

高等学校化学学报 ›› 2015, Vol. 36 ›› Issue (12): 2540.doi: 10.7503/cjcu20150285
王玉春1,2, 郑华艳1, 刘斌1, 张国强1, 李忠1( )
)
收稿日期:2015-04-13
出版日期:2015-12-10
发布日期:2015-10-12
作者简介:联系人简介: 李 忠, 男, 博士, 教授, 博士生导师, 主要从事多相催化研究. E-mail:基金资助:
WANG Yuchun1,2, ZHENG Huayan1, LIU Bin1, ZHANG Guoqiang1, LI Zhong1,*( )
)
Received:2015-04-13
Online:2015-12-10
Published:2015-10-12
Contact:
LI Zhong
E-mail:lizhong@tyut.edu.cn
Supported by:摘要:
以乙酰丙酮铜Cu(acac)2为铜源、 NH4Y分子筛为载体, 采用固相反应制备了无氯CuY催化剂, 考察了在甲醇氧化羰基化合成碳酸二甲酯过程中固相反应温度和Cu负载量对CuY催化剂催化性能的影响, 分析了CuY催化剂物相结构、 可还原性Cu物种和织构性质对催化性能的影响. 结果表明, 随着固相反应温度的升高, 与NH4Y中N
中图分类号:
TrendMD:
王玉春, 郑华艳, 刘斌, 张国强, 李忠. 固相反应制备无氯CuY催化剂及其催化氧化羰基化—固相反应温度和铜负载量的影响. 高等学校化学学报, 2015, 36(12): 2540.
WANG Yuchun, ZHENG Huayan, LIU Bin, ZHANG Guoqiang, LI Zhong. Chloride-free CuY Catalyst Prepared by Solid State Reaction for Oxidative Carbonylation of Methanol† — Effect of Solid State Reactive Temperature and Copper Loading. Chem. J. Chinese Universities, 2015, 36(12): 2540.
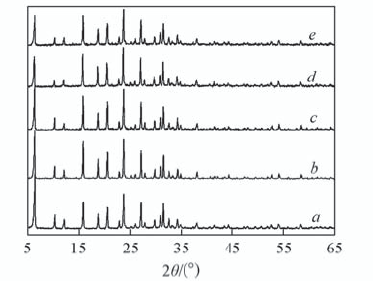
Fig.1 XRD patterns of NH4Y and catalysts 10CuY with different temperature of solid state reaction(SSR) a. NH4Y; b. 10CuY170; c.10CuY200; d. 10CuY250;e. 10CuY280.
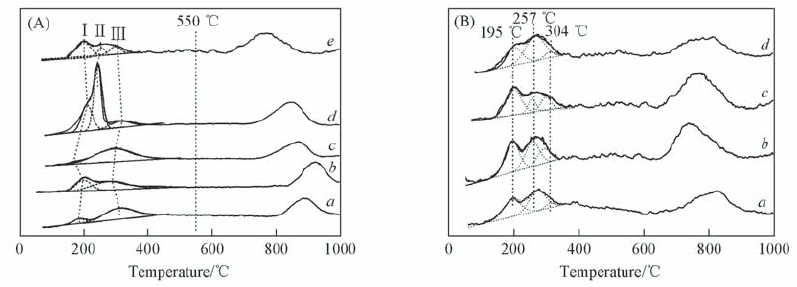
Fig.2 H2-TPR patterns and Gaussian fitting of low temperature peaks (A) a. Cu2+-Y(6.3); b. CuY(6.3); c. 6CuY250; d. CuY(10.6); e. 10CuY250.(B) a. 10CuY170; b. 10CuY200; c. 10CuY250; d. 10CuY280.
| Catalyst | Mass fraction(%) | ||||
|---|---|---|---|---|---|
| Cu2+(Supercage) | Cu2+(Sodalite cage) | CuO | Cu+ | Cusum | |
| 10CuY170 | 2.99 | 0.84 | 2.36 | 3.30 | 9.49 |
| 10CuY200 | 3.30 | 1.39 | 1.31 | 3.39 | 9.39 |
| 10CuY250 | 3.38 | 1.40 | 0.98 | 3.76 | 9.52 |
| 10CuY280 | 2.78 | 0.91 | 2.68 | 3.04 | 9.41 |
Table 1 Cu species content of 10CuYT catalysts
| Catalyst | Mass fraction(%) | ||||
|---|---|---|---|---|---|
| Cu2+(Supercage) | Cu2+(Sodalite cage) | CuO | Cu+ | Cusum | |
| 10CuY170 | 2.99 | 0.84 | 2.36 | 3.30 | 9.49 |
| 10CuY200 | 3.30 | 1.39 | 1.31 | 3.39 | 9.39 |
| 10CuY250 | 3.38 | 1.40 | 0.98 | 3.76 | 9.52 |
| 10CuY280 | 2.78 | 0.91 | 2.68 | 3.04 | 9.41 |
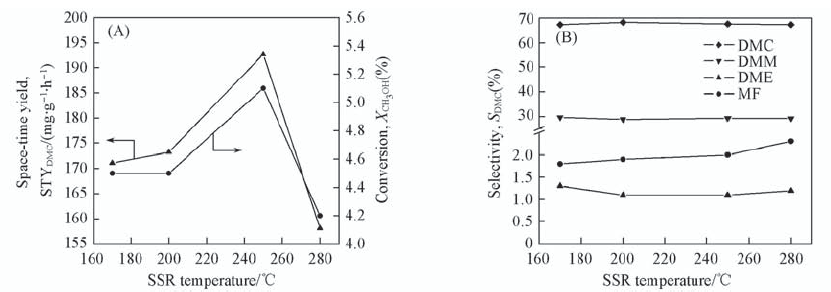
Fig.3 STYDMC and conversion(A) and selectivities of DMC, DME, DMM and MF(B) vs. SSR temperature Feed composition(volume fraction): 31.8%CH3OH, 62.5%CO, 5.7%O2; GHSV 3250 h-1.
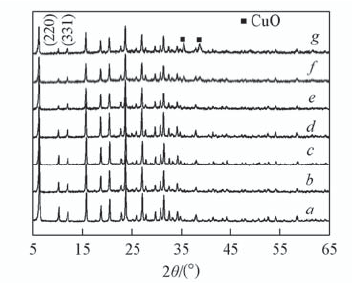
Fig.4 XRD patterns of NH4Y and catalysts with different copper loadings a. NH4Y; b. 6CuY250; c. 8CuY250; d. 10CuY250; e. 12CuY250; f. 15CuY250; g. 20CuY250.
| Catalyst | SBET/(m2·g-1) | Smicro/(m2·g-1) | Smeso/(m2·g-1) | Vmicro/(cm3·g-1) | Vmeso/(cm3·g-1) |
|---|---|---|---|---|---|
| NH4Y | 791.9 | 746.4 | 45.5 | 0.36 | 0.10 |
| 6CuY250 | 737.5 | 690.9 | 46.7 | 0.34 | 0.13 |
| 8CuY250 | 718.0 | 668.4 | 49.6 | 0.33 | 0.18 |
| 10CuY250 | 705.0 | 656.3 | 48.9 | 0.32 | 0.12 |
| 12CuY250 | 560.3 | 514.2 | 46.1 | 0.26 | 0.12 |
| 15CuY250 | 549.6 | 507.9 | 41.7 | 0.25 | 0.12 |
| 20CuY250 | 530.1 | 481.9 | 48.2 | 0.24 | 0.12 |
Table 2 Specific surface area and pore volume of catalysts with different Cu loadings*
| Catalyst | SBET/(m2·g-1) | Smicro/(m2·g-1) | Smeso/(m2·g-1) | Vmicro/(cm3·g-1) | Vmeso/(cm3·g-1) |
|---|---|---|---|---|---|
| NH4Y | 791.9 | 746.4 | 45.5 | 0.36 | 0.10 |
| 6CuY250 | 737.5 | 690.9 | 46.7 | 0.34 | 0.13 |
| 8CuY250 | 718.0 | 668.4 | 49.6 | 0.33 | 0.18 |
| 10CuY250 | 705.0 | 656.3 | 48.9 | 0.32 | 0.12 |
| 12CuY250 | 560.3 | 514.2 | 46.1 | 0.26 | 0.12 |
| 15CuY250 | 549.6 | 507.9 | 41.7 | 0.25 | 0.12 |
| 20CuY250 | 530.1 | 481.9 | 48.2 | 0.24 | 0.12 |
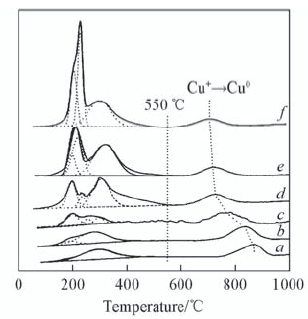
Fig.7 Hydrogen TPR profiles for the catalysts with different copper loadings a. 6CuY250; b. 8CuY250; c. 10CuY250; d. 12CuY250;e. 15CuY250; f. 20CuY250.
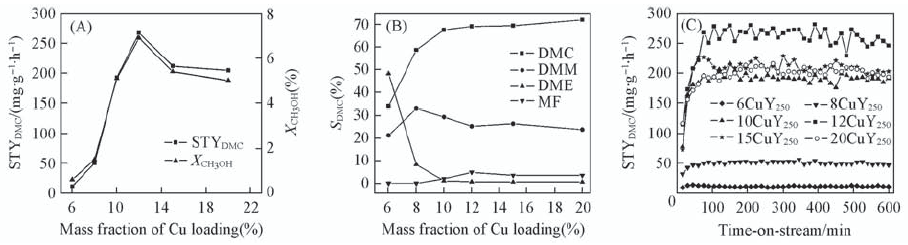
Fig.9 Influence on catalysis of copper loading STYDMC and XCH3OH vs. copper loading(A), selectivities of DMC, DME, DMM and MF vs. copper loading(B) and time-on-stream performance of xCuY250 catalysts(C) Feed composition(volume fraction): 31.8% CH3OH, 62.5% CO, 5.7% O2; GHSV 3250 h-1.
| Catalyst | Preparation method | Mass fraction of Cu loading(%) | STYDMC /(mg·g-1·h-1) | SDMC(%) | |
|---|---|---|---|---|---|
| 6CuY250 | SSR | 6.1 | 11.3a | 33.9 | 0.6 |
| 8CuY250 | SSR | 8.0 | 50.5a | 58.7 | 1.5 |
| 10CuY250 | SSR | 9.5 | 192.7a | 67.6 | 5.1 |
| 12CuY250 | SSR | 11.3 | 267.3a | 69.2 | 6.9 |
| 15CuY250 | SSR | 13.9 | 211.7a | 69.6 | 5.4 |
| 20CuY250 | SSR | 16.1 | 204.5a | 72.1 | 5.0 |
| CuHY-3[ | IEIM | 11.9 | 200.5b | 51.7 | 13.0 |
| CuCl2/HY[ | SSIE | 12.2 | 97.3b | 74.6 | 4.4 |
| Cu2(OH)3Cl/AC[ | DP | 18.7 | 139.1b | 67.3 | 6.9 |
| 12Cu- | DP | 12.0 | 152.1c | 56.3 | 8.4 |
Table 3 Catalytic activities of CuY catalysts in the oxidative carbonylation of methanol
| Catalyst | Preparation method | Mass fraction of Cu loading(%) | STYDMC /(mg·g-1·h-1) | SDMC(%) | |
|---|---|---|---|---|---|
| 6CuY250 | SSR | 6.1 | 11.3a | 33.9 | 0.6 |
| 8CuY250 | SSR | 8.0 | 50.5a | 58.7 | 1.5 |
| 10CuY250 | SSR | 9.5 | 192.7a | 67.6 | 5.1 |
| 12CuY250 | SSR | 11.3 | 267.3a | 69.2 | 6.9 |
| 15CuY250 | SSR | 13.9 | 211.7a | 69.6 | 5.4 |
| 20CuY250 | SSR | 16.1 | 204.5a | 72.1 | 5.0 |
| CuHY-3[ | IEIM | 11.9 | 200.5b | 51.7 | 13.0 |
| CuCl2/HY[ | SSIE | 12.2 | 97.3b | 74.6 | 4.4 |
| Cu2(OH)3Cl/AC[ | DP | 18.7 | 139.1b | 67.3 | 6.9 |
| 12Cu- | DP | 12.0 | 152.1c | 56.3 | 8.4 |
| [1] | Xu J., Long K. Z., Wang Y., Xue B. Li Y. X., Appl. Catal. A,2015, 496, 1—8 |
| [2] | Ren J., Wang D. L., Pei Y. L., Qin Z. F., Lin J. Y., Li Z., Chem. J. Chinese Universities,2013, 34(11), 2594—2600 |
| (任军, 王冬蕾, 裴永丽, 秦志峰, 林建英, 李忠. 高等学校化学学报,2013, 34(11), 2594—2600) | |
| [3] | Keller N., Rebmann G. Keller V., J. Mol. Catal. A,2010, 317(1/2), 1—18 |
| [4] | Wang P., Liu S., Zhou F., Yang B., Alshammari A. S., Lu L. Deng Y., Fuel Process. Technol., 2014, 126, 359—365 |
| [5] | Delledonne D., Rivetti F. Romano U., Appl. Catal. A,2001, 221(1/2), 241—251 |
| [6] | Zhu D., Mei F., Chen L., Li T., Mo W., Li G., Energy Fuels,2009, 23(5), 2359—2363 |
| [7] | Li Z., Wen C. M., Zheng H. Y., Xie K. C., Chem. J. Chinese Universities,2010, 31(1), 145—152 |
| (李忠, 文春梅, 郑华艳, 谢克昌. 高等学校化学学报,2010, 31(1), 145—152) | |
| [8] | Saada R., Kellici S., Heil T., Morgan D. Saha B., Appl. Catal. B,2015, 168/169, 353—362 |
| [9] | Du G. F., Guo H., Wang Y., Li W. J., Shi W. J., Dai B., J. Saudi Chemical Society,2015, 19(1), 112—115 |
| [10] | Nam J. K., Choi M. J., Cho D. H., Suh J. K., Kim S. B., J. Mol. Catal. A,2013, 370, 7—13 |
| [11] | King S. T., J. Catal., 1996, 161(2), 530—538 |
| [12] | King S. T., Catal. Today, 1997, 33(1—3), 173—182 |
| [13] | Li Z., Fu T.J., Zheng H. Y.,Chinese J. Inorg. Chem., 2011, (8), 1483—1490 |
| (李忠, 付廷俊, 郑华艳. 无机化学学报, 2011, (8), 1483—1490) | |
| [14] | Li Z., Fu T. J., Wang R. Y., Niu Y. Y., Zheng H. Y., Chem. J. Chinese Universities,2011, 32(6), 1366—1372 |
| (李忠, 付廷俊, 王瑞玉, 牛燕燕, 郑华艳. 高等学校化学学报,2011, 32(6), 1366—1372) | |
| [15] | Richter M., Fait M. J. G., Eckelt R., Schreier E., Schneider M., Pohl M. M., Fricke R., Appl. Catal. B,2007, 73(3/4), 269—281 |
| [16] | Richter M., Fait M. J. G., Eckelt R., Schneider M., Radnik J., Heidemann D., Fricke R., J. Catal., 2007, 245(1), 11—24 |
| [17] | Drake I. J., Zhang Y., Briggs D., Lim B., Chau T., Bell A. T., J. Phys. Chem. B,2006, 110(24), 11654—11664 |
| [18] | Zhang Y. Bell A. T., J. Catal., 2008, 255 (2), 153—161 |
| [19] | Zheng H., Qi J., Zhang R., Li Z., Wang B., Ma X., Fuel Process. Technol., 2014, 128, 310—318 |
| [20] | Zhang Y. Q., Zheng H. Y., Zhang R. G., Li Z., Wang B. J., Zhao Q. Y., Chem. J. Chinese Universities,2015, 36(10), 1945—1953 |
| (张艳表, 郑华艳, 章日光, 李忠, 王宝俊, 赵秋男. 高等学校化学学报,2015, 36(10), 1945—1953) | |
| [21] | Engeldinger J., Domke C., Richter M., Bentrup U., Appl. Catal. A,2010, 382(2), 303—311 |
| [22] | Engeldinger J., Richter M. Bentrup U., Phys. Chem. Chem. Phys., 2012, 14(7), 2183—2191 |
| [23] | Ribeiro da Silva M. A. V., Monte M. J. S. Huinink J., J. Chem. Thermodynamics,1995, 27(2), 175—190 |
| [24] | Goel P., Duragasi G. Singh J. P.,J. Mater. Sci., 2013, 48(14), 4876—4882 |
| [25] | Nasibulin A. G., Richard O., Kauppinen E. I., Brown D. P., Jokiniemi J. K. Altman I. S., Aerosol Sci. Technol., 2002, 36(8), 899—911 |
| [26] | Sun S., Wu Y. Y., Luo S. Z., Chu W., Ni H. Z., Chem. J. Chinese Universities,2011, 32(8), 1794—1798 |
| (孙思, 吴永永, 罗仕忠, 储伟, 倪宏志. 高等学校化学学报,2011, 32(8), 1794—1798) | |
| [27] | Fu T.J., Zheng H. Y., Niu Y.Y., Wang R. Y., Li Z.,Acta Chimica Sinica, 2011, (15), 1765—1772 |
| (付廷俊, 郑华艳, 牛燕燕, 王瑞玉, 李忠. 化学学报, 2011, (15), 1765—1772) | |
| [28] | Bulánek R., Wichterlová B., Sobalík Z., Tichy J., Appl. Catal. B,2001. 31(1), 13—25 |
| [29] | Sultana A., Nanba T., Sasaki M., Haneda M., Suzuki K., Hamada H., Catal. Today,2011, 164(1), 495—499 |
| [30] | Kieger S., Delahay G., Coq B., Neveu B., J. Catal., 1999, 183(2), 267—280 |
| [31] | Torre-Abreu C., Henriques C., Ribeiro F. R., Delahay G., Ribeiro M. F., Catal. Today,1999, 54(4), 407—418 |
| [32] | Song H., Wan X., Dai M., Zhang J., Li F., Song H., Fuel Process. Technol., 2013, 116, 52—62 |
| [33] | Antunes A. P., Ribeiro M. F., Silva J. M., Ribeiro F. R., Magnoux P., Guisnet M., Appl. Catal. B,2001, 33(2), 149—164 |
| [34] | Wang R.Y., Li Z.,J. Fuel Chem. Technol., 2013, (11), 1361—1366 |
| (王瑞玉, 李忠. 燃料化学学报, 2013, (11), 1361—1366) | |
| [35] | Alonso F., Melkonian T., Moglie Y., Yus M., Eur. J. Org. Chem., 2011, 2011(13), 2524—2530 |
| [36] | Li X., Zhang X., Lei L., Sep. Purif. Technol., 2009, 64(3), 326—331 |
| [37] | Zahmakıran M., Durap F., Özkar S., Int. J. Hydrogen Energy,2010, 35(1), 187—197 |
| [38] | Fei J., Hou Z., Zhu B., Lou H. Zheng X., Appl. Catal. A,2006, 304, 49—54 |
| [39] | Berthomieu D., Jardillier N., Delahay G., Coq B. Goursot A., Catal. Today,2005, 110(3/4), 294—302 |
| [40] | Wang J.Z., Study on Controlling Cu Catalytic Active Center and Catalytic Performance of CuY Catalyst, Taiyuan University of Technology, 2013 |
| (王佳臻. CuY催化剂中Cu活性中心调控及催化性能的研究, 太原: 太原理工大学, 2013 ) | |
| [41] | Li Z., Wang R. Y., Zheng H. Y., Xie K. C., Fuel, 2010, 89(7), 1339—1343 |
| [42] | Wang R.Y., Li Z., Zheng H. Y., Xie K. C.,Chinese. J. Catal., 2009, (10), 1068—1072 |
| (王瑞玉, 李忠, 郑华艳, 谢克昌.催化学报, 2009, (10), 1068—1072) |
| [1] | 王蔓, 王鑫, 周静, 高国华. 聚离子液体催化碳酸乙烯酯与甲醇的酯交换反应[J]. 高等学校化学学报, 2021, 42(12): 3701. |
| [2] | 张国强, 孙宇辰, 史亚波, 郑华艳, 李忠, 上官炬, 刘守军, 史鹏政. Ce1-xMnxO2表面性质对催化CO2和甲醇直接合成DMC反应活性的影响[J]. 高等学校化学学报, 2020, 41(9): 2061. |
| [3] | 尹娇, 张国强, 阎立飞, 贾东森, 郑华艳, 李忠. 反应过程中Cu物种演变对其催化甲醇氧化羰基化反应活性的影响[J]. 高等学校化学学报, 2019, 40(7): 1510. |
| [4] | 张艳青, 郑华艳, 章日光, 李忠, 王宝俊, 赵秋勇. 碱金属阳离子对Cu+Y催化甲醇氧化羰基化性能影响的密度泛函理论研究[J]. 高等学校化学学报, 2015, 36(10): 1945. |
| [5] | 郑华艳, 章日光, 李忠. 甲醇氧化羰基化反应中CO和CH3O在CuCl(111)表面上吸附作用的理论研究[J]. 高等学校化学学报, 2014, 35(9): 1926. |
| [6] | 任军, 王冬蕾, 裴永丽, 秦志峰, 林建英, 李忠. 助剂含量对CuLi/AC 催化剂结构及甲醇氧化羰基化反应性能的影响[J]. 高等学校化学学报, 2013, 34(11): 2594. |
| [7] | 裴义霞, 李会泉, 柳海涛, 张懿. 铅化合物催化二氨基二苯甲烷与碳酸二甲酯反应机理的红外光谱研究[J]. 高等学校化学学报, 2012, 33(03): 598. |
| [8] | 杜治平, 肖艳华, 王公应. 二丁基锡磺酸酯催化酯交换合成碳酸二苯酯[J]. 高等学校化学学报, 2009, 30(9): 1798. |
| [9] | 李忠, 文春梅, 王瑞玉, 郑华艳, 谢克昌. 醋酸铜热解制备无氯Cu2O/AC催化剂及其催化氧化羰基化[J]. 高等学校化学学报, 2009, 30(10): 2024. |
| [10] | 于琴琴, 王庶, 白荣献, 梅付名, 李光兴. Zn-Al水滑石催化碳酸二甲酯与苯酚酯交换反应的研究[J]. 高等学校化学学报, 2005, 26(8): 1502. |
| [11] | 钟顺和, 程庆彦, 黎汉生. 负载型Sn2(OMe)2Cl2/SiO2催化剂的制备、表征与催化合成碳酸二甲酯[J]. 高等学校化学学报, 2003, 24(1): 125. |
| [12] | 王少成, 曹勇, 杨平, 胡建国, 吴东, 孙予罕, 邓景发. TBAB修饰的负载型Wacker催化剂催化甲醇羰基化合成碳酸二甲酯[J]. 高等学校化学学报, 2002, 23(12): 2363. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||