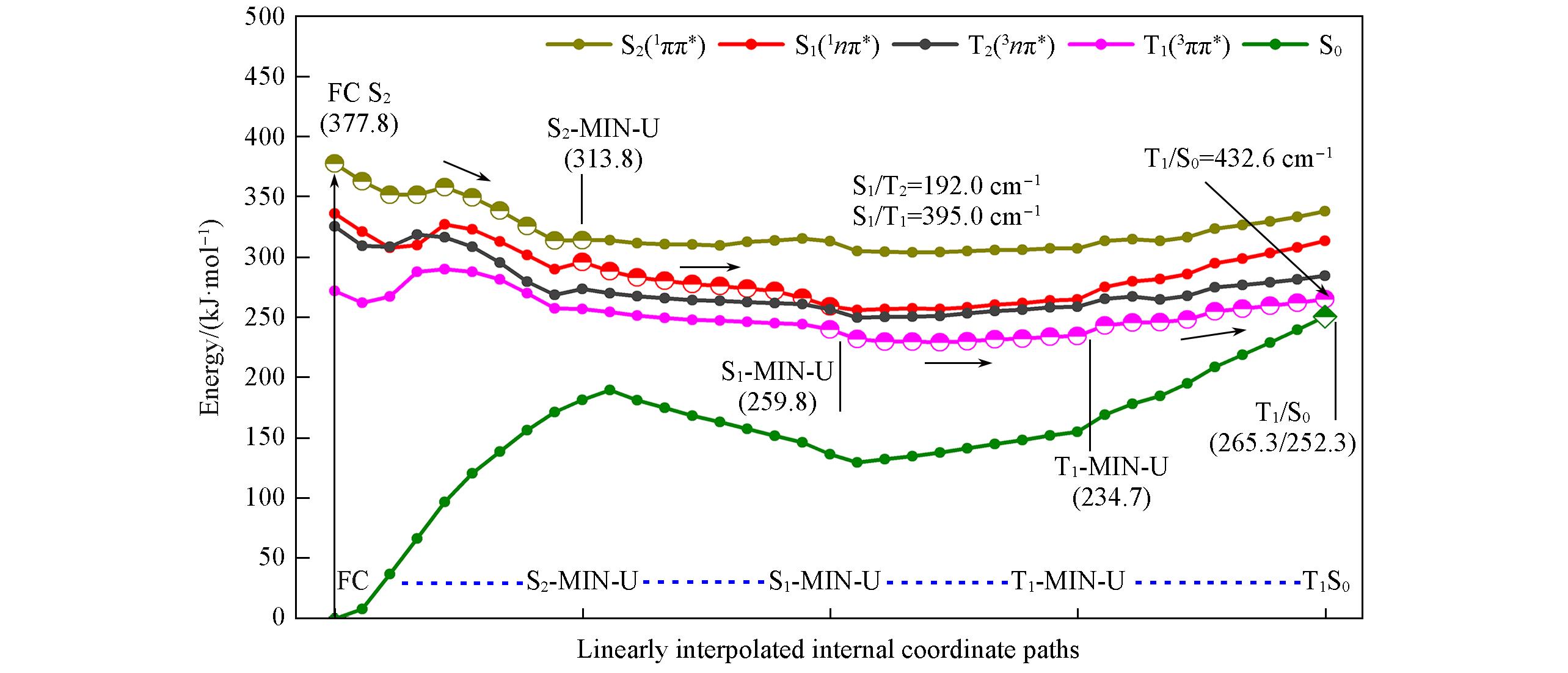
Fig.8 Main excited relaxation path of 2SeT?A in DNA calculated by QM(MS?CASPT2)/MM(the conformation of C─Se bond up along the pyrimidine ring plane)See supporting information for excited relaxation paths in other conformations.
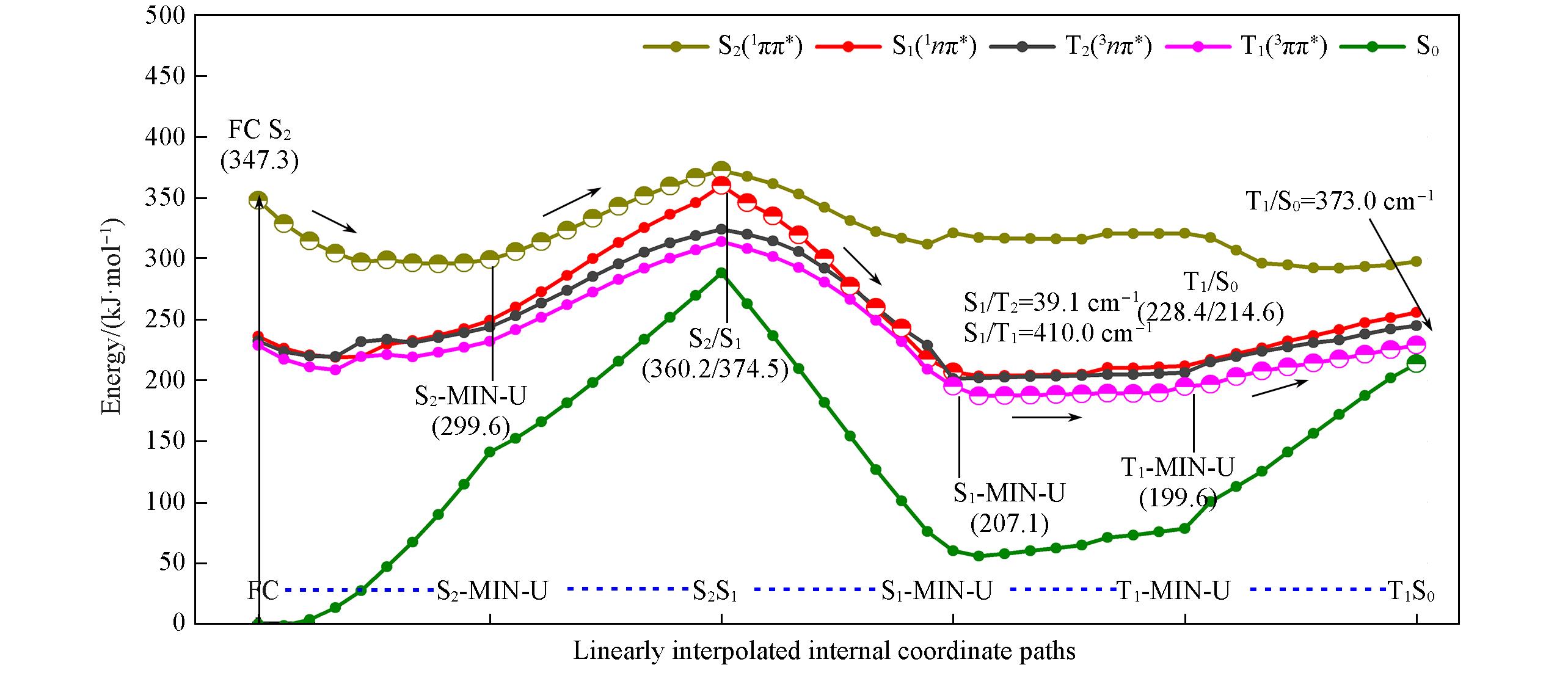
Fig.9 Main excited relaxation path of 2SeT?A in DNA calculated by QM(MS?CASPT2)/MM(the conformation of C―Se bond up along the pyrimidine ring plane)See supporting information for excited relaxation paths in other conformations.
| 1 | Watson J. D., Crick F. H. C., Nature, 1953, 171, 737―738 |
| 2 | Schreier W. J., Gilch, P., Zinth W., Annu. Rev. Phys. Chem., 2015, 66, 497―519 |
| 3 | Crespo⁃Hernández C. E., Cohen B., Hare P. M., Kohler B., Chem. Rev., 2004, 104, 1977―2019 |
| 4 | Schultz T., Samoylova E., Radloff W., Hertel I. V., Sobolewski A. L., Domcke W., Science, 2004, 306, 1765―1768 |
| 5 | Cohen B., Hare P. M., Kohler B., J. Am. Chem. Soc., 2003, 125, 13594―13601 |
| 6 | Kang H., Lee K. T., Jung B., Ko Y. J., Kim S. K., J. Am. Chem. Soc., 2002, 124, 12958―12959 |
| 7 | Boggio⁃Pasqua M., Groenhof G., Schäfer L. V., Grubmüeller H., Robb M. A., J. Am. Chem. Soc., 2007, 129, 10996―10997 |
| 8 | Barbatti M., Aquino A. J. A., Szymczak J. J., Nachtigallová D., Hobza P., Lischka H., Proc. Natl. Acad. Sci. U.S.A., 2010, 107, 21453―21458 |
| 9 | Improta R., Santoro F., Blancafort L., Chem. Rev., 2016, 116, 3540―3593 |
| 10 | Blancafort L., J. Am. Chem. Soc., 2006, 128, 210―219 |
| 11 | Milder S. J., Kliger D. S., J. Am. Chem. Soc., 1985, 107, 7365―7373 |
| 12 | Favre A., Saintomé C., Fourrey J. L., Clivio P., Laugâa P., J. Photochem. Photobiol. B, 1998, 42, 109―124 |
| 13 | Taras⁃Goślińka K., Wenska G., Skalski B., Maciejewski A., Burdziński G., Karolczak J., J. Photochem. Photobiol. A, 2004, 168, 227―233 |
| 14 | Kuramochi H., Kobayashi T., Suzuki T., Ichimura T., J. Phys. Chem. B, 2010, 114, 8782―8789 |
| 15 | Khvorostov A., Lapinski L., Rostkowska H., Nowak M. J., J. Phys. Chem. A, 2005, 109, 7700―7707 |
| 16 | Taras⁃Goślińka K., Wenska G., Skalski B., Maciejewski A., Burdziński G., Karolczak J., J. Photochem. Photobiol. A, 2002, 75, 448―456 |
| 17 | Skalski B., Taras⁃Goślińka K., Dembska A., Gdaniec Z., Franzen S., J. Org. Chem., 2010, 75, 621―626 |
| 18 | Reichardt C., Guo C., Crespo⁃Hernández C. E., J. Phys. Chem. B, 2011, 115, 3263―3270 |
| 19 | Reelfs O., Karran P., Young A. R., Photochem. Photobiol. Sci., 2012, 11, 148―154 |
| 20 | Weingart O., Lan Z. G., Koslowski A., Thiel W., J. Phys. Chem. Lett., 2011, 2, 1506―1509 |
| 21 | Vendrell⁃Criado V., Sáez J. A., Lhiaubet⁃Vallet V., Cuquerella M. C., Miranda M. A., Photochem. Photobiol. Sci., 2013, 12, 1460―1465 |
| 22 | Zhang Y. Z., Zhu X. C., Smith J., Haygood M. T., Gao R. M., J. Phys. Chem. B, 2011, 115, 1889―1894 |
| 23 | Puzzarini C., Biczysko M., Barone V., Peña I., Cabezas C., Alonso J. L., Phys. Chem. Chem. Phys., 2013, 15, 16965―16975 |
| 24 | Harada Y., Suzuki T., Ichimura T., Xu Y. Z., J. Phys. Chem. B, 2007, 111, 5518―5524 |
| 25 | Harada Y., Okabe C., Kobayashi T., Suzuki T., Ichimura T., Nishi N., Xu Y. Z., J. Phys. Chem. Lett., 2010, 1, 480―484 |
| 26 | Reichardt C., Crespo⁃Hernández C. E., Chem. Commun., 2010, 46, 5963―5965 |
| 27 | Reichardt C., Crespo⁃Hernández C. E., J. Phys. Chem. Lett., 2010, 1, 2239―2243 |
| 28 | Pollum M., Crespo⁃Hernández C. E., J. Chem. Phys., 2014, 140, 071101 |
| 29 | Jiang J., Zhang T. S., Xue J. D., Zheng X. M., Cui G. L., Fang W. H. J. Chem. Phys., 2015, 143, 175103 |
| 30 | Pollum M., Jockusch S., Crespo⁃Hernández C. E., J. Am. Chem. Soc., 2014, 136, 17930―17933 |
| 31 | Pollum M., Jockusch S., Crespo⁃Hernández C. E., Phys. Chem. Chem. Phys., 2015, 17, 27851―27861 |
| 32 | Pollum M., Martínez⁃Fernández L., Crespo⁃Hernández C. E., Top. Curr. Chem., 2015, 355, 245―327 |
| 33 | Ashwood B., Pollum M., Crespo⁃Hernández C. E., Photochem. Photobiol., 2019, 95, 33―58 |
| 34 | Cui G. L., Fang W. H., J. Chem. Phys., 2013, 138, 044315 |
| 35 | Martínez⁃Fernández L., Corral I., Granucci G., Persico M., Chem. Sci., 2014, 5, 1336―1347 |
| 36 | Gobbo J. P., Borin A. C., J. Phys. Chem. A, 2013, 117, 5589―5596 |
| 37 | Martínez⁃Fernández L., González L., Corral I., Chem. Commun., 2012, 48, 2134―2136 |
| 38 | Cui G. L., Thiel W., J. Phys. Chem. Lett., 2014, 5, 2682―2687 |
| 39 | Mai S., Marquetand P., González L., J. Phys. Chem. A, 2015, 119, 9524―9533 |
| 40 | Mai S., Marquetand P., González L., J. Phys. Chem. Lett., 2016, 7, 1978―1983 |
| 41 | Gobbo J. P., Borin A. C., Comput.Theor. Chem., 2014, 1040/1041, 195―201 |
| 42 | Ruckenbauer M., Mai S., Marquetand P., González L., J. Chem. Phys., 2016, 144, 074303 |
| 43 | Mai S., Pollum M., Martínez⁃Fernández L., Dunn N., Marquetand P., Corral I., Crespo⁃Hernández C. E., González L., Nat. Commun., 2016, 7, 13077 |
| 44 | Bai S. M., Barbatti M., J. Phys. Chem. A, 2016, 120, 6342―6350 |
| 45 | Sánchez⁃Rodríguez J. A., Mohamadzade A., Mai S., Ashwood B., Pollum M., Marquetand P., González L., Crespo⁃Hernández C. E., Ullrich S., Phys. Chem. Chem. Phys., 2017, 19, 19756―19766 |
| 46 | Yu H., Sánchez⁃Rodriguez J. A., Pollum M., Crespo⁃Hernández C. E., Mai S., Marquetand P., González L., Ullrich S., Phys. Chem. Chem. Phys., 2016, 18, 20168―20176 |
| 47 | Xie B. B., Wang Q., Guo W. W., Cui G. L., Phys. Chem. Chem. Phys., 2017, 19, 7689―7698 |
| 48 | Sierant M., Leszczynska G., Sadowska K., Komar P., Radzikowska⁃Cieciura E., Sochacka E., Nawrot B., FEBS Lett., 2018, 592, 2248―2258 |
| 49 | Trujillo C., Mó O., Yáñez M., Org. Biomol. Chem., 2007, 5, 3092―3099 |
| 50 | Caton⁃Williams J., Huang Z., Chem. Biodiversty, 2008, 5, 396―407 |
| 51 | Caton⁃Williams J., Huang Z., Angew. Chem. Int. Ed., 2008, 120, 1747―1749 |
| 52 | Trujillo C., Mó O., Yáñez M., ChemPhysChem, 2008, 9, 1715―1720 |
| 53 | Christofferson A., Zhao L. F., Sun H. Z., Huang Z., Huang N., J. Phys. Chem. B, 2011, 115, 10041―10048 |
| 54 | Vázquez⁃Mayagoitia Á., Huertas O., Brancolini G., Migliore A., Sumpter B. G., Orozco M., Javier Luque F., Di Felice R., Fuentes-Cabrera M., J. Phys. Chem. B, 2009, 113, 14465―14472 |
| 55 | Sun H. Y., Jiang S. B., Caton⁃Williams J., Liu H. H., Huang Z., RNA, 2013, 19, 1309―1314 |
| 56 | Farrell K. M., Brister M. M., Pittelkow M., Sølling T. I., Crespo⁃Hernández C. E., J. Am. Chem. Soc., 2018, 140, 11214―11218 |
| 57 | Salon J., Jiang J. S., Sheng J., Gerlits O. O., Huang Z., Nucleic Acids Res., 2008, 36, 7009―7018 |
| 58 | Pirillo J., de Simone B. C., Russo N., Theor. Chem. Acc., 2015, 135, 8 |
| 59 | Pirillo J., Mazzone G., Russo N., Bertini L., J. Chem. Inf. Model., 2017, 57, 234―242 |
| 60 | Manae M. A., Hazra A., J. Phys. Chem. A, 2017, 121, 8147―8153 |
| 61 | Mai S., Wolf A. P., González L., J. Chem. Theory Comput., 2019, 15, 3730―3742 |
| 62 | Fang Y. G., Peng Q., Fang Q., Fang W. H., Cui G. L., ACS Omega, 2019, 4, 9769―9777 |
| 63 | Peng Q., Zhu Y. H., Zhang T. S., Liu X. Y., Fang W. H., Cui G. L., Phys. Chem. Chem. Phys., 2020, 22, 12120―12128 |
| 64 | Case D. A., Berryman J. T., Betz R. M., Cerutti D. S., Cheatham T. E., Darden T. A., Duke R. E., Giese T. J., Gohlke H., Goetz A. W., Homeyer N., Izadi S., Janowski P., Kaus J., Kovalenko A., Lee T. S., LeGrand S., Li P., Lin C., Luchko T., Luo R., Madej B., Mermelstein D., Merz K. M., Monard G., Nguyen H., Nguyen H. T., Omelyan I., Onufriev A., Roe D. R., Roitberg A., Sagui C., Simmerling C. L., Botello⁃Smith W. M., Swails J., Walker R. C., Wang J., Wolf R. M., Wu X., Xiao L., Kollman P. A., University of California: San Francisco, 2016 |
| 65 | Hornak V., Abel R., Okur A., Strockbine B., Roitberg A., Simmerling C., PROTEINS, 2006, 65, 712―725 |
| 66 | Jorgensen W. L., Chandrasekhar J., Madura J. D., Impey R. W., Klein M. L., J. Chem. Phys., 1983, 79, 926―935 |
| 67 | Warshel A., Levitt M., J. Mol. Biol., 1976, 103, 227―249 |
| 68 | Field M. J., Bash P. A., Karplus M., J. Comput. Chem., 1990, 11, 700―733 |
| 69 | Hu H., Yang W., Annu. Rev. Phys. Chem., 2008, 59, 573―601 |
| 70 | Senn H. M., Thiel W., Angew. Chem. Int. Ed., 2009, 48, 1198―1229 |
| 71 | Andersson K., Malmqvist P. Å., Roos B. O., Sadlej A. J., Wolinski K., J. Phys. Chem., 1990, 94, 5483―5488 |
| 72 | Andersson K., Malmqvist P. Å., Roos B. O., J. Chem. Phys., 1992, 96, 1218―1226 |
| 73 | Försberg N., Malmqvist P. Å., Chem. Phys. Lett., 1997, 274, 196―204 |
| 74 | Zobel J. P., Nogueira J. J., González L., Chem. Sci., 2017, 8, 1482―1499 |
| 75 | Ghigo G., Roos B. O., Malmqvist P. Å., Chem. Phys. Lett., 2004, 396, 142―149 |
| 76 | Aquilante F., Lindh R., Pedersen T. B., J. Chem. Phys., 2007, 127, 114107―114713 |
| 77 | Dunning T. H. Jr., J. Chem. Phys., 1989, 90, 1007―1023 |
| 78 | Wilson A. K., Woon D. E., Peterson K. A., Dunning T. H., J. Chem. Phys., 1999, 110, 7667―7676 |
| 79 | Heβ B. A., Marian C. M., Wahlgren U., Gropen O., Chem. Phys. Lett., 1996, 215, 365―371 |
| 80 | Marian C. M., Wahlgren U., Chem. Phys. Lett., 1996, 251, 357―364 |
| 81 | Marian C. M., Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2012, 2, 187―203 |
| 82 | El⁃Sayed M. A., J. Chem. Phys., 1963, 38, 2834―2837 |
| [1] | 贠吉星, 胡志莉, 李禹蒙, 金晶, 陈冲, 鄢欣, 刘永华, 丁榆, 迟玉贤, 牛淑云. 由芳香羧酸构筑的系列Ni(Ⅱ)配合物的合成、 结构及光电性能[J]. 高等学校化学学报, 2018, 39(10): 2161. |
| [2] | 靳俊玲, 丁祥, 欧利辉, 张向阳, 申有名, 耿允, 苏忠民. N,N-B螯合物光物理性质的密度泛函理论研究[J]. 高等学校化学学报, 2015, 36(5): 962. |
| [3] | 段新丽, 张欣, 雷鸣. 烟酰胺酶催化水解脱氨反应的QM/MM分子动力学模拟[J]. 高等学校化学学报, 2015, 36(12): 2491. |
| [4] | 孙静, 董海亮, 王华, 苗艳勤, 许慧侠, 李洁, 武钰铃, 许并社. 自主体发光的蓝绿色磷光Ir(Ⅲ)配合物的合成及光电特性[J]. 高等学校化学学报, 2015, 36(10): 1859. |
| [5] | 赵伟良, 李琮, 韩旭, 黎挺挺, 张桂菊, 甘欣, 李褔敏, 傅文甫. 硫代多联吡啶铂(Ⅱ)配合物的合成、性质及在光催化制氢中的应用[J]. 高等学校化学学报, 2014, 35(10): 2214. |
| [6] | 段雨爱 耿允 李海斌 杨国春 吴水星 郝立柱 廖奕 苏忠民. 不同桥联二噻吩低带隙给受体共聚物的电子结构和光物理性质的理论研究[J]. 高等学校化学学报, 2011, 32(4): 920. |
| [7] | 孙建平, 翁家宝, 林婷, 马琳璞. 聚(2-甲氧基-5-辛氧基)对苯乙炔/单壁碳纳米管复合材料的光物理性能研究[J]. 高等学校化学学报, 2009, 30(9): 1891. |
| [8] | 徐定国, 鄢国森. L1 β-Lactamase催化反应机理研究[J]. 高等学校化学学报, 2008, 29(12): 2453. |
| [9] | 彭谦, 牛英利, 帅志刚. 二苯多烯分子的光物理性质与共轭长度的关系[J]. 高等学校化学学报, 2008, 29(12): 2435. |
| [10] | 王兆龙,牛淑云,金晶,吕春欣,迟玉贤,杨光弟,叶玲 . Zn-Ln(Ⅲ)(Ln=Eu, Tb)杂核配合物的合成、 结构及光物理性质[J]. 高等学校化学学报, 2007, 28(5): 811. |
| [11] | 李斌, 傅春艳, 苏忠民, 刘景福. 钌(Ⅱ)表面活性剂配合物的合成、表征及其液晶和发光性质[J]. 高等学校化学学报, 2004, 25(6): 1002. |
| [12] | 阎冰, 张洪杰, 王淑彬, 倪嘉缵. 稀土N-苯基邻氨基苯甲酸-1,10-邻菲咯啉二元、三元配合物的合成、表征及其光物理性质[J]. 高等学校化学学报, 1998, 19(5): 671. |
| [13] | 刘志贤, 穆林静, 石双群, 周永洽, 申泮文. 水溶性金属卟啉在水溶液中的光物理与光化学研究[J]. 高等学校化学学报, 1996, 17(10): 1504. |
| [14] | 徐铸德, 陈万喜, 冷拥军, 许小平, 李文铸, 汪茫. 富勒烯掺杂有机光电导材料的光电导性[J]. 高等学校化学学报, 1995, 16(2): 296. |
| [15] | 张先付, 许慧君. 取代锌酞菁的合成及光物理性质[J]. 高等学校化学学报, 1994, 15(6): 917. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||