

高等学校化学学报 ›› 2016, Vol. 37 ›› Issue (7): 1307.doi: 10.7503/cjcu20160098
袁洁1,4, 刘青川2, 徐广灿1,3, 梁光义1,3( ), 徐必学1
), 徐必学1
收稿日期:2016-02-17
接受日期:2016-06-15
出版日期:2016-07-10
发布日期:2016-06-15
基金资助:
YUAN Jie1,4, LIU Qingchuan2, XU Guangcan1,3, LIANG Guangyi1,3,*, XU Bixue1,*( )
)
Received:2016-02-17
Accepted:2016-06-15
Online:2016-07-10
Published:2016-06-15
Contact:
LIANG Guangyi,XU Bixue
E-mail:guangyi_liang@126.com;bixue_xu@126.com
Supported by:摘要:
以D-半乳糖和二缩三乙二醇为原料, 经乙酰化、 糖基化和叠氮化钠取代等反应合成了带叠氮连接臂的半乳糖配基, 通过点击化学反应将其与炔丙基修饰的马蹄金素(MTS)衍生物进行连接, 设计合成了6个具有潜在肝靶向性的半乳糖糖基化MTS衍生物. 通过1H NMR, 13C NMR, 1H-1H COSY, HMQC, DEPT和ESI-MS对其结构进行了表征; 采用HepG2 2.2.15细胞模型初步评价了目标化合物的抗乙型肝炎病毒(HBV)活性. 结果表明, 所有目标化合物对HBV DNA的复制均有抑制作用, 且具有一定的量效关系; 化合物15f在50 μg/mL浓度下对HepG2 2.2.15细胞株的抑制率为83%, 具有进一步研究的价值.
中图分类号:
TrendMD:
袁洁, 刘青川, 徐广灿, 梁光义, 徐必学. 基于点击化学反应的半乳糖糖基化马蹄金素衍生物的合成及抗HBV活性. 高等学校化学学报, 2016, 37(7): 1307.
YUAN Jie, LIU Qingchuan, XU Guangcan, LIANG Guangyi, XU Bixue. Synthesis and Anti-HBV Activity Evaluation of the Galactopyranosyl Derivatives of MTS Based on Click Reaction†. Chem. J. Chinese Universities, 2016, 37(7): 1307.
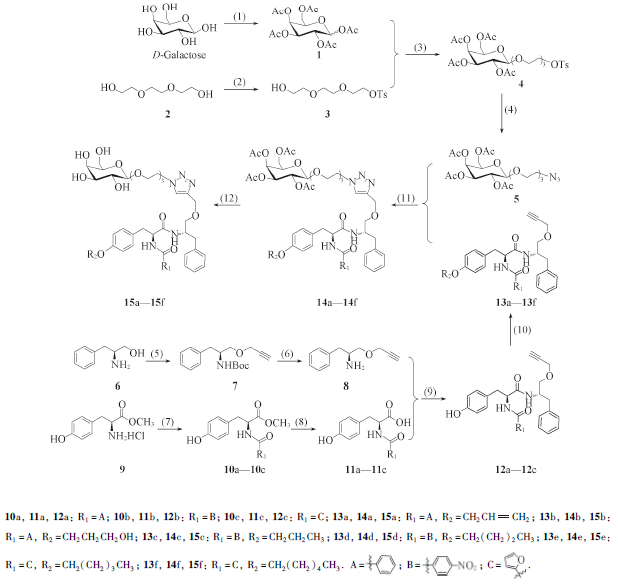
Scheme 1 Synthetic routes of target compounds 15a—15f(1) Ac2O, CH3COONa, 140 ℃; (2) TsCl, pyridine, r. t.; (3) 0.4 nm MS, BF3·Et2O, CH2Cl2, 0 ℃ to r. t.; (4) NaN3, TBAI, DMF, 60 ℃; (5) (a)(Boc)2O, DMF/H2O, r. t.; (b) propargyl bromide(80%, solution in toluene), NaH(60%, dispersion in mineral oil), DMF, 0 ℃ to r. t.; (6) TFA, CH2Cl2, 0 ℃ to r. t.; (7) benzoic acid, 4-nitrobenzoic acid or furoic acid, IBCF, NMM, CH2Cl2/DMF, 0 ℃ to r. t.; (8) 1.0 mol/L NaOH(aq.), DMF, r. t.; (9) IBCF, NMM, CH2Cl2/DMF, 0 ℃ to r. t.; (10) CH3(CH2)5Br, CH3(CH2)4Br, CH3(CH2)3I, CH3(CH2)2I, Br(CH2)3OH or allyl bromide, K2CO3, DMF, r. t.; (11) (+)-sodium L-ascorbate, CuI, DIPEA, DMF/H2O, r. t.; (12) CH3ONa, CH3OH/CH2Cl2, r. t..
| Compd. | Appearance | Yield(%) | m. p./℃ | [α | ESI-MS, m/z |
|---|---|---|---|---|---|
| 3 | Pale oil | 72 | |||
| 4 | Pale oil | 73 | 657.0 [M+Na]+ | ||
| 5 | Pale oil | 94 | 544.2 [M+K]+ | ||
| 8 | Pale oil | 37 | |||
| 12a | White solid | 27 | 160—162 | -61.0 | |
| 12b | Yellow solid | 29 | 200—202 | -104.3 | |
| 12c | White solid | 25 | 98—100 | -24.4 | |
| 13a | White solid | 83 | 167—169 | -51.9 | |
| 13b | White solid | 80 | 135—137 | -29.8 | |
| 13c | Yellow solid | 90 | 232—234 | -22.6 | |
| 13d | Yellow solid | 88 | 219—221 | -35.2 | |
| 13e | White solid | 87 | 123—125 | -50.6 | |
| 13f | White solid | 84 | 124—126 | -35.4 | |
| 14a | Pale oil | 93 | -29.1 | 1024.2 [M+Na]+ | |
| 14b | Pale oil | 90 | -29.0 | 1042.3 [M+Na]+ | |
| 14c | Pale oil | 87 | -24.0 | 1071.2 [M+Na]+ | |
| 14d | Pale oil | 92 | -31.6 | 1063.3 [M+H]+ | |
| 14e | Pale oil | 88 | -32.9 | 1022.3 [M+H]+ | |
| 14f | Pale oil | 86 | -35.9 | 1058.3 [M+Na]+ |
Table 1 Appearance, yields, melting points, specific rotation and ESI-MS data for all intermediates*
| Compd. | Appearance | Yield(%) | m. p./℃ | [α | ESI-MS, m/z |
|---|---|---|---|---|---|
| 3 | Pale oil | 72 | |||
| 4 | Pale oil | 73 | 657.0 [M+Na]+ | ||
| 5 | Pale oil | 94 | 544.2 [M+K]+ | ||
| 8 | Pale oil | 37 | |||
| 12a | White solid | 27 | 160—162 | -61.0 | |
| 12b | Yellow solid | 29 | 200—202 | -104.3 | |
| 12c | White solid | 25 | 98—100 | -24.4 | |
| 13a | White solid | 83 | 167—169 | -51.9 | |
| 13b | White solid | 80 | 135—137 | -29.8 | |
| 13c | Yellow solid | 90 | 232—234 | -22.6 | |
| 13d | Yellow solid | 88 | 219—221 | -35.2 | |
| 13e | White solid | 87 | 123—125 | -50.6 | |
| 13f | White solid | 84 | 124—126 | -35.4 | |
| 14a | Pale oil | 93 | -29.1 | 1024.2 [M+Na]+ | |
| 14b | Pale oil | 90 | -29.0 | 1042.3 [M+Na]+ | |
| 14c | Pale oil | 87 | -24.0 | 1071.2 [M+Na]+ | |
| 14d | Pale oil | 92 | -31.6 | 1063.3 [M+H]+ | |
| 14e | Pale oil | 88 | -32.9 | 1022.3 [M+H]+ | |
| 14f | Pale oil | 86 | -35.9 | 1058.3 [M+Na]+ |
| Compd. | 1H NMR(CDCl3), δ |
|---|---|
| 3 | 7.80(d, J=8.3 Hz, 2H, ArH, 7.35(d, J=8.0 Hz, 2H, ArH), 4.17(t, J=4.8 Hz, 2H, CH2OH), 3.75—3.68(m, 4H, OCH2×2), 3.63—3.55(m, 6H, OCH2×3), 2.45(s, 3H, CH3) |
| 4 | 7.79(d, J=8.3 Hz, 2H, ArH), 7.34(dd, J=8.6, 0.6 Hz, 2H, ArH), 5.38(dd, J=3.4, 1.0 Hz, 1H, 4d-H), 5.20(dd, J=10.5, 8.0 Hz, 1H, 2d-H), 5.02(dd, J=10.5, 3.4 Hz, 1H, 3d-H), 4.56(d, J=8.0 Hz, 1H, 1d-H), 4.20—4.09(m, 4H, 6d-H, OCH2), 3.98—3.89(m, 2H, 5d-H, OCH2), 3.73(ddd, J=11.0, 7.0, 3.9 Hz, 1H, OCH2), 3.70—3.55(m, 8H, OCH2×4), 2.45(s, 3H, CH3), 2.15(s, 3H, CH3CO), 2.05(s, 3H, CH3CO), 2.05(s, 3H, CH3CO), 1.99(s, 3H, CH3CO) |
| 5 | 5.39(dd, J=3.4, 1.1 Hz, 1H, 4d-H), 5.22(dd, J=10.5, 8.0 Hz, 1H, 2d-H), 5.02(dd, J=10.5, 3.4 Hz, 1H, 3d-H), 4.58(d, J=8.0 Hz, 1H, 1d-H), 4.22—4.10(m, 2H, 6d-H), 4.00—3.89(m, 2H, 5d-H, OCH2), 3.76(ddd, J=11.0, 6.9, 4.0 Hz, 1H, OCH2), 3.70—3.64(m, 8H, OCH2×4), 3.41(t, J=5.0 Hz, 2H, CH2N3), 2.15(s, 3H, CH3CO), 2.07(s, 3H, CH3CO), 2.05(s, 3H, CH3CO), 1.99(s, 3H, CH3CO) |
| 8 | 7.32—7.21(m, 5H, 5c-H—9c-H), 4.17(d, J=1.9 Hz, 2H, OCH2C≡≡CH), 3.54(dd, J=9.1, 4.1 Hz, 1H, 1c-H), 3.38(dd, J=8.9, 7.1 Hz, 1H, 1'c-H), 3.29—3.25(m, 1H, 2c-H), 2.81(dd, J=13.5, 5.4 Hz, 1H, 3c-H), 2.58(dd, J=13.3, 8.3 Hz, 1H, 3'c-H), 2.43(t, J=2.4 Hz, 1H, OCH2C≡≡CH) |
Table 2 1H NMR data for intermediates 3—5 and 8*
| Compd. | 1H NMR(CDCl3), δ |
|---|---|
| 3 | 7.80(d, J=8.3 Hz, 2H, ArH, 7.35(d, J=8.0 Hz, 2H, ArH), 4.17(t, J=4.8 Hz, 2H, CH2OH), 3.75—3.68(m, 4H, OCH2×2), 3.63—3.55(m, 6H, OCH2×3), 2.45(s, 3H, CH3) |
| 4 | 7.79(d, J=8.3 Hz, 2H, ArH), 7.34(dd, J=8.6, 0.6 Hz, 2H, ArH), 5.38(dd, J=3.4, 1.0 Hz, 1H, 4d-H), 5.20(dd, J=10.5, 8.0 Hz, 1H, 2d-H), 5.02(dd, J=10.5, 3.4 Hz, 1H, 3d-H), 4.56(d, J=8.0 Hz, 1H, 1d-H), 4.20—4.09(m, 4H, 6d-H, OCH2), 3.98—3.89(m, 2H, 5d-H, OCH2), 3.73(ddd, J=11.0, 7.0, 3.9 Hz, 1H, OCH2), 3.70—3.55(m, 8H, OCH2×4), 2.45(s, 3H, CH3), 2.15(s, 3H, CH3CO), 2.05(s, 3H, CH3CO), 2.05(s, 3H, CH3CO), 1.99(s, 3H, CH3CO) |
| 5 | 5.39(dd, J=3.4, 1.1 Hz, 1H, 4d-H), 5.22(dd, J=10.5, 8.0 Hz, 1H, 2d-H), 5.02(dd, J=10.5, 3.4 Hz, 1H, 3d-H), 4.58(d, J=8.0 Hz, 1H, 1d-H), 4.22—4.10(m, 2H, 6d-H), 4.00—3.89(m, 2H, 5d-H, OCH2), 3.76(ddd, J=11.0, 6.9, 4.0 Hz, 1H, OCH2), 3.70—3.64(m, 8H, OCH2×4), 3.41(t, J=5.0 Hz, 2H, CH2N3), 2.15(s, 3H, CH3CO), 2.07(s, 3H, CH3CO), 2.05(s, 3H, CH3CO), 1.99(s, 3H, CH3CO) |
| 8 | 7.32—7.21(m, 5H, 5c-H—9c-H), 4.17(d, J=1.9 Hz, 2H, OCH2C≡≡CH), 3.54(dd, J=9.1, 4.1 Hz, 1H, 1c-H), 3.38(dd, J=8.9, 7.1 Hz, 1H, 1'c-H), 3.29—3.25(m, 1H, 2c-H), 2.81(dd, J=13.5, 5.4 Hz, 1H, 3c-H), 2.58(dd, J=13.3, 8.3 Hz, 1H, 3'c-H), 2.43(t, J=2.4 Hz, 1H, OCH2C≡≡CH) |
| Compd. | 1H NMR(400 MHz), δa | 13C NMR, δb |
|---|---|---|
| 12a | 7.75—7.69(m, 2H, 3b-H, 7b-H), 7.52(t, J=7.4 Hz, 1H, 5b-H), 7.43(t, J=7.5 Hz, 2H, 4b-H, 6b-H), 7.23—7.04(m, 7H, 5a-H, 9a-H, 5c-H—9c-H), 6.69(d, J=8.4 Hz, 2H, 6a-H, 8a-H), 4.72(t, J=7.5 Hz, 1H, 2a-H), 4.20—4.13(m, 1H, 2c-H), 4.08(dd, J=8.2, 2.4 Hz, 2H, OCH2C≡≡CH), 3.39(dd, J=9.4, 4.7 Hz, 1H, OCH2C≡≡CH), 3.34—3.27(m, 2H, 1c-H), 3.03(dd, J=13.7, 7.1 Hz, 1H, 3a-H), 2.95—2.82(m, 2H, 3'a-H, 3c-H), 2.74(dd, J=13.6, 7.7 Hz, 1H, 3'c-H) | 172.8(C1a), 169.6(C1b), 157.0(C7a), 139.1(C4c), 135.0(C2b), 132.7(C5b), 131.3(2C), 130.3(2C), 129.4(2C), 129.2(2C), 128.8(C4a), 128.2(C3b, C7b), 127.2(C7c), 116.1(C6a, C8a), 80.3(OCH2C≡≡CH), 76.0(OCH2C≡≡CH), 71.0(C1c), 59.0(OCH2·C≡≡CH), 56.6(C2a), 51.6(C2c), 38.2(C3a), 38.0(C3c) |
| 12b | 9.19(s, 1H, OH), 8.80(d, J=8.5 Hz, 1H, NHCO), 8.29(d, J=8.9 Hz, 2H, 4b-H, 6b-H), 8.09(d, J=8.4 Hz, 1H, NHCO), 8.00(d, J=9.0 Hz, 2H, 3b-H, 7b-H), 7.23—7.11(m, 5H, 5c-H—9c-H), 7.08(d, J=8.5 Hz, 2H, 5a-H, 9a-H), 6.62(d, J=8.5 Hz, 2H, 6a-H, 8a-H), 4.67—4.56(m, 1H, 2a-H), 4.13(d, J=2.4 Hz, 2H, OCH2C≡≡CH), 4.11—4.01(m, 1H, 2c-H), 3.49—3.31(m, 3H, OCH2C≡≡CH, 1c-H), 2.91(dd, J=13.8, 4.6 Hz, 1H, 3a-H), 2.86—2.77(m, 2H, 3'a-H, 3c-H), 2.70(dd, J=13.7, 8.2 Hz, 1H, 3'a-H) | 170.8(C1a), 164.4(C1b), 155.7(C7a), 149.0(C5b), 139.7(C4c), 138.4(C2b), 130.1(2C), 129.2(2C), 129.0(2C), 128.2, 128.1(2C), 126.1(C7c), 123.4(C4b, C6b), 114.9(C6a, C8a), 80.2(OCH2C≡≡CH), 77.4(OCH2C≡≡CH), 70.3(C1c), 57.7(OCH2C≡≡CH), 55.3(C2a), 50.0(C2c), 36.6(C3a, C3c) |
| 12c | 9.19(s, 1H, OH), 8.13—8.02(m, 2H, NHCO×2), 7.81(dd, J=1.7, 0.7 Hz, 1H, 5b-H), 7.23—7.10(m, 6H, 3b-H, 5c-H—9c-H), 7.02(d, J=8.5 Hz, 2H, 5a-H, 9a-H), 6.63—6.57(m, 3H, 4b-H, 6a-H, 8a-H), 4.59—4.49(m, 1H, 2a-H), 4.13(d, J=2.4 Hz, 2H, OCH2C≡≡CH), 4.08—3.99(m, 1H, 2c-H), 3.48—3.33(m, 3H, OCH2C≡≡CH, 1c-H), 2.89—2.76(m, 3H, 3a-H, 3c-H), 2.69(dd, J=13.7, 8.1 Hz, 1H, 3'c-H) | 170.8(C1a), 157.3, 155.8(C1b, C7a), 147.5(C2b), 145.1(C5b), 138.4(C4c), 130.1(2C), 129.2(2C), 128.1(2C), 128.0, 126.1(C7c), 114.9(C6a, C8a), 113.7, 111.9(C3b, C4b), 80.2(OCH2C≡≡CH), 77.4(OCH2·C≡≡CH), 70.3(C1c), 57.7(OCH2C≡≡CH), 54.2(C2a), 50.0(C2c), 36.7(C3a, C3c) |
| 13a | 8.42(d, J=8.5 Hz, 1H, NHCO), 8.04(d, J=8.4 Hz, 1H, NHCO), 7.80—7.75(m, 2H, 3b-H, 7b-H), 7.54—7.40(m, 3H, 4b-H—6b-H), 7.27—7.07(m, 7H, 5a-H, 9a-H, 5c-H—9c-H), 6.81(d, J=8.7 Hz, 2H, 6a-H, 8a-H), 6.04—5.94(m, 1H, OCH2CH | 171.3(C1a), 166.5(C1b), 156.9(C7a), 138.6(C4c), 134.2(C2b), 134.0(OCH2·CH |
| 13b | 8.41(d, J=8.5 Hz, 1H, NHCO), 8.04(d, J=8.4 Hz, 1H, NHCO), 7.81—7.74(m, 2H, H-3b, 7b-H), 7.54—7.41(m, 3H, 4b-H—6b-H), 7.31—7.02(m, 7H, H-5a, 9a-H, 5c-H—9c-H), 6.79(d, J=8.7 Hz, 2H, 6a-H, 8a-H), 4.71—4.54(m, 1H, 2a-H), 4.13(d, J=2.3 Hz, 2H, OCH2C≡≡CH), 4.09—4.01(m, 1H, 2c-H), 3.94(t, J=6.4 Hz, 2H, OCH2CH2CH2OH), 3.70—3.24(m, 5H, OCH2CH2CH2OH, OCH2C≡≡CH, 1c-H), 2.97—2.76(m, 3H, 3a-H, 3c-H), 2.74—2.66(m, 1H, 3'c-H), 1.86—1.75(m, 2H, OCH2CH2CH2OH) | 171.2(C1a), 166.3(C1b), 157.3(C7a), 138.5(C4c), 134.2(C2b), 131.4(C5b), 130.3(2C), 130.1(C4a), 129.3(2C), 128.3(2C), 128.3(2C), 127.5(C3b, C7b), 126.2(C7c), 114.1(C6a, C8a), 80.3(OCH2C≡≡CH), 77.4(OCH2C≡≡CH), 70.5(C1c), 64.5(OCH2·CH2CH2OH), 58.6(OCH2CH2CH2OH), 57.8(OCH2C≡≡ CH), 55.2(C2a), 50.1(C2c), 36.7(C3a), 36.6(C3c), 32.2(OCH2CH2CH2OH) |
| 13c | 8.83(d, J=8.2 Hz, 1H, NHCO), 8.29(d, J=8.4 Hz, 2H, 4b-H, 6b-H), 8.12(d, J=8.0 Hz, 1H, NHCO), 8.00(d, J=8.2 Hz, 2H, 3b-H, 7b-H), 7.29—7.09(m, 7H, 5a-H, 9a-H, 5c-H—9c-H), 6.79(d, J=8.0 Hz, 2H, 6a-H, 8a-H), 4.70—4.59(m, 1H, 2a-H), 4.17—4.02(m, 3H, OCH2C≡≡CH, 2c-H), 3.83(t, J=5.8 Hz, 2H, OCH2CH2CH3), 3.61—3.24(m, 3H, OCH2C≡≡CH, 1c-H), 3.03—2.76(m, 3H, 3a-H, 3c-H), 2.76—2.66(m, 1H, 3'c-H), 1.76—1.59(m, 2H, OCH2CH2CH3), 0.93(t, J=7.1 Hz, 3H, OCH2CH2CH3) | 170.9(C1a), 164.6(C1b), 157.3(C7a), 149.1(C5b), 139.8(C4c), 138.5(C2b), 130.3(2C), 130.0(C4a), 129.3(2C), 129.0(C3b, C7b), 128.3(2C), 126.2(C7c), 123.6(C4b, C6b), 114.1(C6a, C8a), 80.3(OCH2·C≡≡CH), 77.4(OCH2C≡≡CH), 70.4(C1c), 69.0(OCH2CH2CH3), 57.8(OCH2C≡≡CH), 55.4(C2a), 50.1(C2c), 36.7(C3a), 36.7(C3c), 22.2(OCH2CH2CH3), 10.6(OCH2CH2CH3) |
| 13d | 8.83(d, J=8.5 Hz, 1H, NHCO), 8.29(d, J=8.9 Hz, 2H, 4b-H, 6b-H), 8.12(d, J=8.4 Hz, 1H, NHCO), 8.00(d, J=8.9 Hz, 2H, H-3b, 7b-H), 7.25—7.10(m, 7H, 5a-H, 9a-H, 5c-H—9c-H), | 170.8(C1a), 164.6(C1b), 157.3(C7a), 149.1(C5b), 140.0(C4c), 138.5(C2b), 130.3(2C), 129.8(C4a), 129.3(2C), 129.0(2C), |
| 13d | 6.79(d, J=8.7 Hz, 2H, 6a-H, 8a-H), 4.68—4.60(m, 1H, 2a-H), 4.13(d, J=2.4 Hz, 2H, OCH2C≡≡CH), 4.10—4.03(m, 1H, 2c-H), 3.87(t, J=6.5 Hz, 2H, OCH2CH2CH2CH3), 3.55—3.27(m, 3H, OCH2C≡≡CH, 1c-H), 3.00—2.79(m, 3H, 3a-H, 3c-H), 2.75—2.67(m, 1H, 3'c-H), 1.68—1.58(m, 2H, OCH2CH2·CH2CH3), 1.45—1.32(m, 2H, OCH2CH2CH2CH3), 0.89(t, J=7.4 Hz, 3H, OCH2CH2CH2CH3) | 128.2(2C), 126.2(C7c), 123.5(C4b, C6b), 114.1(C6a, C8a), 80.3(OCH2C≡≡CH), 77.3(OCH2C≡≡CH), 70.4(C1c), 67.0(OCH2·CH2CH2CH3), 57.8(OCH2C≡≡CH), 55.3(C2a), 50.0(C2c), 36.7(C3a), 36.6(C3c), 30.9(OCH2CH2CH2CH3), 18.8(OCH2CH2·CH2CH3), 13.8(OCH2CH2CH2CH3) |
| 13e | 8.14(d, J=8.6 Hz, 1H, NHCO), 8.09(d, J=8.4 Hz, 1H, NHCO), 7.80(dd, J=1.7, 0.8 Hz, 1H, 5b-H), 7.22—7.08(m, 8H, 3b-H, 5a-H, 9a-H, 5c-H—9c-H), 6.76(d, J=8.7 Hz, 2H, 6a-H, 8a-H), 6.60(dd, J=3.5, 1.8 Hz, 1H, 4b-H), 4.60—4.52(m, 1H, 2a-H), 4.12(d, J=2.3 Hz, 2H, OCH2C≡≡CH), 4.09—4.00(m, 1H, 2c-H), 3.86(t, J=6.5 Hz, 2H, OCH2CH2CH2CH2CH3), 3.59—3.31(m, 3H, OCH2C≡≡CH, 1c-H), 2.92—2.76(m, 3H, 3a-H, 3c-H), 2.73—2.64(m, 1H, 3'c-H), 1.70—1.61(m, 2H, OCH2·CH2CH2CH2CH3), 1.41—1.25(m, 4H, OCH2CH2CH2CH2CH3), 0.86(t, J=7.1 Hz, 3H, OCH2CH2CH2CH2CH3) | 170.9(C1a), 157.5, 157.4(C1b, C7a), 147.5(C2b), 145.3(C5b), 138.5(C4c), 130.3(2C), 129.7(C4a), 129.4(2C), 128.3(2C), 126.3(C7c), 114.1(C6a, C8a), 114.0, 112.1(C3b, C4b), 80.3(OCH2C≡≡CH), 77.5(OCH2C≡≡CH), 70.5(C1c), 67.4(OCH2·CH2CH2CH2CH3), 57.9(OCH2C≡≡CH), 54.3(C2a), 50.1(C2c), 36.8(C3a), 36.7(C3c), 28.6(OCH2CH2CH2CH2CH3), 27.9(OCH2·CH2CH2CH2CH3), 22.1(OCH2CH2CH2CH2·CH3), 14.1(OCH2CH2CH2CH2CH3) |
| 13f | 8.14(d, J=8.5 Hz, 1H, NHCO), 8.09(d, J=8.4 Hz, 1H, NHCO), 7.81(dd, J=1.7, 0.8 Hz, 1H, 5b-H), 7.29—7.05(m, 8H, 3b-H, 5a-H, 9a-H, 5c-H—9c-H), 6.76(d, J=8.7 Hz, 2H, 6a-H, 8a-H), 6.60(dd, J=3.5, 1.8 Hz, 1H, 4b-H), 4.61—4.52(m, 1H, 2a-H), 4.13(d, J=2.3 Hz, 2H, OCH2C≡≡CH), 4.09—3.99(m, 1H, 2c-H), 3.86(t, J=6.5 Hz, 2H, OCH2CH2CH2CH2CH2CH3), 3.61—3.27(m, 3H, OCH2C≡≡CH, 1c-H), 2.94—2.76(m, 3H, 3a-H, 3c-H), 2.74—2.63(m, 1H, 3'c-H), 1.71—1.58(m, 2H, OCH2CH2CH2CH2CH2CH3), 1.42—1.32(m, 2H, OCH2CH2CH2·CH2CH2CH3), 1.32—1.21(m, 4H, OCH2CH2CH2CH2CH2CH3), 0.85(t, J=7.0 Hz, 3H, OCH2CH2CH2CH2CH2CH3) | 170.8(C1a), 157.5, 157.3(C1b, C7a), 147.5(C2b), 145.3(C5b), 138.5(C4c), 130.3(2C), 129.6(C4a), 129.3(2C), 128.3(2C), 126.2(C7c), 114.1(C6a, C8a), 113.9, 112.0,(C3b, C4b), 80.3(OCH2C≡≡CH), 77.4(OCH2C≡≡CH), 70.4(C1c), 67.4(OCH2·CH2CH2CH2CH2CH3), 57.8(OCH2C≡≡CH), 54.2(C2a), 50.1(C2c), 36.7(C3a), 36.7(C3c), 31.1(OCH2CH2CH2CH2CH2CH3),28.8(OCH2CH2CH2CH2CH2CH3), 25.4(OCH2·CH2CH2CH2CH2CH3), 22.2(OCH2CH2CH2·CH2CH2CH3), 14.1(OCH2CH2CH2CH2·CH2CH3) |
| 14a | 7.72(d, J=7.7 Hz, 2H, 3b-H, 7b-H), 7.69(s, 1H, OCH2C | 170.6, 170.4, 170.3, 170.2, 170.0(C1a, CH3CO×4), 167.0(C1b), 157.7(C7a), 144.6(OCH2C |
| Compd. | 1H NMR(400 MHz), δa | 13C NMR, δb |
| 14b | 7.77—7.74(m, 2H, 3b-H, 7b-H), 7.65(s, 1H, OCH2C | 170.6, 170.4, 170.3, 170.1, 169.6(C1a, CH3CO×4), 166.9(C1b), 158.0(C7a), 144.7(OCH2C |
| 14c | 8.27(d, J=8.8 Hz, 2H, 4b-H, 6b-H), 7.90(d, J=8.9 Hz, 2H, 3b-H, 7b-H), 7.69(s, 1H, OCH2C | 170.6, 170.4, 170.3, 169.9, 169.6(C1a, CH3CO×4), 164.9(C1b), 158.4(C7a), 149.8(C5b), 144.5(OCH2C |
| 14d | 8.27(d, J=8.9 Hz, 2H, 4b-H, 6b-H), 7.90(d, J=8.9 Hz, 2H, 3b-H, 7b-H), 7.69(s, 1H, OCH2C | 170.6, 170.4, 170.3, 169.9, 169.6(C1a, CH3CO×4), 164.9(C1b), 158.4(C7a), 149.8(C5b), 144.5(OCH2C |
| 14e | 7.68(s, 1H, OCH2C | 170.4, 170.2, 170.2, 169.8, 169.4(C1a, CH3CO×4), 158.0, 157.8(C1b, C7a), 147.3(C2b), 144.4, 144.2(C5b, OCH2C |
| 14f | 7.67(s, 1H, OCH2C | 170.5, 170.4, 170.3, 170.0, 169.6(C1a, CH3CO×4), 158.2, 158.0(C1b, C7a), 147.5(C2b), 144.6, 144.4(C5b, OCH2C |
Table 3 1H NMR and 13C NMR data for intermediates 12a—12c, 13a—13f and 14a—14f
| Compd. | 1H NMR(400 MHz), δa | 13C NMR, δb |
|---|---|---|
| 12a | 7.75—7.69(m, 2H, 3b-H, 7b-H), 7.52(t, J=7.4 Hz, 1H, 5b-H), 7.43(t, J=7.5 Hz, 2H, 4b-H, 6b-H), 7.23—7.04(m, 7H, 5a-H, 9a-H, 5c-H—9c-H), 6.69(d, J=8.4 Hz, 2H, 6a-H, 8a-H), 4.72(t, J=7.5 Hz, 1H, 2a-H), 4.20—4.13(m, 1H, 2c-H), 4.08(dd, J=8.2, 2.4 Hz, 2H, OCH2C≡≡CH), 3.39(dd, J=9.4, 4.7 Hz, 1H, OCH2C≡≡CH), 3.34—3.27(m, 2H, 1c-H), 3.03(dd, J=13.7, 7.1 Hz, 1H, 3a-H), 2.95—2.82(m, 2H, 3'a-H, 3c-H), 2.74(dd, J=13.6, 7.7 Hz, 1H, 3'c-H) | 172.8(C1a), 169.6(C1b), 157.0(C7a), 139.1(C4c), 135.0(C2b), 132.7(C5b), 131.3(2C), 130.3(2C), 129.4(2C), 129.2(2C), 128.8(C4a), 128.2(C3b, C7b), 127.2(C7c), 116.1(C6a, C8a), 80.3(OCH2C≡≡CH), 76.0(OCH2C≡≡CH), 71.0(C1c), 59.0(OCH2·C≡≡CH), 56.6(C2a), 51.6(C2c), 38.2(C3a), 38.0(C3c) |
| 12b | 9.19(s, 1H, OH), 8.80(d, J=8.5 Hz, 1H, NHCO), 8.29(d, J=8.9 Hz, 2H, 4b-H, 6b-H), 8.09(d, J=8.4 Hz, 1H, NHCO), 8.00(d, J=9.0 Hz, 2H, 3b-H, 7b-H), 7.23—7.11(m, 5H, 5c-H—9c-H), 7.08(d, J=8.5 Hz, 2H, 5a-H, 9a-H), 6.62(d, J=8.5 Hz, 2H, 6a-H, 8a-H), 4.67—4.56(m, 1H, 2a-H), 4.13(d, J=2.4 Hz, 2H, OCH2C≡≡CH), 4.11—4.01(m, 1H, 2c-H), 3.49—3.31(m, 3H, OCH2C≡≡CH, 1c-H), 2.91(dd, J=13.8, 4.6 Hz, 1H, 3a-H), 2.86—2.77(m, 2H, 3'a-H, 3c-H), 2.70(dd, J=13.7, 8.2 Hz, 1H, 3'a-H) | 170.8(C1a), 164.4(C1b), 155.7(C7a), 149.0(C5b), 139.7(C4c), 138.4(C2b), 130.1(2C), 129.2(2C), 129.0(2C), 128.2, 128.1(2C), 126.1(C7c), 123.4(C4b, C6b), 114.9(C6a, C8a), 80.2(OCH2C≡≡CH), 77.4(OCH2C≡≡CH), 70.3(C1c), 57.7(OCH2C≡≡CH), 55.3(C2a), 50.0(C2c), 36.6(C3a, C3c) |
| 12c | 9.19(s, 1H, OH), 8.13—8.02(m, 2H, NHCO×2), 7.81(dd, J=1.7, 0.7 Hz, 1H, 5b-H), 7.23—7.10(m, 6H, 3b-H, 5c-H—9c-H), 7.02(d, J=8.5 Hz, 2H, 5a-H, 9a-H), 6.63—6.57(m, 3H, 4b-H, 6a-H, 8a-H), 4.59—4.49(m, 1H, 2a-H), 4.13(d, J=2.4 Hz, 2H, OCH2C≡≡CH), 4.08—3.99(m, 1H, 2c-H), 3.48—3.33(m, 3H, OCH2C≡≡CH, 1c-H), 2.89—2.76(m, 3H, 3a-H, 3c-H), 2.69(dd, J=13.7, 8.1 Hz, 1H, 3'c-H) | 170.8(C1a), 157.3, 155.8(C1b, C7a), 147.5(C2b), 145.1(C5b), 138.4(C4c), 130.1(2C), 129.2(2C), 128.1(2C), 128.0, 126.1(C7c), 114.9(C6a, C8a), 113.7, 111.9(C3b, C4b), 80.2(OCH2C≡≡CH), 77.4(OCH2·C≡≡CH), 70.3(C1c), 57.7(OCH2C≡≡CH), 54.2(C2a), 50.0(C2c), 36.7(C3a, C3c) |
| 13a | 8.42(d, J=8.5 Hz, 1H, NHCO), 8.04(d, J=8.4 Hz, 1H, NHCO), 7.80—7.75(m, 2H, 3b-H, 7b-H), 7.54—7.40(m, 3H, 4b-H—6b-H), 7.27—7.07(m, 7H, 5a-H, 9a-H, 5c-H—9c-H), 6.81(d, J=8.7 Hz, 2H, 6a-H, 8a-H), 6.04—5.94(m, 1H, OCH2CH | 171.3(C1a), 166.5(C1b), 156.9(C7a), 138.6(C4c), 134.2(C2b), 134.0(OCH2·CH |
| 13b | 8.41(d, J=8.5 Hz, 1H, NHCO), 8.04(d, J=8.4 Hz, 1H, NHCO), 7.81—7.74(m, 2H, H-3b, 7b-H), 7.54—7.41(m, 3H, 4b-H—6b-H), 7.31—7.02(m, 7H, H-5a, 9a-H, 5c-H—9c-H), 6.79(d, J=8.7 Hz, 2H, 6a-H, 8a-H), 4.71—4.54(m, 1H, 2a-H), 4.13(d, J=2.3 Hz, 2H, OCH2C≡≡CH), 4.09—4.01(m, 1H, 2c-H), 3.94(t, J=6.4 Hz, 2H, OCH2CH2CH2OH), 3.70—3.24(m, 5H, OCH2CH2CH2OH, OCH2C≡≡CH, 1c-H), 2.97—2.76(m, 3H, 3a-H, 3c-H), 2.74—2.66(m, 1H, 3'c-H), 1.86—1.75(m, 2H, OCH2CH2CH2OH) | 171.2(C1a), 166.3(C1b), 157.3(C7a), 138.5(C4c), 134.2(C2b), 131.4(C5b), 130.3(2C), 130.1(C4a), 129.3(2C), 128.3(2C), 128.3(2C), 127.5(C3b, C7b), 126.2(C7c), 114.1(C6a, C8a), 80.3(OCH2C≡≡CH), 77.4(OCH2C≡≡CH), 70.5(C1c), 64.5(OCH2·CH2CH2OH), 58.6(OCH2CH2CH2OH), 57.8(OCH2C≡≡ CH), 55.2(C2a), 50.1(C2c), 36.7(C3a), 36.6(C3c), 32.2(OCH2CH2CH2OH) |
| 13c | 8.83(d, J=8.2 Hz, 1H, NHCO), 8.29(d, J=8.4 Hz, 2H, 4b-H, 6b-H), 8.12(d, J=8.0 Hz, 1H, NHCO), 8.00(d, J=8.2 Hz, 2H, 3b-H, 7b-H), 7.29—7.09(m, 7H, 5a-H, 9a-H, 5c-H—9c-H), 6.79(d, J=8.0 Hz, 2H, 6a-H, 8a-H), 4.70—4.59(m, 1H, 2a-H), 4.17—4.02(m, 3H, OCH2C≡≡CH, 2c-H), 3.83(t, J=5.8 Hz, 2H, OCH2CH2CH3), 3.61—3.24(m, 3H, OCH2C≡≡CH, 1c-H), 3.03—2.76(m, 3H, 3a-H, 3c-H), 2.76—2.66(m, 1H, 3'c-H), 1.76—1.59(m, 2H, OCH2CH2CH3), 0.93(t, J=7.1 Hz, 3H, OCH2CH2CH3) | 170.9(C1a), 164.6(C1b), 157.3(C7a), 149.1(C5b), 139.8(C4c), 138.5(C2b), 130.3(2C), 130.0(C4a), 129.3(2C), 129.0(C3b, C7b), 128.3(2C), 126.2(C7c), 123.6(C4b, C6b), 114.1(C6a, C8a), 80.3(OCH2·C≡≡CH), 77.4(OCH2C≡≡CH), 70.4(C1c), 69.0(OCH2CH2CH3), 57.8(OCH2C≡≡CH), 55.4(C2a), 50.1(C2c), 36.7(C3a), 36.7(C3c), 22.2(OCH2CH2CH3), 10.6(OCH2CH2CH3) |
| 13d | 8.83(d, J=8.5 Hz, 1H, NHCO), 8.29(d, J=8.9 Hz, 2H, 4b-H, 6b-H), 8.12(d, J=8.4 Hz, 1H, NHCO), 8.00(d, J=8.9 Hz, 2H, H-3b, 7b-H), 7.25—7.10(m, 7H, 5a-H, 9a-H, 5c-H—9c-H), | 170.8(C1a), 164.6(C1b), 157.3(C7a), 149.1(C5b), 140.0(C4c), 138.5(C2b), 130.3(2C), 129.8(C4a), 129.3(2C), 129.0(2C), |
| 13d | 6.79(d, J=8.7 Hz, 2H, 6a-H, 8a-H), 4.68—4.60(m, 1H, 2a-H), 4.13(d, J=2.4 Hz, 2H, OCH2C≡≡CH), 4.10—4.03(m, 1H, 2c-H), 3.87(t, J=6.5 Hz, 2H, OCH2CH2CH2CH3), 3.55—3.27(m, 3H, OCH2C≡≡CH, 1c-H), 3.00—2.79(m, 3H, 3a-H, 3c-H), 2.75—2.67(m, 1H, 3'c-H), 1.68—1.58(m, 2H, OCH2CH2·CH2CH3), 1.45—1.32(m, 2H, OCH2CH2CH2CH3), 0.89(t, J=7.4 Hz, 3H, OCH2CH2CH2CH3) | 128.2(2C), 126.2(C7c), 123.5(C4b, C6b), 114.1(C6a, C8a), 80.3(OCH2C≡≡CH), 77.3(OCH2C≡≡CH), 70.4(C1c), 67.0(OCH2·CH2CH2CH3), 57.8(OCH2C≡≡CH), 55.3(C2a), 50.0(C2c), 36.7(C3a), 36.6(C3c), 30.9(OCH2CH2CH2CH3), 18.8(OCH2CH2·CH2CH3), 13.8(OCH2CH2CH2CH3) |
| 13e | 8.14(d, J=8.6 Hz, 1H, NHCO), 8.09(d, J=8.4 Hz, 1H, NHCO), 7.80(dd, J=1.7, 0.8 Hz, 1H, 5b-H), 7.22—7.08(m, 8H, 3b-H, 5a-H, 9a-H, 5c-H—9c-H), 6.76(d, J=8.7 Hz, 2H, 6a-H, 8a-H), 6.60(dd, J=3.5, 1.8 Hz, 1H, 4b-H), 4.60—4.52(m, 1H, 2a-H), 4.12(d, J=2.3 Hz, 2H, OCH2C≡≡CH), 4.09—4.00(m, 1H, 2c-H), 3.86(t, J=6.5 Hz, 2H, OCH2CH2CH2CH2CH3), 3.59—3.31(m, 3H, OCH2C≡≡CH, 1c-H), 2.92—2.76(m, 3H, 3a-H, 3c-H), 2.73—2.64(m, 1H, 3'c-H), 1.70—1.61(m, 2H, OCH2·CH2CH2CH2CH3), 1.41—1.25(m, 4H, OCH2CH2CH2CH2CH3), 0.86(t, J=7.1 Hz, 3H, OCH2CH2CH2CH2CH3) | 170.9(C1a), 157.5, 157.4(C1b, C7a), 147.5(C2b), 145.3(C5b), 138.5(C4c), 130.3(2C), 129.7(C4a), 129.4(2C), 128.3(2C), 126.3(C7c), 114.1(C6a, C8a), 114.0, 112.1(C3b, C4b), 80.3(OCH2C≡≡CH), 77.5(OCH2C≡≡CH), 70.5(C1c), 67.4(OCH2·CH2CH2CH2CH3), 57.9(OCH2C≡≡CH), 54.3(C2a), 50.1(C2c), 36.8(C3a), 36.7(C3c), 28.6(OCH2CH2CH2CH2CH3), 27.9(OCH2·CH2CH2CH2CH3), 22.1(OCH2CH2CH2CH2·CH3), 14.1(OCH2CH2CH2CH2CH3) |
| 13f | 8.14(d, J=8.5 Hz, 1H, NHCO), 8.09(d, J=8.4 Hz, 1H, NHCO), 7.81(dd, J=1.7, 0.8 Hz, 1H, 5b-H), 7.29—7.05(m, 8H, 3b-H, 5a-H, 9a-H, 5c-H—9c-H), 6.76(d, J=8.7 Hz, 2H, 6a-H, 8a-H), 6.60(dd, J=3.5, 1.8 Hz, 1H, 4b-H), 4.61—4.52(m, 1H, 2a-H), 4.13(d, J=2.3 Hz, 2H, OCH2C≡≡CH), 4.09—3.99(m, 1H, 2c-H), 3.86(t, J=6.5 Hz, 2H, OCH2CH2CH2CH2CH2CH3), 3.61—3.27(m, 3H, OCH2C≡≡CH, 1c-H), 2.94—2.76(m, 3H, 3a-H, 3c-H), 2.74—2.63(m, 1H, 3'c-H), 1.71—1.58(m, 2H, OCH2CH2CH2CH2CH2CH3), 1.42—1.32(m, 2H, OCH2CH2CH2·CH2CH2CH3), 1.32—1.21(m, 4H, OCH2CH2CH2CH2CH2CH3), 0.85(t, J=7.0 Hz, 3H, OCH2CH2CH2CH2CH2CH3) | 170.8(C1a), 157.5, 157.3(C1b, C7a), 147.5(C2b), 145.3(C5b), 138.5(C4c), 130.3(2C), 129.6(C4a), 129.3(2C), 128.3(2C), 126.2(C7c), 114.1(C6a, C8a), 113.9, 112.0,(C3b, C4b), 80.3(OCH2C≡≡CH), 77.4(OCH2C≡≡CH), 70.4(C1c), 67.4(OCH2·CH2CH2CH2CH2CH3), 57.8(OCH2C≡≡CH), 54.2(C2a), 50.1(C2c), 36.7(C3a), 36.7(C3c), 31.1(OCH2CH2CH2CH2CH2CH3),28.8(OCH2CH2CH2CH2CH2CH3), 25.4(OCH2·CH2CH2CH2CH2CH3), 22.2(OCH2CH2CH2·CH2CH2CH3), 14.1(OCH2CH2CH2CH2·CH2CH3) |
| 14a | 7.72(d, J=7.7 Hz, 2H, 3b-H, 7b-H), 7.69(s, 1H, OCH2C | 170.6, 170.4, 170.3, 170.2, 170.0(C1a, CH3CO×4), 167.0(C1b), 157.7(C7a), 144.6(OCH2C |
| Compd. | 1H NMR(400 MHz), δa | 13C NMR, δb |
| 14b | 7.77—7.74(m, 2H, 3b-H, 7b-H), 7.65(s, 1H, OCH2C | 170.6, 170.4, 170.3, 170.1, 169.6(C1a, CH3CO×4), 166.9(C1b), 158.0(C7a), 144.7(OCH2C |
| 14c | 8.27(d, J=8.8 Hz, 2H, 4b-H, 6b-H), 7.90(d, J=8.9 Hz, 2H, 3b-H, 7b-H), 7.69(s, 1H, OCH2C | 170.6, 170.4, 170.3, 169.9, 169.6(C1a, CH3CO×4), 164.9(C1b), 158.4(C7a), 149.8(C5b), 144.5(OCH2C |
| 14d | 8.27(d, J=8.9 Hz, 2H, 4b-H, 6b-H), 7.90(d, J=8.9 Hz, 2H, 3b-H, 7b-H), 7.69(s, 1H, OCH2C | 170.6, 170.4, 170.3, 169.9, 169.6(C1a, CH3CO×4), 164.9(C1b), 158.4(C7a), 149.8(C5b), 144.5(OCH2C |
| 14e | 7.68(s, 1H, OCH2C | 170.4, 170.2, 170.2, 169.8, 169.4(C1a, CH3CO×4), 158.0, 157.8(C1b, C7a), 147.3(C2b), 144.4, 144.2(C5b, OCH2C |
| 14f | 7.67(s, 1H, OCH2C | 170.5, 170.4, 170.3, 170.0, 169.6(C1a, CH3CO×4), 158.2, 158.0(C1b, C7a), 147.5(C2b), 144.6, 144.4(C5b, OCH2C |
| Compd. | Appearance | Yield(%) | [α | HRMS(calcd.), m/z |
|---|---|---|---|---|
| 15a | Pale oil | 92 | -26.1 | 856.3751(856.3745) [M+Na]+ |
| 15b | Pale oil | 95 | -24.0 | 874.3842(874.3851) [M+Na]+ |
| 15c | Pale oil | 90 | -16.0 | 903.3759(903.3752) [M+Na]+ |
| 15d | Pale oil | 95 | -29.9 | 917.3917(917.3909) [M+Na]+ |
| 15e | Pale oil | 86 | -32.0 | 854.4193(854.4188) [M+H]+ |
| 15f | Pale oil | 85 | -40.0 | 890.4172(890.4164) [M+Na]+ |
Table 4 Appearance, yields, specific rotation and HRMS data for target compounds 15a—15f
| Compd. | Appearance | Yield(%) | [α | HRMS(calcd.), m/z |
|---|---|---|---|---|
| 15a | Pale oil | 92 | -26.1 | 856.3751(856.3745) [M+Na]+ |
| 15b | Pale oil | 95 | -24.0 | 874.3842(874.3851) [M+Na]+ |
| 15c | Pale oil | 90 | -16.0 | 903.3759(903.3752) [M+Na]+ |
| 15d | Pale oil | 95 | -29.9 | 917.3917(917.3909) [M+Na]+ |
| 15e | Pale oil | 86 | -32.0 | 854.4193(854.4188) [M+H]+ |
| 15f | Pale oil | 85 | -40.0 | 890.4172(890.4164) [M+Na]+ |
| Compd. | 1H NMR(400 MHz), δ | 13C NMR(101 MHz), δ |
|---|---|---|
| 15a | 7.97(s, 1H, OCH2C | 173.1(C1a), 169.8(C1b), 158.9(C7a), 145.6(OCH2C |
| 15b | 7.98(s, 1H, OCH2C | 173.1(C1a), 169.8(C1b), 159.4(C7a), 145.6(OCH2C |
| 15c | 8.81(d, J=8.5 Hz, 1H, NHCO), 8.28(d, J=8.9 Hz, 2H, 4b-H, 6b-H), 8.11(d, J=8.4 Hz, 1H, NHCO), 8.04(s, 1H, OCH2·C | 170.9(C1a), 164.7(C1b), 157.3(C7a), 149.2(C5b), 143.8(OCH2C |
| 15d | 8.80(d, J=8.5 Hz, 1H, NHCO), 8.28(d, J=8.9 Hz, 2H, 4b-H, 6b-H), 8.09(d, J=8.4 Hz, 1H, NHCO), 8.04(s, 1H, OCH2·C | 170.9(C1a), 164.6(C1b), 157.3(C7a), 149.1(C5b), 143.8(OCH2C |
| 15e | 7.99(s, 1H, OCH2C | 172.7(C1a), 160.1(C1b), 159.4(C7a), 148.5(C2b), 146.5(C5b), 145.5(OCH2C |
| 15f | 7.99(s, 1H, OCH2C | 172.7(C1a), 160.1(C1b), 159.4(C7a), 148.5(C2b), 146.5(C5b), 145.5(OCH2C |
Table 5 1H NMR and 13C NMR data for target compounds 15a—15f*
| Compd. | 1H NMR(400 MHz), δ | 13C NMR(101 MHz), δ |
|---|---|---|
| 15a | 7.97(s, 1H, OCH2C | 173.1(C1a), 169.8(C1b), 158.9(C7a), 145.6(OCH2C |
| 15b | 7.98(s, 1H, OCH2C | 173.1(C1a), 169.8(C1b), 159.4(C7a), 145.6(OCH2C |
| 15c | 8.81(d, J=8.5 Hz, 1H, NHCO), 8.28(d, J=8.9 Hz, 2H, 4b-H, 6b-H), 8.11(d, J=8.4 Hz, 1H, NHCO), 8.04(s, 1H, OCH2·C | 170.9(C1a), 164.7(C1b), 157.3(C7a), 149.2(C5b), 143.8(OCH2C |
| 15d | 8.80(d, J=8.5 Hz, 1H, NHCO), 8.28(d, J=8.9 Hz, 2H, 4b-H, 6b-H), 8.09(d, J=8.4 Hz, 1H, NHCO), 8.04(s, 1H, OCH2·C | 170.9(C1a), 164.6(C1b), 157.3(C7a), 149.1(C5b), 143.8(OCH2C |
| 15e | 7.99(s, 1H, OCH2C | 172.7(C1a), 160.1(C1b), 159.4(C7a), 148.5(C2b), 146.5(C5b), 145.5(OCH2C |
| 15f | 7.99(s, 1H, OCH2C | 172.7(C1a), 160.1(C1b), 159.4(C7a), 148.5(C2b), 146.5(C5b), 145.5(OCH2C |
| [1] | Lozano R., Naghavi M., Foreman K., Lim S., Shibuya K. J., Aboyans V., Lancet,2012, 380(9859), 2095—2128 |
| [2] | Goulis I., Karatapanis S., Akriviadis E., Deutsch M., Dalekos G. N., Raptopoulou-Gigi M., Mimidis K., Germanidis G., Drakoulis C., Triantos C., Zintzaras E., Bakalos G., Papatheodoridis G., Liver Int., 2015, 35(5), 1540—1548 |
| [3] | Liang G. P., Hu Z. X., Liu Q. C., Huang Z. M., Zhang J. X., Liang G. Y., Xu B. X., Chem. J. Chinese Universities,2014, 35(11), 2353—2359(梁光平, 胡占兴, 刘青川, 黄正明, 张建新, 梁光义, 徐必学. 高等学校化学学报, 2014, 35(11), 2353—2359) |
| [4] | Qiu J. Y., Xu B. X., Huang Z. M., Pan W. D., Cao P. X., Liu C. X., Hao X. J., Song B. A., Liang G. Y., Bioorg. Med. Chem., 2011, 19(18), 5352—5360 |
| [5] | Qiu J. Y., Huang Z. M., Pan W. D., Cao P. X., Liang G. Y., J. Chin. Pharm. Univ., 2012, 43(5), 390—394(邱净英, 黄正明, 潘卫东, 曹佩雪, 梁光义. 中国药科大学学报, 2012, 43(5), 390—394) |
| [6] | Liang G. P., Cao P. X., Yang X. X., Huang Z. M., Liu Q. C., Liang G. Y., Xu B. X., Chin. J. Org. Chem., 2014, 34(5), 973—979(梁光平, 曹佩雪, 杨秀虾, 黄正明, 刘青川, 梁光义, 徐必学. 有机化学, 2014, 34(5), 973—979) |
| [7] | Spiess M., Biochem., 1990, 29(43), 10009—10018 |
| [8] | Ding J. X., Xiao C. S., Li Y., Cheng Y. L., Wang N. N., He C. L., Zhuang X. L., Zhu X. J., Chen X. S., J. Control. Release,2013, 169(3), 193—203 |
| [9] | Wang Y. Q., Su J., Cai W. W., Lu P., Yuan L. F., Jin T., Chen S. Y., Sheng J., Drug. Des. Devel. Ther., 2013, 7(3), 211—221 |
| [10] | Naicker K., Ariatti M., Singh M., Colloids Surfaces B: Biointerfaces,2014, 122, 482—490 |
| [11] | Tao Y. F., He J. L., Zhang M. Z., Hao Y., Liu J., Ni P. H., Polym. Chem., 2014, 5(10), 3443—3452 |
| [12] | Yuan J., Liu Q. C., Xu G. C., Hu Z. X., Liang G. P., Huang Z. M., Liu C. X., Liang G. Y., Xu B. X., Chin. J. Org. Chem., 2015, 35(10), 2176—2183(袁洁, 刘青川, 徐广灿, 胡占兴, 梁光平, 黄正明, 刘昌孝, 梁光义, 徐必学. 有机化学, 2015, 35(10), 2176—2183) |
| [13] | Khorev O., Stokmaier D., Schwardt O., Cutting B., Ernst B., Bioorg. Med. Chem., 2008, 16(9), 5216—5231 |
| [14] | LaBell R. Y., Jacobsen N. E., Gervay-Hague J., O’Brian D. F., Bioconjugate Chem., 2002, 13(1), 143—149 |
| [15] | Percec V., Leowanawat P., Sun H. J., Kulikov O., Nusbaum C. D., Tran T. M., Bertin A., Wilson D. A., Peterca M., Zhang S. D., Kamat N. P., Vargo K., Moock D., Johnston E. D., Hammer D. A., Pochan D. J., Chen Y. X., Chabre Y. M., Shiao T. C., Bergeron-Blerk M., André S., Roy R., Gabius H. J., Heiney P. A., J. Am. Chem. Soc., 2013, 135(24), 9055—9077 |
| [16] | Zhang S. D., Moussodia R. O., Sun H. J., Leowanawat P., Muncan A., Nusbaum C. D., Chelling K. M., Heiney P. A., Klein M. L., André S., Roy R., Gabius H. J., Percec V., Angew. Chem. Int. Ed., 2014, 53(41), 10899—10903 |
| [17] | Zhao J., Xuan L. N., Zhao H. C., Cheng J., Fu X. Y., Jing F. M., Chem. Res. Chinese Universities,2014, 30(5), 764—769 |
| [18] | LiY. D., Mao W. T., Fan Z. J., Fang Z., Ji X. T., Zong G. N., Li F. Y., Chem. Res. Chinese Universities,2014, 30(3), 390—395 |
| [19] | Salameh B. A., Cumpstey I., Sundin A., Leffler H., Nilsson U. J., Bioorg. Med. Chem., 2010, 18(14), 5367—5378 |
| [20] | Zhang S. S., Wan J., Li X. M., Li C. L., Xu L. Z., Chem. Res. Chinese Universities,2007, 23(1), 120—124 |
| [21] | Johansson J. R., Lincoln P., Nordén B., Kann N., J. Org. Chem.2011, 76(7), 2355—2359 |
| [22] | Mishra K. B., Tiwari V. K., J. Org. Chem., 2014, 79(12), 5752—5762 |
| [23] | Li L. F., Chang K. C., Zhou Y. M., Shieh B., Ponder J., Abraham A. D., Ali H., Snow A., Mark Petrash J., LaBarbera D. V., J. Med. Chem., 2014, 57(1), 71—77 |
| [24] | Cai M.S., Li Z. J., Carbohydrate Chemistry: Fundamentals, Reactions, Synthesis, Isolation and Structure, Chemical Industry Press, Beijing, 2006, 370—373 |
| (蔡孟深, 李中军. 糖化学—基础、 反应、 合成、 分离及结构, 北京: 化学工业出版社, 2006, 370—373) |
| [1] | 赵军强, 闫彩霞, 陈泽, 杨宁, 冯霞, 赵义平, 陈莉. 细胞内氧化还原响应型肝靶向嵌段聚合物的合成及纳米自组装性能[J]. 高等学校化学学报, 2018, 39(7): 1592. |
| [2] | 徐广灿, 袁洁, 刘青川, 黄正明, 梁光义, 徐必学. 间接糖基化马蹄金素衍生物的合成及抗乙肝病毒活性研究[J]. 高等学校化学学报, 2016, 37(8): 1451. |
| [3] | 陈龙, 吴刚, 黄超, 王佳慧. 利用点击化学反应修饰聚氨酯[J]. 高等学校化学学报, 2014, 35(4): 853. |
| [4] | 王静云, 付雪, 包永明. 原子转移自由基聚合(ATRP)阳离子化菊粉作为非病毒基因载体的研究[J]. 高等学校化学学报, 2014, 35(10): 2124. |
| [5] | 朱建勤, 陈晖, 王少戎, 李燕, 方唯硕. 熊果酸半乳糖苷偶联物的合成及保肝活性[J]. 高等学校化学学报, 2013, 34(7): 1660. |
| [6] | 杨木泉, 毛骏, 董志鑫, 王大鹏, 姬相玲. 两亲性PS-b-PEG嵌段共聚物刷的合成及响应行为[J]. 高等学校化学学报, 2012, 33(12): 2816. |
| [7] | 徐宠恩, 杨静, 罗丙红, 李建华, 徐湾, 周长忍. 侧链含炔丙基的PLGA-PEG-PLGA三嵌段共聚物的合成与表征[J]. 高等学校化学学报, 2012, 33(10): 2138. |
| [8] | 施玲丽, 李剑波, 王成, 贾丽娜, 汪勇先, 张岚. 整合素αvβ3 靶向PET探针 18 F-c(RGDfK) 在Cu(Ⅰ)催化体系中的点击合成[J]. 高等学校化学学报, 2012, 33(07): 1486. |
| [9] | 于雷, 华小辉, 翟延君, 杨丽敏, 赵国忠, 孟田华, 翁诗甫, 徐怡庄, 刘克新, 吴瑾光, 陈佳洱. 两种半乳糖醇氯化钆配合物的制备与表征[J]. 高等学校化学学报, 2011, 32(6): 1244. |
| [10] | 吕丰, 李艳周, 武莉, 刘天军. 用于肿瘤成像的半乳糖/酞菁近红外荧光探针[J]. 高等学校化学学报, 2011, 32(5): 1010. |
| [11] | 高遐 张馥 陈志春 林贤福. 含乳糖聚电解质靶向微胶囊的层层自组装构筑[J]. 高等学校化学学报, 2011, 32(4): 957. |
| [12] | 黄微 王平 王蔚 张玥 张闯年 田秦 王秀华 刘媛 袁直. 甘草次酸修饰PEG-PLGA纳米粒的制备及与肝癌细胞亲和性[J]. 高等学校化学学报, 2011, 32(2): 416. |
| [13] | 张奇 王彬 黎霞 侯洁 白芳 孙丹 白钢. 基于点击化学反应的免疫荧光检测方法的建立和应用[J]. 高等学校化学学报, 2011, 32(2): 281. |
| [14] | 王富强, 郝俊霞, 戴小军, 龚波林. 原子转移自由基聚合制备新型接枝聚α-D-吡喃半乳糖苷色谱固定相及其性能[J]. 高等学校化学学报, 2011, 32(12): 2757. |
| [15] | 张涛, 武元鹏, 郑朝晖, 丁小斌, 彭宇行. 基于点击化学和活性自由基聚合方法制备双重响应金纳米粒子[J]. 高等学校化学学报, 2010, 31(11): 2303. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||