

高等学校化学学报 ›› 2016, Vol. 37 ›› Issue (8): 1451.doi: 10.7503/cjcu20160222
徐广灿1,2, 袁洁1,4, 刘青川3, 黄正明3, 梁光义1,2( ), 徐必学1(
), 徐必学1( )
)
收稿日期:2016-05-20
出版日期:2016-07-19
发布日期:2016-07-19
作者简介:联系人简介: 梁光义, 男, 博士, 教授, 博士生导师, 主要从事天然产物化学方面的研究. E-mail:
基金资助:
XU Guangcan1,2, YUAN Jie1,4, LIU Qingchuan3, HUANG Zhengming3, LIANG Guangyi1,2,*( ), XU Bixue1,*(
), XU Bixue1,*( )
)
Received:2016-05-20
Online:2016-07-19
Published:2016-07-19
Contact:
LIANG Guangyi,XU Bixue
E-mail:guangyi_liang@126.com;bixue_xu@126.com
Supported by:摘要:
以二缩三乙二醇或三缩四乙二醇为连接臂, 通过间接引入具有肝靶向性的D-半乳糖配基的方法, 设计合成了6个半乳糖糖基化修饰的肝靶向马蹄金素(MTS)衍生物; 通过1H NMR, 13C NMR, 1H-1H COSY, HMQC和ESI-MS对其结构进行了表征; 并以HepG2 2.2.15为细胞模型初步评价了目标化合物的抗乙肝病毒(HBV)活性. 结果表明, 所有目标化合物对HBV DNA的复制均有抑制作用, 且具有一定的量效关系. 化合物15b的体外抗HBV活性最好, 后期研究将选用其进行小鼠体内组织分布实验, 并与原型化合物以及Y101体内组织分布情况进行比较研究, 以验证半乳糖基的引入能否提高MTS衍生物的肝靶向性.
中图分类号:
TrendMD:
徐广灿, 袁洁, 刘青川, 黄正明, 梁光义, 徐必学. 间接糖基化马蹄金素衍生物的合成及抗乙肝病毒活性研究. 高等学校化学学报, 2016, 37(8): 1451.
XU Guangcan,YUAN Jie,LIU Qingchuan,HUANG Zhengming,LIANG Guangyi,XU Bixue. Synthesis and Anti-HBV Activities of the Indirect Galactopyranosyl Derivatives of Matijin-Su†. Chem. J. Chinese Universities, 2016, 37(8): 1451.
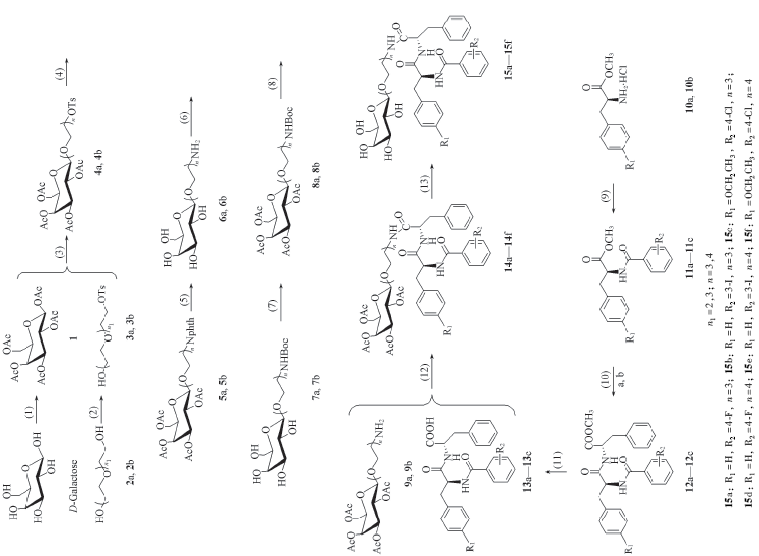
Scheme 1 Synthetic routes of target compounds 15a—15f (1) Ac2O, CH3COONa, 140 ℃; (2) 4-methylphenylsulfonyl chloride, pyridine, r.t.; (3) 0.4 nm molecular sieves, BF3·Et2O, CH2Cl2, 0 ℃ to r.t.; (4) phthalimide potassium, DMF, 110 ℃; (5) N2H4·H2O, MeOH, H2O, 80 ℃; (6)(Boc)2O, NaHCO3, EtOH, r.t.; (7) Ac2O, pyridine, r.t.; (8) TFA, CH2Cl2, 0 ℃; (9) IBCF, NMM, CH2Cl2/DMF, 0 ℃ to r.t.; (10) (a) NaOH, DMF, r.t.; (b) IBCF, NMM, CH2Cl2/DMF, 0 ℃ to r.t.; (11) NaOH, DMF, r.t.; (12) IBCF, NMM, CH2Cl2/DMF, 0 ℃ to r.t.; (13) CH3ONa, CH3OH/CH2Cl2, r.t..
| Compd. | Appearance | Yield(%) | m.p./℃ | [α | ESI-MS, m/z |
|---|---|---|---|---|---|
| 3a | Pale oil | 40 | |||
| 3b | Pale oil | 55 | |||
| 4a | Pale oil | 49 | 657.0[M+Na]+ | ||
| 4b | Pale oil | 45 | |||
| 5a | Pale oil | 87 | |||
| 5b | Pale oil | 84 | |||
| 8a | Pale oil | 79 | 580.1[M+H]+ | ||
| 8b | Pale oil | 82 | 626.5[M+Na]+ | ||
| 9a | Pale oil | 502.0[M+Na]+ | |||
| 9b | Pale oil | 524.1[M+H]+ | |||
| 14a | White solid | 79 | 130—132 | -29.89 | 896.4[M+H]+ |
| 14b | White solid | 83 | 133—134 | -31.37 | 1026.3[M+Na]+ |
| 14c | White solid | 65 | 145—146 | -43.17 | 978.4[M+Na]+ |
| 14d | White solid | 97 | 128—129 | -27.82 | 962.2[M+Na]+ |
| 14e | White solid | 79 | 132—135 | -33.39 | 1048.3[M+H]+ |
| 14f | White solid | 68 | 146—148 | -34.68 | 1022.3[M+Na]+ |
Table 1 Appearance, yields, melting points, specific rotation and ESI-MS data for all the intermediates*
| Compd. | Appearance | Yield(%) | m.p./℃ | [α | ESI-MS, m/z |
|---|---|---|---|---|---|
| 3a | Pale oil | 40 | |||
| 3b | Pale oil | 55 | |||
| 4a | Pale oil | 49 | 657.0[M+Na]+ | ||
| 4b | Pale oil | 45 | |||
| 5a | Pale oil | 87 | |||
| 5b | Pale oil | 84 | |||
| 8a | Pale oil | 79 | 580.1[M+H]+ | ||
| 8b | Pale oil | 82 | 626.5[M+Na]+ | ||
| 9a | Pale oil | 502.0[M+Na]+ | |||
| 9b | Pale oil | 524.1[M+H]+ | |||
| 14a | White solid | 79 | 130—132 | -29.89 | 896.4[M+H]+ |
| 14b | White solid | 83 | 133—134 | -31.37 | 1026.3[M+Na]+ |
| 14c | White solid | 65 | 145—146 | -43.17 | 978.4[M+Na]+ |
| 14d | White solid | 97 | 128—129 | -27.82 | 962.2[M+Na]+ |
| 14e | White solid | 79 | 132—135 | -33.39 | 1048.3[M+H]+ |
| 14f | White solid | 68 | 146—148 | -34.68 | 1022.3[M+Na]+ |
| Compd. | 1H NMR(CDCl3), δ |
|---|---|
| 3a | 7.80(d, J=8.3 Hz, 2H), 7.35(d, J=8.0 Hz, 2H), 4.17(t, J=4.8 Hz, 2H), 3.75—3.68(m, 4H), 3.63—3.55(m, 6H), 2.45(s, 3H) |
| 3b | 7.79(d, J=8.3 Hz, 2H), 7.33(d, J=8.0 Hz, 2H), 4.15(t, J=4.8 Hz, 2H), 3.75—3.56(m, 14H), 2.44(s, 3H) |
| 4a | 7.79(d, J=8.3 Hz, 2H), 7.34(d, J=7.9 Hz, 2H), 5.38(dd, J=3.4, 1.0 Hz, 1H), 5.20(dd, J=10.5, 8.0 Hz, 1H), 5.02(dd, J=10.5, 3.4 Hz, 1H), 4.56(d, J=8.0 Hz, 1H), 4.20—4.09(m, 4H), 3.98—3.89(m, 2H), 3.73(ddd, J=11.0, 7.0, 3.9 Hz, 1H), 3.70—3.55(m, 8H), 2.45(s, 3H), 2.15(s, 3H), 2.05(s, 3H), 2.05(s, 3H), 1.99(s, 3H) |
| 4b | 7.79(d, J=8.3 Hz, 2H), 7.35(d, J=8.1 Hz, 2H), 5.38(dd, J=2.6, 0.8 Hz, 1H), 5.19(dd, J=10.5, 8.0 Hz, 1H), 5.01(dd, J=10.5, 3.4 Hz, 1H), 4.56(d, J=8.0 Hz, 1H), 4.19—4.08(m, 4H), 4.00—3.84(m, 2H), 3.80—3.55(m, 13H), 2.44(s, 3H), 2.14(s, 3H), 2.04(d, J=3.1 Hz, 6H), 1.97(s, 3H) |
Table 2 1H NMR data for intermediates 3 and 4*
| Compd. | 1H NMR(CDCl3), δ |
|---|---|
| 3a | 7.80(d, J=8.3 Hz, 2H), 7.35(d, J=8.0 Hz, 2H), 4.17(t, J=4.8 Hz, 2H), 3.75—3.68(m, 4H), 3.63—3.55(m, 6H), 2.45(s, 3H) |
| 3b | 7.79(d, J=8.3 Hz, 2H), 7.33(d, J=8.0 Hz, 2H), 4.15(t, J=4.8 Hz, 2H), 3.75—3.56(m, 14H), 2.44(s, 3H) |
| 4a | 7.79(d, J=8.3 Hz, 2H), 7.34(d, J=7.9 Hz, 2H), 5.38(dd, J=3.4, 1.0 Hz, 1H), 5.20(dd, J=10.5, 8.0 Hz, 1H), 5.02(dd, J=10.5, 3.4 Hz, 1H), 4.56(d, J=8.0 Hz, 1H), 4.20—4.09(m, 4H), 3.98—3.89(m, 2H), 3.73(ddd, J=11.0, 7.0, 3.9 Hz, 1H), 3.70—3.55(m, 8H), 2.45(s, 3H), 2.15(s, 3H), 2.05(s, 3H), 2.05(s, 3H), 1.99(s, 3H) |
| 4b | 7.79(d, J=8.3 Hz, 2H), 7.35(d, J=8.1 Hz, 2H), 5.38(dd, J=2.6, 0.8 Hz, 1H), 5.19(dd, J=10.5, 8.0 Hz, 1H), 5.01(dd, J=10.5, 3.4 Hz, 1H), 4.56(d, J=8.0 Hz, 1H), 4.19—4.08(m, 4H), 4.00—3.84(m, 2H), 3.80—3.55(m, 13H), 2.44(s, 3H), 2.14(s, 3H), 2.04(d, J=3.1 Hz, 6H), 1.97(s, 3H) |
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(100 MHz, CDCl3), δ |
|---|---|---|
| 5a | 7.84(dd, J=5.5, 3.0 Hz, 2H), 7.71(dd, J=5.5, 3.0 Hz, 2H), 5.38(dd, J=3.4, 0.9 Hz, 1H), 5.19(dd, J=10.5, 8.0 Hz, 1H), 5.02(dd, J=10.5, 3.4 Hz, 1H), 4.55(d, J=8.0 Hz, 1H), 4.20—4.08(m, 2H), 3.95—3.52(m, 13H), 2.14(s, 3H), 2.04(s, 6H), 1.97(s, 3H) | 170.6, 170.4, 170.3, 169.7, 168.4, 168.4, 134.1, 132.2, 123.4, 101.5, 71.0, 70.8, 70.7, 70.5, 70.2, 69.2, 69.0, 68.1, 67.2, 61.5, 37.3, 20.9, 20.8, 20.8, 20.8 |
| 5b | 7.85(dd, J=5.5, 3.0 Hz, 2H), 7.74(dd, J=5.5, 3.0 Hz, 2H), 5.40(dd, J=3.4, 0.8 Hz, 1H), 5.21(dd, J=10.5, 8.0 Hz, 1H), 5.03(dd, J=10.5, 3.4 Hz, 1H), 4.59(d, J=8.0 Hz, 1H), 4.22—4.13(m, 2H), 4.03—3.52(m, 17H), 2.15(s, 3H), 2.06(s, 3H), 2.05(s, 3H), 1.99(s, 3H) | 170.6, 170.4, 170.3, 169.7,168.43, 168.39, 134.1, 132.2, 123.4, 101.5, 71.0, 70.79, 70.76 70.7, 70.5, 70.3, 70.2, 69.2, 69.0, 68.1, 67.2, 61.5, 37.3, 20.9, 20.84, 20.82, 20.8 |
| 8a | 5.26(t, J=8.6 Hz, 1H), 5.10(s, 1H), 4.94(dd, J=10.2, 3.2 Hz, 1H), 4.54(d, J=7.8 Hz, 1H), 4.32(dd, J=6.3, 4.5 Hz, 2H), 4.05(d, J=2.8 Hz, 1H), 3.81—3.51(m, 12H), 2.11(s, 3H), 2.09(s, 3H), 2.06(s, 3H), 1.84(s, 3H),1.45(s, 9H) | 170.6, 170.4, 170.3, 169.6, 156.1, 101.58, 71.0, 70.8, 70.8,70.72, 70.65, 69.2, 69.0, 67.2, 61.4, 40.5, 28.6, 28.0, 21.0, 19.8, 19.7 |
| 8b | 5.37(dd, J=8.4, 1.0 Hz, 1H), 5.20(dd, J=10.5, 8.0 Hz, 1H), 5.01(dd, J=10.5, 3.4 Hz, 1H), 4.56(d, J=8.0 Hz, 1H), 4.21—4.07(m, 2H), 4.00—3.87(m, 2H), 3.81—3.70(m, 1H), 3.70—3.30(m, 14H), 2.14(s, 3H), 2.05(s, 3H), 2.04(s, 3H), 1.97(s, 3H), 1.43(s, 9H) | 170.5, 170.4, 170.3, 169.6, 156.1, 101.5, 71.0, 70.83, 70.77, 70.72, 70.65, 70.41, 70.37, 69.2, 69.0, 67.2, 61.4, 40.5, 28.6, 28.0, 20.9, 20.8, 20.7 |
| 14a | 7.68(dd, J=8.8, 5.3 Hz, 2H), 7.31—7.04(m, 12H), 6.75—6.65(m, 2H), 6.39(t, J=4.4 Hz, 1H), 5.39(d, J=2.6 Hz, 1H), 5.22(dd, J=10.5, 7.9 Hz, 1H), 5.04(dd, J=10.5, 3.4 Hz, 1H), 4.87—4.77(m, 1H), 4.64—4.53(m, 2H), 4.22—4.07(m, 2H), 4.00—3.89(m, 2H), 3.77—3.68(m, 1H), 3.67—3.42(m, 7H), 3.42—3.31(m, 3H), 3.13(d, J=7.1 Hz, 2H), 3.01(d, J=6.9 Hz, 2H), 2.12(s, 3H), 2.04(s, 3H), 2.02(s, 3H), 1.98(s, 3H) | 170.6,170.41, 170.36, 170.3, 169.8, 166.3, 166.2, 163.8, 136.6, 136.4, 129.93, 129.90, 129.7, 129.6, 129.44, 129.42, 128.9, 128.6, 127.3, 127.0, 115.9, 115.7, 101. 4, 71.0, 70.8, 70.7, 70.3, 70.3, 69.7, 69.2, 69.0, 67.2, 61.4, 54.8, 54.6, 39.4, 38.1, 38.2, 20.9, 20.8, 20.80, 20.75 |
| 14b | 7.98(s, 1H), 7.81(d, J=8.1 Hz, 1H), 7.61(d, J=7.9 Hz, 1H), 7.30—7.04(m, 11H), 6.94—6.86(m, 2H), 6.47(t, J=4.9 Hz, 1H), 5.38(d, J=3.3 Hz, 1H), 5.21(dd, J=10.5, 8.0 Hz, 1H), 5.04(dd, J=10.5, 3.4 Hz, 1H), 4.92—4.83(m, 1H), 4.69—4.59(m, 1H), 4.55(d, J=8.0 Hz, 1H), 4.21—4.07(m, 2H), 4.00—3.88(m, 2H), 3.77—3.67(m, 1H), 3.66—3.41(m, 7H), 3.40—3.29(m, 3H), 3.11(d, J=6.9 Hz, 2H), 3.00(d, J=7.0 Hz, 2H), 2.12(s, 3H), 2.03(s, 3H), 2.02(s, 3H), 1.98(s, 3H) | 170.5, 170.4, 170.3,170.21, 170.16, 169.7, 165.6, 140.6, 136.4, 136.3, 136.2, 135.6, 130.1, 129.3, 128.6, 128.4, 127.0, 126.8, 126.2, 101.2, 94.1, 70.8, 70.6, 70.5, 70.1, 70.1, 69.5, 69.0, 68.8, 67.0, 61.2, 54.7, 54.5, 39.2, 38.6, 38.1, 20.8, 20.64, 20.61, 20.57 |
| 14c | 7.61(d, J=8.6 Hz, 2H), 7.39(d, J=8.5 Hz, 2H), 7.20—7.07(m, 7H), 6.80(d, J=8.6 Hz, 2H), 6.71(d, J=7.3 Hz, 1H), 6.62(d, J=7.8 Hz, 1H), 6.37(t, J=4.9 Hz, 1H), 5.39(d, J=2.6 Hz, 1H), 5.22(dd, J=10.5, 8.0 Hz, 1H), 5.05(dd, J=10.5, 3.4 Hz, 1H), 4.80—4.68(m, 1H), 4.63—4.53(m, 2H), 4.23—4.08(m, 2H), 4.02—3.90(m, 4H), 3.77—3.67(m, 1H), 3.65—3.33(m, 10H), 3.10—3.05(m, 2H, 3a-H), 3.02(d, J=6.8 Hz, 2H), 2.13(s, 3H), 2.04(s, 3H), 2.03(s, 3H,), 1.99(s, 3H), 1.39(t, J=7.0 Hz, 3H) | 170.61, 170.59, 170.41, 170.35,170.3, 169.8, 166.2, 158.2, 138.3, 136.6, 132.2, 130.5 129.4, 129.0, 128.7, 128.6, 128.0, 127.0, 114.9, 101.5, 71.0, 70.81, 70.75, 70.4, 70.3, 69.7, 69.3, 69.0, 67.2, 63.5, 61.4, 55.0, 54.6, 39.4, 38.5, 37.3, 21.0, 20.83, 20.80, 20.75, 15.0 |
| 14d | 7.69(dd, J=8.8, 5.3 Hz, 2H), 7.31—7.05(m, 12H), 6.77(d, J=7.6 Hz, 2H), 6.43(t, J=4.3 Hz, 1H), 5.38(d, J=3.4 Hz, 1H,), 5.21(dd, J=10.5, 7.9 Hz, 1H), 5.05(dd, J=10.5, 3.4 Hz, 1H) 4.87—4.77(m, 1H), 4.63—4.55(m, 2H), 4.20—4.08(m, 2H), 3.98—3.90(m, 2H), 3.79—3.69(m, 1H), 3.66—3.43(m, 11H), 3.42—3.30(m, 3H), 3.13(d, J=6.9 Hz, 2H), 3.01(d, J=6.9 Hz, 2H), 2.13(s, 3H), 2.03(s, 3H), 2.02(s, 3H), 1.97(s, 3H) | 170.63, 170.57, 170.41, 170.38,170.3, 169.7, 166.3, 166.2, 163.8, 136.6, 136.5, 129.98, 129.95, 129.7, 129.6, 129.4, 129.0, 128.6, 127.2, 126.9, 115.8, 115.6, 101.3, 71.0, 70.8, 70.7, 70.61, 70.58, 70.32, 70.29, 69.7, 69.1, 68.9, 67.2, 61.4, 54.8, 54.6, 39.4, 38.7, 38.2, 20.9, 20.80, 20.78, 20.75 |
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(100 MHz, CDCl3), δ |
| 14e | 7.98(s, 1H), 7.81(d, J=8.1 Hz, 1H), 7.61(d, J=7.9 Hz, 1H), 7.30—7.04(m, 11H), 6.92(d, J=7.8 Hz, 2H), 6.49(t, J=5.0 Hz, 1H), 5.38(d, J=3.3 Hz, 1H), 5.20(dd, J=10.5, 8.0 Hz, 1H), 5.05(dd, J=10.5, 3.4 Hz, 1H), 4.91—4.82(m, 1H), 4.67—4.56(m, 2H), 4.20—4.07(m, 2H), 3.98—3.90(m, 2H), 3.77—3.69(m, 1H), 3.67—3.48(m, 10H), 3.48—3.29(m, 4H), 3.11(d, J=6.9 Hz, 2H), 3.01(dd, J=6.9, 1.5 Hz, 2H), 2.12(s, 3H), 2.03(s, 3H), 2.01(s, 3H), 1.97(s, 3H) | 170.5, 170.4, 170.23, 170.21, 170.19, 169.5, 165.6, 140.5, 136.4, 136.3, 136.2, 135.7, 130.1, 129.3, 129.2, 128.7, 128.3, 127.0, 126.7, 126.3, 101.1, 94.1, 70.8, 70.5, 70.5, 70.41, 70.38, 70.12, 70.09, 69.4, 68.9, 68.8, 67.0, 61.2, 54.7, 54.4, 39.2, 38.6, 38.1, 20.7, 20.62, 20.60, 20.58 |
| 14f | 7.63(d, J=8.7 Hz, 2H), 7.39(d, J=8.7 Hz, 2H), 7.19—7.06(m, 7H), 6.79(d, J=8.6 Hz, 2H), 5.39(dd, J=3.4, 1.0 Hz, 1H), 5.22(dd, J=10.5, 7.9 Hz, 1H), 5.06(dd, J=10.5, 3.4 Hz, 1H), 4.83—4.76(m, 1H), 4.65—4.57(m, 2H), 4.20—4.09(m, 2H), 4.01—3.92(m, 4H), 3.78—3.70(m, 1H), 3.67—3.29(m, 14H), 3.07(dd, J=6.7, 2.3 Hz, 2H), 3.02(d, J=6.9 Hz, 2H), 2.13(s, 3H), 2.05(s, 3H), 2.03(s, 3H), 1.98(s, 3H), 1.38(t, J=7.0 Hz, 3H) | 170.7, 170.6, 170.39, 170.36, 170.3, 169.7, 166.2, 158.2, 138.2, 136.6, 132.2, 130.4, 129.4, 128.9, 128.7, 128.68, 128.1, 127.0, 114.8, 101.4, 71.0, 70.8, 70.7, 70.6, 70.6, 70.3, 70.3, 69.6, 69.1, 68.9, 67.2, 63.5, 61.4, 55.0, 54.6, 39.3, 38.6, 37.4, 20.9, 20.80, 20.78, 20.7, 15.0 |
Table 3 1H NMR and 13C NMR data for intermediates 5, 8 and 14
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(100 MHz, CDCl3), δ |
|---|---|---|
| 5a | 7.84(dd, J=5.5, 3.0 Hz, 2H), 7.71(dd, J=5.5, 3.0 Hz, 2H), 5.38(dd, J=3.4, 0.9 Hz, 1H), 5.19(dd, J=10.5, 8.0 Hz, 1H), 5.02(dd, J=10.5, 3.4 Hz, 1H), 4.55(d, J=8.0 Hz, 1H), 4.20—4.08(m, 2H), 3.95—3.52(m, 13H), 2.14(s, 3H), 2.04(s, 6H), 1.97(s, 3H) | 170.6, 170.4, 170.3, 169.7, 168.4, 168.4, 134.1, 132.2, 123.4, 101.5, 71.0, 70.8, 70.7, 70.5, 70.2, 69.2, 69.0, 68.1, 67.2, 61.5, 37.3, 20.9, 20.8, 20.8, 20.8 |
| 5b | 7.85(dd, J=5.5, 3.0 Hz, 2H), 7.74(dd, J=5.5, 3.0 Hz, 2H), 5.40(dd, J=3.4, 0.8 Hz, 1H), 5.21(dd, J=10.5, 8.0 Hz, 1H), 5.03(dd, J=10.5, 3.4 Hz, 1H), 4.59(d, J=8.0 Hz, 1H), 4.22—4.13(m, 2H), 4.03—3.52(m, 17H), 2.15(s, 3H), 2.06(s, 3H), 2.05(s, 3H), 1.99(s, 3H) | 170.6, 170.4, 170.3, 169.7,168.43, 168.39, 134.1, 132.2, 123.4, 101.5, 71.0, 70.79, 70.76 70.7, 70.5, 70.3, 70.2, 69.2, 69.0, 68.1, 67.2, 61.5, 37.3, 20.9, 20.84, 20.82, 20.8 |
| 8a | 5.26(t, J=8.6 Hz, 1H), 5.10(s, 1H), 4.94(dd, J=10.2, 3.2 Hz, 1H), 4.54(d, J=7.8 Hz, 1H), 4.32(dd, J=6.3, 4.5 Hz, 2H), 4.05(d, J=2.8 Hz, 1H), 3.81—3.51(m, 12H), 2.11(s, 3H), 2.09(s, 3H), 2.06(s, 3H), 1.84(s, 3H),1.45(s, 9H) | 170.6, 170.4, 170.3, 169.6, 156.1, 101.58, 71.0, 70.8, 70.8,70.72, 70.65, 69.2, 69.0, 67.2, 61.4, 40.5, 28.6, 28.0, 21.0, 19.8, 19.7 |
| 8b | 5.37(dd, J=8.4, 1.0 Hz, 1H), 5.20(dd, J=10.5, 8.0 Hz, 1H), 5.01(dd, J=10.5, 3.4 Hz, 1H), 4.56(d, J=8.0 Hz, 1H), 4.21—4.07(m, 2H), 4.00—3.87(m, 2H), 3.81—3.70(m, 1H), 3.70—3.30(m, 14H), 2.14(s, 3H), 2.05(s, 3H), 2.04(s, 3H), 1.97(s, 3H), 1.43(s, 9H) | 170.5, 170.4, 170.3, 169.6, 156.1, 101.5, 71.0, 70.83, 70.77, 70.72, 70.65, 70.41, 70.37, 69.2, 69.0, 67.2, 61.4, 40.5, 28.6, 28.0, 20.9, 20.8, 20.7 |
| 14a | 7.68(dd, J=8.8, 5.3 Hz, 2H), 7.31—7.04(m, 12H), 6.75—6.65(m, 2H), 6.39(t, J=4.4 Hz, 1H), 5.39(d, J=2.6 Hz, 1H), 5.22(dd, J=10.5, 7.9 Hz, 1H), 5.04(dd, J=10.5, 3.4 Hz, 1H), 4.87—4.77(m, 1H), 4.64—4.53(m, 2H), 4.22—4.07(m, 2H), 4.00—3.89(m, 2H), 3.77—3.68(m, 1H), 3.67—3.42(m, 7H), 3.42—3.31(m, 3H), 3.13(d, J=7.1 Hz, 2H), 3.01(d, J=6.9 Hz, 2H), 2.12(s, 3H), 2.04(s, 3H), 2.02(s, 3H), 1.98(s, 3H) | 170.6,170.41, 170.36, 170.3, 169.8, 166.3, 166.2, 163.8, 136.6, 136.4, 129.93, 129.90, 129.7, 129.6, 129.44, 129.42, 128.9, 128.6, 127.3, 127.0, 115.9, 115.7, 101. 4, 71.0, 70.8, 70.7, 70.3, 70.3, 69.7, 69.2, 69.0, 67.2, 61.4, 54.8, 54.6, 39.4, 38.1, 38.2, 20.9, 20.8, 20.80, 20.75 |
| 14b | 7.98(s, 1H), 7.81(d, J=8.1 Hz, 1H), 7.61(d, J=7.9 Hz, 1H), 7.30—7.04(m, 11H), 6.94—6.86(m, 2H), 6.47(t, J=4.9 Hz, 1H), 5.38(d, J=3.3 Hz, 1H), 5.21(dd, J=10.5, 8.0 Hz, 1H), 5.04(dd, J=10.5, 3.4 Hz, 1H), 4.92—4.83(m, 1H), 4.69—4.59(m, 1H), 4.55(d, J=8.0 Hz, 1H), 4.21—4.07(m, 2H), 4.00—3.88(m, 2H), 3.77—3.67(m, 1H), 3.66—3.41(m, 7H), 3.40—3.29(m, 3H), 3.11(d, J=6.9 Hz, 2H), 3.00(d, J=7.0 Hz, 2H), 2.12(s, 3H), 2.03(s, 3H), 2.02(s, 3H), 1.98(s, 3H) | 170.5, 170.4, 170.3,170.21, 170.16, 169.7, 165.6, 140.6, 136.4, 136.3, 136.2, 135.6, 130.1, 129.3, 128.6, 128.4, 127.0, 126.8, 126.2, 101.2, 94.1, 70.8, 70.6, 70.5, 70.1, 70.1, 69.5, 69.0, 68.8, 67.0, 61.2, 54.7, 54.5, 39.2, 38.6, 38.1, 20.8, 20.64, 20.61, 20.57 |
| 14c | 7.61(d, J=8.6 Hz, 2H), 7.39(d, J=8.5 Hz, 2H), 7.20—7.07(m, 7H), 6.80(d, J=8.6 Hz, 2H), 6.71(d, J=7.3 Hz, 1H), 6.62(d, J=7.8 Hz, 1H), 6.37(t, J=4.9 Hz, 1H), 5.39(d, J=2.6 Hz, 1H), 5.22(dd, J=10.5, 8.0 Hz, 1H), 5.05(dd, J=10.5, 3.4 Hz, 1H), 4.80—4.68(m, 1H), 4.63—4.53(m, 2H), 4.23—4.08(m, 2H), 4.02—3.90(m, 4H), 3.77—3.67(m, 1H), 3.65—3.33(m, 10H), 3.10—3.05(m, 2H, 3a-H), 3.02(d, J=6.8 Hz, 2H), 2.13(s, 3H), 2.04(s, 3H), 2.03(s, 3H,), 1.99(s, 3H), 1.39(t, J=7.0 Hz, 3H) | 170.61, 170.59, 170.41, 170.35,170.3, 169.8, 166.2, 158.2, 138.3, 136.6, 132.2, 130.5 129.4, 129.0, 128.7, 128.6, 128.0, 127.0, 114.9, 101.5, 71.0, 70.81, 70.75, 70.4, 70.3, 69.7, 69.3, 69.0, 67.2, 63.5, 61.4, 55.0, 54.6, 39.4, 38.5, 37.3, 21.0, 20.83, 20.80, 20.75, 15.0 |
| 14d | 7.69(dd, J=8.8, 5.3 Hz, 2H), 7.31—7.05(m, 12H), 6.77(d, J=7.6 Hz, 2H), 6.43(t, J=4.3 Hz, 1H), 5.38(d, J=3.4 Hz, 1H,), 5.21(dd, J=10.5, 7.9 Hz, 1H), 5.05(dd, J=10.5, 3.4 Hz, 1H) 4.87—4.77(m, 1H), 4.63—4.55(m, 2H), 4.20—4.08(m, 2H), 3.98—3.90(m, 2H), 3.79—3.69(m, 1H), 3.66—3.43(m, 11H), 3.42—3.30(m, 3H), 3.13(d, J=6.9 Hz, 2H), 3.01(d, J=6.9 Hz, 2H), 2.13(s, 3H), 2.03(s, 3H), 2.02(s, 3H), 1.97(s, 3H) | 170.63, 170.57, 170.41, 170.38,170.3, 169.7, 166.3, 166.2, 163.8, 136.6, 136.5, 129.98, 129.95, 129.7, 129.6, 129.4, 129.0, 128.6, 127.2, 126.9, 115.8, 115.6, 101.3, 71.0, 70.8, 70.7, 70.61, 70.58, 70.32, 70.29, 69.7, 69.1, 68.9, 67.2, 61.4, 54.8, 54.6, 39.4, 38.7, 38.2, 20.9, 20.80, 20.78, 20.75 |
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(100 MHz, CDCl3), δ |
| 14e | 7.98(s, 1H), 7.81(d, J=8.1 Hz, 1H), 7.61(d, J=7.9 Hz, 1H), 7.30—7.04(m, 11H), 6.92(d, J=7.8 Hz, 2H), 6.49(t, J=5.0 Hz, 1H), 5.38(d, J=3.3 Hz, 1H), 5.20(dd, J=10.5, 8.0 Hz, 1H), 5.05(dd, J=10.5, 3.4 Hz, 1H), 4.91—4.82(m, 1H), 4.67—4.56(m, 2H), 4.20—4.07(m, 2H), 3.98—3.90(m, 2H), 3.77—3.69(m, 1H), 3.67—3.48(m, 10H), 3.48—3.29(m, 4H), 3.11(d, J=6.9 Hz, 2H), 3.01(dd, J=6.9, 1.5 Hz, 2H), 2.12(s, 3H), 2.03(s, 3H), 2.01(s, 3H), 1.97(s, 3H) | 170.5, 170.4, 170.23, 170.21, 170.19, 169.5, 165.6, 140.5, 136.4, 136.3, 136.2, 135.7, 130.1, 129.3, 129.2, 128.7, 128.3, 127.0, 126.7, 126.3, 101.1, 94.1, 70.8, 70.5, 70.5, 70.41, 70.38, 70.12, 70.09, 69.4, 68.9, 68.8, 67.0, 61.2, 54.7, 54.4, 39.2, 38.6, 38.1, 20.7, 20.62, 20.60, 20.58 |
| 14f | 7.63(d, J=8.7 Hz, 2H), 7.39(d, J=8.7 Hz, 2H), 7.19—7.06(m, 7H), 6.79(d, J=8.6 Hz, 2H), 5.39(dd, J=3.4, 1.0 Hz, 1H), 5.22(dd, J=10.5, 7.9 Hz, 1H), 5.06(dd, J=10.5, 3.4 Hz, 1H), 4.83—4.76(m, 1H), 4.65—4.57(m, 2H), 4.20—4.09(m, 2H), 4.01—3.92(m, 4H), 3.78—3.70(m, 1H), 3.67—3.29(m, 14H), 3.07(dd, J=6.7, 2.3 Hz, 2H), 3.02(d, J=6.9 Hz, 2H), 2.13(s, 3H), 2.05(s, 3H), 2.03(s, 3H), 1.98(s, 3H), 1.38(t, J=7.0 Hz, 3H) | 170.7, 170.6, 170.39, 170.36, 170.3, 169.7, 166.2, 158.2, 138.2, 136.6, 132.2, 130.4, 129.4, 128.9, 128.7, 128.68, 128.1, 127.0, 114.8, 101.4, 71.0, 70.8, 70.7, 70.6, 70.6, 70.3, 70.3, 69.6, 69.1, 68.9, 67.2, 63.5, 61.4, 55.0, 54.6, 39.3, 38.6, 37.4, 20.9, 20.80, 20.78, 20.7, 15.0 |
| Compd. | Appearance | Yield (%) | m.p./℃ | [α (c=0.5) | HRMS(calcd.)b, m/z | IR(KBr), |
|---|---|---|---|---|---|---|
| 15a | White solid | 88 | 156—157 | -80.73 | 750.3010(750.3014) | 3283, 2880, 1634, 1542, 1507, 1076, 700 |
| 15b | White solid | 89 | 168—169 | -46.64 | 858.2074(858.2075) | 3278, 2875, 1635, 1540, 1508, 1075, 698 |
| 15c | White solid | 88 | 182—184 | -36.09 | 810.3001(810.2981) | 3284, 2924, 1632, 1542, 1512, 1073, 677 |
| 15d | White solid | 74 | 151—152 | -36.99 | 794.3265(794.3276) | 3282, 2875, 1635, 1542, 1506, 1077, 699 |
| 15e | White solid | 73 | 166—167 | -39.71 | 902.2334(902.2337) | 3273, 2873, 1635, 1534, 1506, 1077, 698 |
| 15f | White solid | 78 | 186—189 | -43.17 | 832.3429(832.3423) | 3279, 2873, 1632, 1539, 1512, 1091, 702 |
Table 4 Appearance, yields, melting points, specific rotation, MS and IR data for the target compounds 15a—15f
| Compd. | Appearance | Yield (%) | m.p./℃ | [α (c=0.5) | HRMS(calcd.)b, m/z | IR(KBr), |
|---|---|---|---|---|---|---|
| 15a | White solid | 88 | 156—157 | -80.73 | 750.3010(750.3014) | 3283, 2880, 1634, 1542, 1507, 1076, 700 |
| 15b | White solid | 89 | 168—169 | -46.64 | 858.2074(858.2075) | 3278, 2875, 1635, 1540, 1508, 1075, 698 |
| 15c | White solid | 88 | 182—184 | -36.09 | 810.3001(810.2981) | 3284, 2924, 1632, 1542, 1512, 1073, 677 |
| 15d | White solid | 74 | 151—152 | -36.99 | 794.3265(794.3276) | 3282, 2875, 1635, 1542, 1506, 1077, 699 |
| 15e | White solid | 73 | 166—167 | -39.71 | 902.2334(902.2337) | 3273, 2873, 1635, 1534, 1506, 1077, 698 |
| 15f | White solid | 78 | 186—189 | -43.17 | 832.3429(832.3423) | 3279, 2873, 1632, 1539, 1512, 1091, 702 |
| Compd. | 1H NMR(400 MHz, DMSO-d6), δ | 13C NMR(100 MHz, DMSO-d6), δ |
|---|---|---|
| 15a | 8.55(d, J=8.4 Hz, 1H), 8.08(d, J=8.2 Hz, 1H), 8.01(t, J=5.5 Hz, 1H), 7.79(dd, J=8.7, 5.6 Hz, 2H), 7.29—7.06(m, 12H), 4.80(d, J=4.3 Hz, 1H), 4.69—4.59(m, 2H), 4.54(t, J=5.7 Hz, 1H), 4.47(dd, J=13.7, 8.3 Hz, 1H), 4.33(d, J=4.5 Hz, 1H), 4.05(d, J=7.0 Hz, 1H), 3.85—3.73(m, 1H), 3.62—3.09(m, 17H), 3.05—2.75(m, 4H) | 171.1, 170.8, 165.2, 162.7, 138.3, 137.6, 130.4, 130.1, 130.0, 129.3, 129.2,128.07, 128.05, 126.3, 126.2, 115.3, 115.1, 103.6, 75.2, 73.5, 70.5, 69.78, 69.75, 69.7, 69.0, 68.2, 67.7, 60.5, 54.9, 54.0, 38.7, 37.9, 36.8 |
| 15b | 8.64(d, J=8.5 Hz, 1H), 8.12—8.05(m, 2H), 8.01(t, J=5.6 Hz, 1H), 7.85(d, J=7.8 Hz, 1H), 7.71(d, J=7.9 Hz, 1H), 7.28—7.08(m, 11H), 4.80(d, J=4.4 Hz, 1H), 4.69—4.60(m, 2H), 4.54(t, J=5.6 Hz, 1H), 4.47(dd, J=13.6, 8.3 Hz, 1H), 4.33(d, J=4.5 Hz, 1H), 4.05(d, J=7.1 Hz, 1H), 3.85—3.75(m, 1H), 3.61—3.10(m, 17H), 3.06—2.76(m, 4H) | 170.9, 170.8, 164.8, 139.9, 138.3, 137.6, 136.0, 135.8, 130.5, 129.3, 129.1, 128.1, 128.0, 126.9, 126.3, 103.6, 94.6, 75.2, 73.5, 70.5, 69.8, 69.7, 69.0, 68.2, 67.7, 60.5, 54.8, 54.0, 38.7, 37.9, 36.8 |
| 15c | 8.58(d, J=8.4 Hz, 1H), 8.10(d, J=8.0 Hz, 1H), 8.01(t, J=5.7 Hz, 1H), 7.77(d, J =8.7 Hz, 2H), 7.52(d, J=8.7 Hz, 2H, 7.21—7.10(m, 7H), 6.75(d, J=8.7 Hz, 2H), 4.81(d, J=4.5 Hz, 1H), 4.68(d, J=5.2 Hz), 4.63—4.56(m, 1H), 4.55(t, J=5.7 Hz, 1H), 4.52—4.48(m, | 171.3, 171.0, 165.4, 157.2, 137.8, 136.4, 132.9, 130.3, 130.2, 129.6, 129.5, 128.5, 128.2, 126.5, 114.1, 103.8, 75.4, 73.7, 70.7,69.98, 69.94, |
| Compd. | 1H NMR(400 MHz, DMSO-d6), δ | 13C NMR(100 MHz, DMSO-d6), δ |
| 15c | 1H), 4.34(d, J=4.5 Hz, 1H), 4.07(d, J=7.3 Hz, 1H), 3.91(q, J=7.0 Hz, 2Ha), 3.85—3.77(m, 1H), 3.62—3.58(m, 1H), 3.57—3.44(m, 11H), 3.39—3.10(m, 4H), 2.99—2.91(m, 2H), 2.82(dd, J=13.8, 9.4 Hz, 2H), 1.25(t, J=7.0 Hz, 3H) | 69.9, 69.2, 68.4, 67.9, 63.0, 60.7, 55.3, 54.2, 39.9, 38.6, 37.9, 36.1 |
| 15d | 7.74(dd, J=8.7, 5.4 Hz, 2H), 7.30—7.08(m, 12H), 4.79(dd, J=9.4, 5.7 Hz, 1H), 4.60(t, J=7.3 Hz, 1H), 4.24(d, J=7.5 Hz, 1H), 4.02—3.95(m, 1H), 3.83—3.22(m, 21H), 3.21—3.05(m, 2H), 3.03—2.85(m, 2H) | 173.3, 173.1, 169.0, 167.5, 165.0, 138.6, 138.2, 131.5, 131.2, 131.1, 130.4, 130.3, 129.5, 129.4, 127.8, 116.4, 116.2, 105.1, 76.7, 74.9, 72.5, 71.6, 71.5, 71.3, 70.4, 70.3, 69.6, 62.5, 56.6, 56.0, 40.4, 39.2, 38.4 |
| 15e | 8.01(s, 1H), 7.87(d, J=8.0 Hz, 1H), 7.65(d, J=8.2 Hz, 1H), 7.28—7.10(m, 11H), 4.79(dd, J=9.4, 5.6 Hz, 1H, 2-H), 4.61(dd, J=8.1, 6.3 Hz, 1H,), 4.24(d, J=7.5 Hz, 1H), 4.03—3.96(m, 1H), 3.85—3.23(m, 21H), 3.21—3.07(m, 2H), 3.01—2.87(m, 2H) | 173.2, 173.1, 168.4, 141.8, 138.6, 138.2, 137.6, 137.2, 131.3, 130.4, 130.3, 129.5, 129.5, 127.8, 105.1, 94.6, 76.7, 74.9, 72.5,71.54, 71.48, 71.3, 70.4, 70.3, 69.6, 62.5, 56.6, 56.0, 40.4, 39.2, 38.3 |
| 15f | 8.60(d, J=8.4 Hz, 1H), 8.12(d, J=8.0 Hz, 1H), 8.02(t, J=5.4 Hz, 1H), 7.79(d, J=8.6 Hz, 2H), 7.53(d, J=8.6 Hz, 2H), 7.24—7.12(m, 7H), 6.77(d, J=8.6 Hz, 2H), 4.83(d, J=4.4 Hz, 1H), 4.70(d, J=5.0 Hz, 1H), 4.66—4.45(m, 3H), 4.36(d, J=4.6 Hz, 1H), 4.08(d, J=7.0 Hz, 1H), 3.93(q, J=7.0 Hz, 2H), 3.88—3.55(m, 2H), 3.59—3.10(m, 20H), 3.03—2.92(m, 2H), 2.90—2.79(m, 2H), 1.27(t, J=7.0 Hz, 3H) | 171.0, 170.7, 165.2, 157.0, 137.5, 136.2, 136.0, 132.7, 130.1, 129.9, 129.3, 129.2, 128.3, 128.0, 126.3, 113.9, 103.6, 75.2, 73.5, 70.5, 69.8, 69.7, 69.6, 68.9, 68.2, 67.7, 62.8, 60.4, 55.1, 54.0, 38.6, 37.9, 36.0, 14.7 |
Table 5 1H NMR and 13C NMR data for the target compounds 15a—15f
| Compd. | 1H NMR(400 MHz, DMSO-d6), δ | 13C NMR(100 MHz, DMSO-d6), δ |
|---|---|---|
| 15a | 8.55(d, J=8.4 Hz, 1H), 8.08(d, J=8.2 Hz, 1H), 8.01(t, J=5.5 Hz, 1H), 7.79(dd, J=8.7, 5.6 Hz, 2H), 7.29—7.06(m, 12H), 4.80(d, J=4.3 Hz, 1H), 4.69—4.59(m, 2H), 4.54(t, J=5.7 Hz, 1H), 4.47(dd, J=13.7, 8.3 Hz, 1H), 4.33(d, J=4.5 Hz, 1H), 4.05(d, J=7.0 Hz, 1H), 3.85—3.73(m, 1H), 3.62—3.09(m, 17H), 3.05—2.75(m, 4H) | 171.1, 170.8, 165.2, 162.7, 138.3, 137.6, 130.4, 130.1, 130.0, 129.3, 129.2,128.07, 128.05, 126.3, 126.2, 115.3, 115.1, 103.6, 75.2, 73.5, 70.5, 69.78, 69.75, 69.7, 69.0, 68.2, 67.7, 60.5, 54.9, 54.0, 38.7, 37.9, 36.8 |
| 15b | 8.64(d, J=8.5 Hz, 1H), 8.12—8.05(m, 2H), 8.01(t, J=5.6 Hz, 1H), 7.85(d, J=7.8 Hz, 1H), 7.71(d, J=7.9 Hz, 1H), 7.28—7.08(m, 11H), 4.80(d, J=4.4 Hz, 1H), 4.69—4.60(m, 2H), 4.54(t, J=5.6 Hz, 1H), 4.47(dd, J=13.6, 8.3 Hz, 1H), 4.33(d, J=4.5 Hz, 1H), 4.05(d, J=7.1 Hz, 1H), 3.85—3.75(m, 1H), 3.61—3.10(m, 17H), 3.06—2.76(m, 4H) | 170.9, 170.8, 164.8, 139.9, 138.3, 137.6, 136.0, 135.8, 130.5, 129.3, 129.1, 128.1, 128.0, 126.9, 126.3, 103.6, 94.6, 75.2, 73.5, 70.5, 69.8, 69.7, 69.0, 68.2, 67.7, 60.5, 54.8, 54.0, 38.7, 37.9, 36.8 |
| 15c | 8.58(d, J=8.4 Hz, 1H), 8.10(d, J=8.0 Hz, 1H), 8.01(t, J=5.7 Hz, 1H), 7.77(d, J =8.7 Hz, 2H), 7.52(d, J=8.7 Hz, 2H, 7.21—7.10(m, 7H), 6.75(d, J=8.7 Hz, 2H), 4.81(d, J=4.5 Hz, 1H), 4.68(d, J=5.2 Hz), 4.63—4.56(m, 1H), 4.55(t, J=5.7 Hz, 1H), 4.52—4.48(m, | 171.3, 171.0, 165.4, 157.2, 137.8, 136.4, 132.9, 130.3, 130.2, 129.6, 129.5, 128.5, 128.2, 126.5, 114.1, 103.8, 75.4, 73.7, 70.7,69.98, 69.94, |
| Compd. | 1H NMR(400 MHz, DMSO-d6), δ | 13C NMR(100 MHz, DMSO-d6), δ |
| 15c | 1H), 4.34(d, J=4.5 Hz, 1H), 4.07(d, J=7.3 Hz, 1H), 3.91(q, J=7.0 Hz, 2Ha), 3.85—3.77(m, 1H), 3.62—3.58(m, 1H), 3.57—3.44(m, 11H), 3.39—3.10(m, 4H), 2.99—2.91(m, 2H), 2.82(dd, J=13.8, 9.4 Hz, 2H), 1.25(t, J=7.0 Hz, 3H) | 69.9, 69.2, 68.4, 67.9, 63.0, 60.7, 55.3, 54.2, 39.9, 38.6, 37.9, 36.1 |
| 15d | 7.74(dd, J=8.7, 5.4 Hz, 2H), 7.30—7.08(m, 12H), 4.79(dd, J=9.4, 5.7 Hz, 1H), 4.60(t, J=7.3 Hz, 1H), 4.24(d, J=7.5 Hz, 1H), 4.02—3.95(m, 1H), 3.83—3.22(m, 21H), 3.21—3.05(m, 2H), 3.03—2.85(m, 2H) | 173.3, 173.1, 169.0, 167.5, 165.0, 138.6, 138.2, 131.5, 131.2, 131.1, 130.4, 130.3, 129.5, 129.4, 127.8, 116.4, 116.2, 105.1, 76.7, 74.9, 72.5, 71.6, 71.5, 71.3, 70.4, 70.3, 69.6, 62.5, 56.6, 56.0, 40.4, 39.2, 38.4 |
| 15e | 8.01(s, 1H), 7.87(d, J=8.0 Hz, 1H), 7.65(d, J=8.2 Hz, 1H), 7.28—7.10(m, 11H), 4.79(dd, J=9.4, 5.6 Hz, 1H, 2-H), 4.61(dd, J=8.1, 6.3 Hz, 1H,), 4.24(d, J=7.5 Hz, 1H), 4.03—3.96(m, 1H), 3.85—3.23(m, 21H), 3.21—3.07(m, 2H), 3.01—2.87(m, 2H) | 173.2, 173.1, 168.4, 141.8, 138.6, 138.2, 137.6, 137.2, 131.3, 130.4, 130.3, 129.5, 129.5, 127.8, 105.1, 94.6, 76.7, 74.9, 72.5,71.54, 71.48, 71.3, 70.4, 70.3, 69.6, 62.5, 56.6, 56.0, 40.4, 39.2, 38.3 |
| 15f | 8.60(d, J=8.4 Hz, 1H), 8.12(d, J=8.0 Hz, 1H), 8.02(t, J=5.4 Hz, 1H), 7.79(d, J=8.6 Hz, 2H), 7.53(d, J=8.6 Hz, 2H), 7.24—7.12(m, 7H), 6.77(d, J=8.6 Hz, 2H), 4.83(d, J=4.4 Hz, 1H), 4.70(d, J=5.0 Hz, 1H), 4.66—4.45(m, 3H), 4.36(d, J=4.6 Hz, 1H), 4.08(d, J=7.0 Hz, 1H), 3.93(q, J=7.0 Hz, 2H), 3.88—3.55(m, 2H), 3.59—3.10(m, 20H), 3.03—2.92(m, 2H), 2.90—2.79(m, 2H), 1.27(t, J=7.0 Hz, 3H) | 171.0, 170.7, 165.2, 157.0, 137.5, 136.2, 136.0, 132.7, 130.1, 129.9, 129.3, 129.2, 128.3, 128.0, 126.3, 113.9, 103.6, 75.2, 73.5, 70.5, 69.8, 69.7, 69.6, 68.9, 68.2, 67.7, 62.8, 60.4, 55.1, 54.0, 38.6, 37.9, 36.0, 14.7 |
| Compd. | Inhibition ratio(%) | ||
|---|---|---|---|
| 12.5 μg/mL | 25 μg/mL | 50 μg/mL | |
| 15a | 19.08±0.71 | 40.61±2.13 | 43.88±0.55 |
| 15b | 65.57±2.41 | 70.27±7.73 | 72.24±2.07 |
| 15c | 29.89±1.68 | 25.60±1.04 | 56.49±0.73 |
| 15d | 16.48±3.66 | 29.19±1.90 | 41.28±1.63 |
| 15e | 11.04±0.19 | 12.54±2.60 | 33.05±1.00 |
| 15f | 25.07±0.73 | 16.97±1.12 | 65.24±2.23 |
| Lamivudine | 92.57±1.03 | ||
Table 6 Inhibition ratios for the replication of HBV DNA of compounds 15a—15f*
| Compd. | Inhibition ratio(%) | ||
|---|---|---|---|
| 12.5 μg/mL | 25 μg/mL | 50 μg/mL | |
| 15a | 19.08±0.71 | 40.61±2.13 | 43.88±0.55 |
| 15b | 65.57±2.41 | 70.27±7.73 | 72.24±2.07 |
| 15c | 29.89±1.68 | 25.60±1.04 | 56.49±0.73 |
| 15d | 16.48±3.66 | 29.19±1.90 | 41.28±1.63 |
| 15e | 11.04±0.19 | 12.54±2.60 | 33.05±1.00 |
| 15f | 25.07±0.73 | 16.97±1.12 | 65.24±2.23 |
| Lamivudine | 92.57±1.03 | ||
| [1] | Li G. , Viral Hepatitis , People’s Medical Publishing House, Beijing, 2002, 22— 41 |
| ( 李刚. 病毒性肝炎, 北京: 人卫出版社, 2002, 22— 41) | |
| [2] |
Zoulim, F. , Liver Int., 2013, 31( S1), 111- 116
doi: 10.1111/liv.12069 URL pmid: 23286855 |
| [3] |
Feng D., R. , Liang, S. , Wang Y., Y. , Xu Y., J. , Chem. Res. Chinese Universities, 2014, 30( 5), 749- 754
doi: 10.1016/j.biomaterials.2014.03.014 URL pmid: 24685267 |
| [4] |
Pardo, M. , Bartolome, J. , Carreno, V. , Arch. Med. Res., 2007, 38( 6), 661- 677
doi: 10.1016/j.arcmed.2006.12.013 URL pmid: 17613358 |
| [5] |
刘玉明, 梁光义, 徐必学. 天然产物研究与开发, 2003, 15( 1), 15- 17
doi: 10.3969/j.issn.1001-6880.2003.01.005 URL |
|
Liu Y., M. , Liang G., Y. , Xu B., X. , Nat. Prod. Res. Dev., 2003, 15( 1), 15- 17
doi: 10.3969/j.issn.1001-6880.2003.01.005 URL |
|
| [6] | Xu B., X. , Huang Z., M. , Liu C., X. , Cai Z., G. , Pan W., D. , Cao P., X. , Hao X., J. , Liang G., Y. , Bioorg. Med. Chem., 2009, 17( 8), 3118- 3125 |
| [7] | Qiu J., Y. , Xu B., X. , Huang Z., M. , Pan W., D. , Cao P., X. , Liu C., X. , Hao X., J. , Song B. A.. Liang G., Y. , Bioorg. Med. Chem., 2011, 19( 18), 5352- 5360 |
| [8] | 邱净英, 黄正明, 潘卫东, 曹佩雪, 梁光义. 中国药科大学学报, 2012, 43( 5), 390- 394 |
| Qiu J., Y. , Huang Z., M. , Pan W., D. , Cao P., X. , Liang G., Y. , J. Chin. Pharm. Univ., 2012, 43( 5), 390- 394 | |
| [9] |
梁光平, 胡占兴, 刘青川, 黄正明, 张建新, 梁光义, 徐必学. 高等学校化学学报, 2014, 35( 11), 2353- 2359
doi: 10.7503/cjcu20140223 |
|
Liang G., P. , Hu Z., X. , Liu Q., C. , Huang Z., M. , Zhang J., X. , Liang G., Y. , Xu B., X. , Chem. J. Chinese Universities, 2014, 35( 11), 2353- 2359
doi: 10.7503/cjcu20140223 |
|
| [10] | Ashwell, G. , Harford, J. , Annu. Rev. Biochem., 1982, 51( 4), 531- 554 |
| [11] | Morell A., G. , Gregoriadis, G. , Scheinberg I., H. , Hickman, J. , Ashwell, G. , J. Biol. Chem., 1971, 246( 5), 1461- 1467 |
| [12] |
Steirer L., M. , Park E., I. , Townsend R., R. , Jacques U., B. , J. Biol. Chem., 2009, 284( 6), 3777- 3783
doi: 10.1074/jbc.M808689200 URL pmid: 19075021 |
| [13] |
Hashida, M. , Nishikawa, M. , Takakura, Y. , J. Controlled Release, 1995, 36( 12), 99- 107
doi: 10.1016/0168-3659(95)00050-I URL |
| [14] | Wang Y., Q. , Su, J. , Cai W., W. , Lu, P. , Yuan L., F. , Jin, T. , Chen S., Y. , Sheng, J. , Drug, Design , Development and Therapy, 2013, 7( 3), 211- 221 |
| [15] |
吴卫, 程怡, 吴琼. 南京医科大学学报, 2012, 32( 2), 168- 171
doi: 10.7655 URL |
|
Wu, W. , Cheng, Y. , Wu, Q. , J. Nanjing. Med. Univ., 2012, 32( 2), 168- 171
doi: 10.7655 URL |
|
| [16] |
吴超, 郭伟英. 辽宁医学院学报, 2008, 29( 6), 490- 491
doi: 10.3969/j.issn.1674-0424.2008.06.004 URL |
|
Wu, C. , Guo W., Y. , J. Liaoning Med. Univ., 2008, 29( 6), 490- 491
doi: 10.3969/j.issn.1674-0424.2008.06.004 URL |
|
| [17] |
袁洁, 刘青川, 徐广灿, 胡占兴, 梁光平, 黄正明, 刘昌孝, 梁光义, 徐必学. 有机化学, 2015, 35( 10), 2176- 2183
doi: 10.6023/cjoc201505011 |
|
Yuan, J. , Liu Q., C. , Xu G., C. , Hu Z., X. , Liang G., P. , Huang Z., M. , Liu C., X. , Liang G., Y. , Xu B., X. , Chin. J. Org. Chem., 2015, 35( 10), 2176- 2183
doi: 10.6023/cjoc201505011 |
|
| [18] | Lee Y., C. , Lee R., T. , Carbohydrates in Chemistry and, Biology , Wiley-VCH, Weinheim, 2000, 549-592 |
| [19] | Biessen E., A. , Beuting D., M. , Roelen H., C. , Marel G. A. V., D. , Boom J. H., V. , Berkel T. J., V. , J. Med. Chem., 1995, 38( 9), 1538- 1546 |
| [20] |
Dean, B. , Oguchi, H. , Cai, S. , Otsuji, E. , Tashiro, K. , Hakomori, S. , Toyokuni, T. , Carbohydr. Res., 1993, 245( 2), 175- 192
doi: 10.1016/0008-6215(93)80071-L URL pmid: 8370021 |
| [21] |
徐广灿, 刘青川, 袁洁, 胡占兴, 马芳芳, 梁光义, 徐必学. 有机化学, 2016, 36( 7), 1617- 1625
doi: 10.6023/cjoc201512051 |
|
Xu G., C. , Liu Q., C. , Yuan, J. , Hu Z., X. , Ma F., F. , Liang G., Y. , Xu B., X. , J. Org. Chem., 2016, 36( 7), 1617- 1625
doi: 10.6023/cjoc201512051 |
|
| [22] | Percec, V. , Leowanawat, P. , Sun H., J. , Kulikov, O. , Nusbaum C., D. , Tran T., M. , Bertin, A. , Wilson D., A. , Peterca, M. , Zhang S., D. , Kamat N., P. , Vargo, K. , Moock, D. , Johnston E., D. , Hammer D., A. , Pochan D., J. , Chen Y., X. , Chabre Y., M. , Shiao T., C. , Bergeron-Blerk, M. , André, S. , Roy, R. , Gabius H., J. , Heiney P., A. , J. Am. Chem. Soc., 2013, 135( 24), 9055- 9077 |
| [23] | Zhang S., D. , Moussodia R., O , Sun H., J. , Leowanawat, P. , Muncan, A. , Nusbaum C., D. , Chelling K., M. , Heiney P., A. , Klein M., L. , Andre, S. , Roy, R. , Gabius H., J. , Perec, V. , Angew. Chem. Int. Ed., 2014, 53( 41), 10899- 10903 |
| [24] |
Ligeour, C. , Dupin, L. , Marra, A. , Eur. J. Org. Chem., 2014, 2014( 34), 7621- 7630
doi: 10.1002/ejoc.201402902 URL |
| [25] | Li Y., X. , Wang F., J. , Chen, W. , Wan Y., Y. , Li Z., M. , Chem. Res. Chinese Universities, 2015, 31( 6), 952- 957 |
| [26] |
Alker, D. , Arrowsmith J., E. , Eur. J. Med. Chem., 1991, 26( 91), 907- 913
doi: 10.1016/0223-5234(91)90132-7 URL |
| [27] |
Chen, N. , Xie, J. , J. Org. Chem., 2014, 79( 21), 10716- 10724
doi: 10.1021/jo502128h URL pmid: 25318074 |
| [28] | Cai M. S., Li Z. J., Carbohydrate Chemistry: Fundamentals, Reactions, Synthesis, Isolation and Structure, Chemical Industry Press, Beijing, 2006, 370— 373 |
| ( 蔡孟深, 李中军. 糖化学 基础、 反应、 合成、 分离及结构 , 北京: 化学工业出版社, 2006, 370— 373) |
| [1] | 赵军强, 闫彩霞, 陈泽, 杨宁, 冯霞, 赵义平, 陈莉. 细胞内氧化还原响应型肝靶向嵌段聚合物的合成及纳米自组装性能[J]. 高等学校化学学报, 2018, 39(7): 1592. |
| [2] | 袁洁, 刘青川, 徐广灿, 梁光义, 徐必学. 基于点击化学反应的半乳糖糖基化马蹄金素衍生物的合成及抗HBV活性[J]. 高等学校化学学报, 2016, 37(7): 1307. |
| [3] | 王静云, 付雪, 包永明. 原子转移自由基聚合(ATRP)阳离子化菊粉作为非病毒基因载体的研究[J]. 高等学校化学学报, 2014, 35(10): 2124. |
| [4] | 朱建勤, 陈晖, 王少戎, 李燕, 方唯硕. 熊果酸半乳糖苷偶联物的合成及保肝活性[J]. 高等学校化学学报, 2013, 34(7): 1660. |
| [5] | 于雷, 华小辉, 翟延君, 杨丽敏, 赵国忠, 孟田华, 翁诗甫, 徐怡庄, 刘克新, 吴瑾光, 陈佳洱. 两种半乳糖醇氯化钆配合物的制备与表征[J]. 高等学校化学学报, 2011, 32(6): 1244. |
| [6] | 吕丰, 李艳周, 武莉, 刘天军. 用于肿瘤成像的半乳糖/酞菁近红外荧光探针[J]. 高等学校化学学报, 2011, 32(5): 1010. |
| [7] | 高遐 张馥 陈志春 林贤福. 含乳糖聚电解质靶向微胶囊的层层自组装构筑[J]. 高等学校化学学报, 2011, 32(4): 957. |
| [8] | 黄微 王平 王蔚 张玥 张闯年 田秦 王秀华 刘媛 袁直. 甘草次酸修饰PEG-PLGA纳米粒的制备及与肝癌细胞亲和性[J]. 高等学校化学学报, 2011, 32(2): 416. |
| [9] | 王富强, 郝俊霞, 戴小军, 龚波林. 原子转移自由基聚合制备新型接枝聚α-D-吡喃半乳糖苷色谱固定相及其性能[J]. 高等学校化学学报, 2011, 32(12): 2757. |
| [10] | 贺晓凌, 董友玉, 聂萍萍, 冯霞, 陈莉. 聚苯乙烯细胞培养板表面的糖化温敏修饰及其对细胞行为的影响[J]. 高等学校化学学报, 2009, 30(12): 2502. |
| [11] | 查瑞涛,贺晓婷,杜田,袁直 . 肝靶向甘草次酸修饰的壳聚糖纳米粒子的合成和表征[J]. 高等学校化学学报, 2007, 28(6): 1098. |
| [12] | 王银松,韩月莲,李英霞,王玉玫,李荣珊 . 甲氨喋呤-乳糖酰基壳聚糖的制备及其体外实验[J]. 高等学校化学学报, 2007, 28(6): 1092. |
| [13] | 查瑞涛, 杜田, 袁直 . 端基为肝靶向基团的聚谷氨酸苄酯的合成及表征[J]. 高等学校化学学报, 2006, 27(5): 885. |
| [14] | 杨丽敏, 赵莹, 苏允兰, 王哲明, 严纯华, 吴瑾光. 半乳糖醇与氯化稀土配合物的合成及荧光光谱研究[J]. 高等学校化学学报, 2002, 23(8): 1475. |
| [15] | 丁雄军, 卓仁禧, 付功成. 二乙三胺五乙酸吡哆醇酯配体及其钆配合物的合成、弛豫率和肝靶向性研究[J]. 高等学校化学学报, 2002, 23(1): 49. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||