

高等学校化学学报 ›› 2015, Vol. 36 ›› Issue (5): 932.doi: 10.7503/cjcu20141136
收稿日期:2014-12-29
出版日期:2015-05-10
发布日期:2015-04-14
作者简介:联系人简介: 王长生, 男, 博士, 教授, 博士生导师, 主要从事理论与计算化学研究. E-mail: 基金资助:
WANG Xiaowen, LI Shuang, JIANG Xiaonan, WANG Changsheng*( )
)
Received:2014-12-29
Online:2015-05-10
Published:2015-04-14
Contact:
WANG Changsheng
E-mail:chwangcs@lnnu.edu.cn
Supported by:摘要:
本文优化得到了16个由槲皮素与腺嘌呤形成的氢键复合物的稳定结构, 并计算了它们的结合能. 研究发现, 在气相和水相中, 槲皮素均通过qu1位点与腺嘌呤作用形成稳定的氢键复合物. 比较了腺嘌呤与槲皮素形成的氢键复合物、 腺嘌呤与胸腺嘧啶形成的Watson-Crick碱基对的相对稳定性. 在气相条件下Watson-Crick碱基对更稳定, 在水相条件下腺嘌呤与槲皮素形成的氢键复合物更稳定, 说明水相条件下腺嘌呤与槲皮素之间的相互作用强于与胸腺嘧啶之间的相互作用. 基于标准反应Gibbs自由能变的计算结果估算了水相条件下腺嘌呤与槲皮素形成的氢键复合物和Watson-Crick碱基对的相对平衡浓度.
中图分类号:
TrendMD:
王晓雯, 李爽, 姜笑楠, 王长生. 槲皮素与腺嘌呤氢键作用的最佳位点. 高等学校化学学报, 2015, 36(5): 932.
WANG Xiaowen, LI Shuang, JIANG Xiaonan, WANG Changsheng. Site-preference of Quercetin Hydrogen Bonding to Adenine†. Chem. J. Chinese Universities, 2015, 36(5): 932.
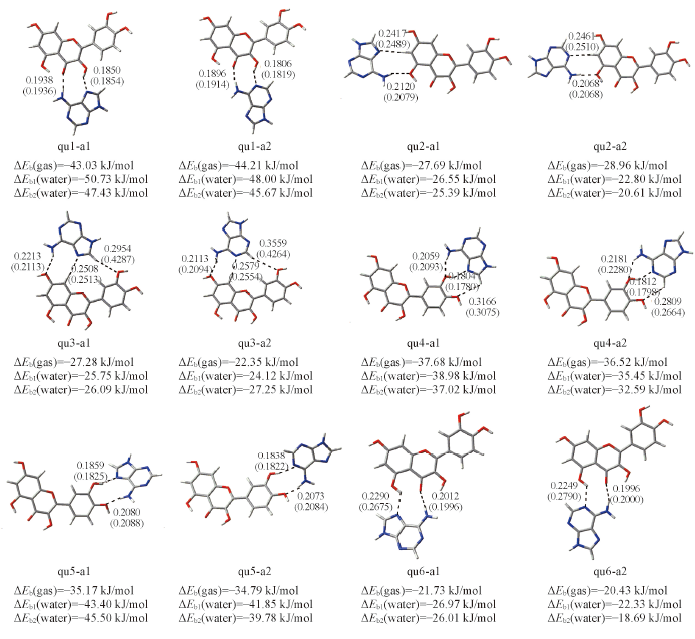
Fig.2 Optimal structures of twelve hydrogen-bonded quercetin-adenine complexesThe hydrogen bond distances in gas phase and in water solvent are given in the corresponding position and in parentheses, respectively. ΔEb(gas) is the binding energy in gas phase. ΔEb1(water) and ΔEb2(water) are the binding energies in water solvent using the optimal structure in gas phase and the optimal structure in water solvent, respectively. The hydrogen bond distance units are in nm.
| Complex | Hydrogen bond | R(Y…H)/nm | ΔE(2)/(kJ·mol-1) | ∑ΔE(2)/(kJ·mol-1) | ρc/a.u. | ∑ρc/a.u. |
|---|---|---|---|---|---|---|
| qu1-a1 | N—H…O═C | 0.1938 | 41.21 | 135.22 | 0.023 | 0.059 |
| O—H…N | 0.1850 | 94.01 | 0.036 | |||
| qu1-a2 | N—H…O═C | 0.1896 | 52.47 | 164.14 | 0.025 | 0.067 |
| O—H…N | 0.1806 | 111.67 | 0.041 | |||
| qu2-a1 | N—H…O | 0.2120 | 27.32 | 45.73 | 0.016 | 0.028 |
| C—H…N | 0.2417 | 18.41 | 0.012 | |||
| qu2-a2 | N—H…O | 0.2068 | 33.39 | 50.42 | 0.017 | 0.029 |
| C—H…N | 0.2461 | 17.03 | 0.011 | |||
| qu3-a1 | N—H…O | 0.2213 | 19.08 | 34.87 | 0.013 | 0.026 |
| C—H…O | 0.2954 | 1.23 | 0.003 | |||
| C—H…N | 0.2508 | 14.56 | 0.010 | |||
| qu3-a2 | N—H…O | 0.2113 | 28.28 | 40.20 | 0.016 | 0.025 |
| C—H…O | 0.3559 | 0.00 | 0.000 | |||
| C—H…N | 0.2579 | 11.92 | 0.008 | |||
| qu4-a1 | N—H…O | 0.2059 | 29.87 | 139.45 | 0.018 | 0.059 |
| C—H…O | 0.3166 | 0.00 | 0.000 | |||
| O—H…N | 0.1804 | 109.58 | 0.041 | |||
| qu4-a2 | N—H…O | 0.2181 | 15.56 | 125.26 | 0.014 | 0.060 |
| C—H…O | 0.2809 | 1.17 | 0.005 | |||
| O—H…N | 0.1812 | 108.53 | 0.041 | |||
| qu5-a1 | O—H…N | 0.1859 | 89.12 | 122.84 | 0.035 | 0.053 |
| N—H…O | 0.2080 | 33.72 | 0.018 | |||
| qu5-a2 | O—H…N | 0.1838 | 98.53 | 131.58 | 0.038 | 0.057 |
| N—H…O | 0.2073 | 33.05 | 0.018 | |||
| qu6-a1 | N—H…O═C | 0.2012 | 32.59 | 55.60 | 0.019 | 0.033 |
| O—H…N | 0.2290 | 23.01 | 0.014 | |||
| qu6-a2 | N—H…O═C | 0.1996 | 36.11 | 61.42 | 0.019 | 0.035 |
| O—H…N | 0.2249 | 25.31 | 0.016 |
Table 1 n→σ* second-order stabilization energies ΔE(2) obtained at the B3LYP/6-31G(d,p) level and the electron densities(ρc) at the hydrogen bond critical points obtained at the B3LYP/6-311+G(d,p) level
| Complex | Hydrogen bond | R(Y…H)/nm | ΔE(2)/(kJ·mol-1) | ∑ΔE(2)/(kJ·mol-1) | ρc/a.u. | ∑ρc/a.u. |
|---|---|---|---|---|---|---|
| qu1-a1 | N—H…O═C | 0.1938 | 41.21 | 135.22 | 0.023 | 0.059 |
| O—H…N | 0.1850 | 94.01 | 0.036 | |||
| qu1-a2 | N—H…O═C | 0.1896 | 52.47 | 164.14 | 0.025 | 0.067 |
| O—H…N | 0.1806 | 111.67 | 0.041 | |||
| qu2-a1 | N—H…O | 0.2120 | 27.32 | 45.73 | 0.016 | 0.028 |
| C—H…N | 0.2417 | 18.41 | 0.012 | |||
| qu2-a2 | N—H…O | 0.2068 | 33.39 | 50.42 | 0.017 | 0.029 |
| C—H…N | 0.2461 | 17.03 | 0.011 | |||
| qu3-a1 | N—H…O | 0.2213 | 19.08 | 34.87 | 0.013 | 0.026 |
| C—H…O | 0.2954 | 1.23 | 0.003 | |||
| C—H…N | 0.2508 | 14.56 | 0.010 | |||
| qu3-a2 | N—H…O | 0.2113 | 28.28 | 40.20 | 0.016 | 0.025 |
| C—H…O | 0.3559 | 0.00 | 0.000 | |||
| C—H…N | 0.2579 | 11.92 | 0.008 | |||
| qu4-a1 | N—H…O | 0.2059 | 29.87 | 139.45 | 0.018 | 0.059 |
| C—H…O | 0.3166 | 0.00 | 0.000 | |||
| O—H…N | 0.1804 | 109.58 | 0.041 | |||
| qu4-a2 | N—H…O | 0.2181 | 15.56 | 125.26 | 0.014 | 0.060 |
| C—H…O | 0.2809 | 1.17 | 0.005 | |||
| O—H…N | 0.1812 | 108.53 | 0.041 | |||
| qu5-a1 | O—H…N | 0.1859 | 89.12 | 122.84 | 0.035 | 0.053 |
| N—H…O | 0.2080 | 33.72 | 0.018 | |||
| qu5-a2 | O—H…N | 0.1838 | 98.53 | 131.58 | 0.038 | 0.057 |
| N—H…O | 0.2073 | 33.05 | 0.018 | |||
| qu6-a1 | N—H…O═C | 0.2012 | 32.59 | 55.60 | 0.019 | 0.033 |
| O—H…N | 0.2290 | 23.01 | 0.014 | |||
| qu6-a2 | N—H…O═C | 0.1996 | 36.11 | 61.42 | 0.019 | 0.035 |
| O—H…N | 0.2249 | 25.31 | 0.016 |
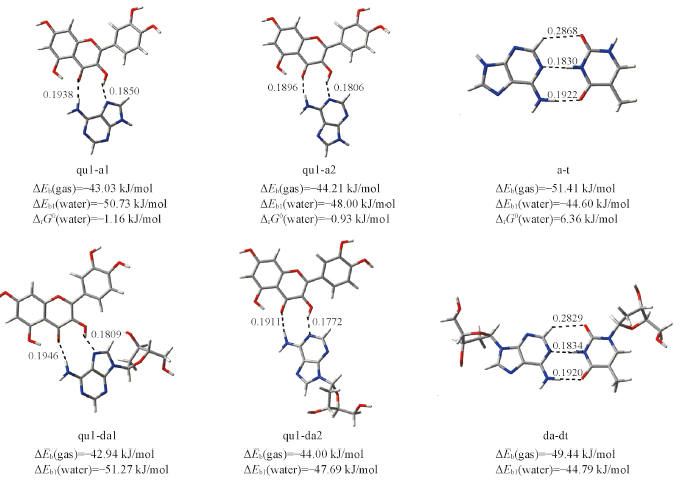
Fig.3 Optimal structures of six hydrogen-bonded complexesThe hydrogen bond distances are given in the corresponding position. ΔEb(gas) and ΔEb1(water) are the binding energies in gas phase and in water solvent using the optimal structure in gas phase. ΔrG0(water) is the standard Gibbs free energy change in water solvent using the optimal structure in water solvent. The bond distance units are in nm.
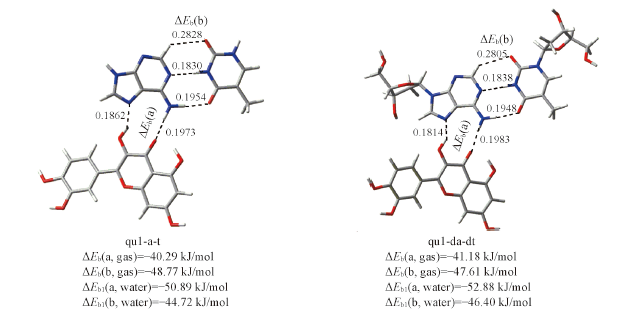
Fig.4 Optimal structures of the hydrogen-bonded quercetin-adenine-thymine and quercetin-deoxyadenosine-deoxythymidine complexesThe hydrogen bond distances are given in the corresponding position. ΔEb(gas) and ΔEb1(water) are the binding energies in gas phase and in water solvent using the optimal structure in gas phase. The bond distance units are in nm.
| [1] | Rachel E., A. , Neil, O. , Chem. Res. Toxicol., 2014, 27( 5), 787- 793 |
| [2] | Soumya S., M. , Somnath S., R. , Sayantani, C. , Sudin, B. , Subhash C., B. , J. Phys. Chem. B, 2013, 117( 47), 14655- 14665 |
| [3] | Eileen S., K. , Zhong J., C. , James A., H. , Biochemistry, 2013, 52( 9), 1559- 1567 |
| [4] | 张长胜, 来鲁华. 物理化学学报, 2012, 28( 10), 2363- 2380 |
| Zhang C., S. , Lai L., H. , Acta Phys.-Chim. Sin., 2012, 28( 10), 2363- 2380 | |
| [5] | 于楠, 刘朋, 王长生. 物理化学学报, 2013, 29( 6), 1173- 1182 |
| Yu, N. , Liu, P. , Wang C., S. , Acta Phys.-Chim. Sin., 2013, 29( 6), 1173- 1182 | |
| [6] | Adam C., K. , William A., D. , David E., G. , Neil, O. , Biochemistry, 2012, 51( 8), 1730- 1739 |
| [7] | Steven L., P. , Michae J., J. , Maria, D. , Daniel, D. , David E., G. , Neil, O. , Biochemistry, 2011, 50( 22), 5058- 5066 |
| [8] | Mohajeri, A. , Nobandegani F., F. , J. Phys. Chem. A, 2008, 112( 2), 281- 295 |
| [9] | Kawahara, S. , Uchimaru, T. , Tairi, K. , Sekine, M. , J. Phys. Chem. A, 2002, 106( 13), 3207- 3212 |
| [10] | 蓝蓉, 李浩然, 韩世钧. 化学学报, 2005, 63( 14), 1288- 1292 |
| Lan, R. , Li H., R. , Han S., J. , Acta Chim. Sinica, 2005, 63( 14), 1288- 1292 | |
| [11] | Asensio, A. , Kobko, N. , Dannenberg J., J. , J. Phys. Chem. A, 2003, 107( 33), 6441- 6443 |
| [12] | 林雪飞, 孙成科, 杨思娅, 余仕问, 姚立峰, 陈益山. 化学学报, 2011, 69( 23), 2787- 2795 |
| Lin X., F. , Sun C., K. , Yang S., Y. , Yu S., W. , Yao L., F. , Chen Y., S. , Acta Chim. Sinica, 2011, 69( 23), 2787- 2795 | |
| [13] | Li, Y. , Wang C., S. , Sci. China Ser. Chem., 2011, 54( 11), 1759- 1769 |
| [14] | Huang C., Y. , Li, Y. , Wang C., S. , Sci. China Chem., 2013, 56( 2), 238- 248 |
| [15] | Jiang X., N. , Wang C., S. , Sci. China Ser. Chem., 2010, 53( 8), 1754- 1761 |
| [16] | Zhao G., J. , Liu J., Y. , Zhou L., C. , Han K., L. , J. Phys. Chem. B, 2007, 111( 30), 8940- 8945 |
| [17] | Zhao G., J. , Han K., L. , Accounts Chem. Res., 2012, 45( 3), 404- 413 |
| [18] | Li S., S. , Huang C., Y. , Hao J., J. , Wang C., S. , J. Comput. Chem., 2014, 35( 6), 415- 506 |
| [19] | Dong, H. , Hua W., J. , Li S., H. , J. Phys. Chem. A, 2007, 111( 15), 2941- 2945 |
| [20] | Hao J., J. , Li S., S. , Jiang X., N. , Li X., L. , Wang C., S. , Theor. Chem. Acc., 2014, 133, 1516- 1527 |
| [21] | Wu Y., D. , Zhao Y., L. , J. Am. Chem. Soc., 2001, 123( 22), 5313- 5319 |
| [22] | 刘朋, 李书实, 王长生. 高等学校化学学报, 2014, 35( 1), 154- 160 |
| Liu, P. , Li S., S. , Wang C., S. , Chem. J. Chinese Universities, 2014, 35( 1), 154- 160 | |
| [23] | Li, Y. , Wang C., S. , J. Comput Chem., 2011, 32( 13), 2765- 2773 |
| [24] | Hao J., J. , Wang C., S. , RSC Adv., 2015, 5, 6452- 6461 |
| [25] | Riham I., E. , Noelia, R. , Julie T. W., W. , Wafa T., A. , Maxime, B. , Houmam, K. , Muniba, N. , Rebecca, K. , Vincenzo, A. , Fré, déric L. , Sara, B. , Gustaaf V., T. , Amany O., K. , Gehanne A. S., A. , Nahed D., M. , Khuloud T., A. , ACS Nano, 2014, 8( 2), 1384- 1401 |
| [26] | Yu C., H. , Hui T., Y. , Nan W., S. , J. Agric. Food Chem., 2012, 60( 10), 2674- 2681 |
| [27] | Lespade, L. , Bercion, S. , J. Phys. Chem. B, 2010, 114( 2), 921- 928 |
| [28] | Lekka C., E. , Ren, J. , Meng, S. , Kaxiras, E. , J. Phys. Chem. B, 2009, 113( 18), 6478- 6483 |
| [29] | Havsteen B., H. , Pharmacol. Ther., 2002, 96( 2/3), 67- 202 |
| [30] | Leopoldini, M. , Marino, T. , Russo, N. , Toscano, M. , Theor. Chem. Acc. , 2004, 111( 2/6), 210- 216 |
| [31] | Bouktaib, M. , Lebrun, S. , Atmani, A. , Rolando, C. , Tetrahedron., 2002, 58( 50), 10001- 10009 |
| [32] | Hä, mäläinen M. , Nieminen, R. , Vuorela, P. , Mediators Inflamm., 2007, 2007, 45673- 45682 |
| [33] | Bidisha, S. , Samantha M., R. , Donald E. D., J. , Kisa, H. , Randy M., W. , Denise, W. , D’, Asia G. , Cari, H. , J. Phys. Chem. B, 2015, 119( 6), 2546- 2556 |
| [34] | Omari J., B. , Sara J., C. , Neil, O. , Chem. Res. Toxicol., 2008, 21, 1253- 1260 |
| [35] | Zhang X., M. , Huang S., P. , Xu, Q. , Cancer Chemother. Pharmacol., 2004, 53( 1), 82- 88 |
| [36] | Becke A., D. , J. Chem. Phys., 1993, 98, 5648- 5652 |
| [37] | Lee, C. , Yang W., T. , Parr R., G. , Phys. Rev. B, 1988, 37, 785- 789 |
| [38] | Mϕ, ller C. , Plesset M., S. , Phys. Rev., 1934, 46, 618 |
| [39] | York D., M. , Karplus, M. , J. Phys. Chem. A, 1999, 103( 50), 11060- 11079 |
| [40] | Frisch M., J. , Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Mennucci B., Petersson G. A., Nakatsuji H., Caricato M., Li X., Hratchian H. P., Izmaylov A. F., Bloino J., Zheng G., Sonnenberg J. L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T. J. A., Montgomery J., Peralta J. E., Ogliaro F., Bearpark M., Heyd J. J., Brothers E., Kudin K. N., Staroverov V. N., Keith T., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Rega N., Millam J. M., Klene M., Knox J. E., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Martin R. L., Morokuma K., Zakrzewski V. G., Voth G. A., Salvador P., Dannenberg J. J., Dapprich S., Daniels A. D., Farkas O., Foresman J. B., Ortiz J. V., Cioslowski J., Fox D. J., Gaussian 09, Revision D. 01, Gaussian, Inc., Wallingford CT, 2013. |
| [41] | Biegler K., F. , Schonbohm, J. , Bayles, D. , J. Comput. Chem., 2001, 22( 5), 545- 559 |
| [1] | 李晓蕾, 孙云娇, 唐颖, 王长生. 醇及脱氧核糖与水分子间三体作用强度的快速准确计算[J]. 高等学校化学学报, 2021, 42(12): 3664. |
| [2] | 李菲, 李小轩, 李一峻, 何锡文, 陈朗星, 张玉奎. 表面定向磁性印迹聚合物的制备及对槲皮素的选择性识别[J]. 高等学校化学学报, 2021, 42(12): 3606. |
| [3] | 颜范勇, 孙中慧, 庞纪平, 江英霞, 陈圆. 苯并噻嗪衍生物功能化的碳点用于检测银杏叶茶中的槲皮素[J]. 高等学校化学学报, 2020, 41(8): 1768. |
| [4] | 王梦雨, 曹思敏, 李昊阳, 张梦婕, 李栋, 赵泽楠, 徐建华. 辅酶NADH与色氨酸共振能量转移的荧光动力学研究[J]. 高等学校化学学报, 2020, 41(11): 2473. |
| [5] | 刘冰彤, 庄永亮. 肽钙螯合物VGLPNSR-Ca的结构表征及在Caco-2单细胞层的促钙吸收性能[J]. 高等学校化学学报, 2019, 40(8): 1643. |
| [6] | 刘海春, 卢帅, 张艳敏, 周伟能, 尹凌枫, 朱露, 赵珺楠, 陆涛, 陈亚东. 分子动力学模拟研究Fedratinib-JAK2/JAK3选择性[J]. 高等学校化学学报, 2018, 39(7): 1540. |
| [7] | 李金星, 邢晓凤, 齐中囡, 艾洪奇. 3种改性小分子对不同Aβ42纤维结构稳定性的影响机制研究[J]. 高等学校化学学报, 2018, 39(10): 2230. |
| [8] | 李蕾, 黄翠英, 姜笑楠, 高希婵, 王长生. 精氨酸侧链和核酸碱基间离子氢键作用强度分析[J]. 高等学校化学学报, 2016, 37(8): 1460. |
| [9] | 孙小丽, 霍瑞萍, 步宇翔, 李吉来. 氢气吸附的密度泛函理论方法的基准研究[J]. 高等学校化学学报, 2015, 36(8): 1570. |
| [10] | 买买提·吐尔逊, 古丽巴哈尔·达吾提, 热萨莱提·伊敏, 楚刚辉, 买合木提江·杰力, 木合塔尔·吐尔洪. 基于活性自由基聚合的槲皮素分子印迹聚合物的合成及在维药祖卡木颗粒活性成分分析中的应用[J]. 高等学校化学学报, 2015, 36(12): 2402. |
| [11] | 刘翠, 张千慧, 宫利东, 卢丽男, 杨忠志. Fapy-G对碱基氢键复合物影响的理论研究[J]. 高等学校化学学报, 2014, 35(12): 2645. |
| [12] | 李书实, 姜笑楠, 王长生. 含酰胺和尿嘧啶的氢键复合物的三体效应[J]. 高等学校化学学报, 2014, 35(11): 2403. |
| [13] | 刘朋, 李书实, 王长生. 取代基对腺嘌呤与胸腺嘧啶氢键复合物体系结合能的影响[J]. 高等学校化学学报, 2014, 35(1): 154. |
| [14] | 王蕾, 符玲, 敬林林, 阿有梅, 贾陆. 月季花抗氧化活性成分研究[J]. 高等学校化学学报, 2012, 33(11): 2457. |
| [15] | 陈哲, KWONG Anna Ka-Yee, 杨振军, 张亮仁, LEE Hon Cheung, 张礼和. 烟酰胺腺嘌呤二核苷酸类CD38抑制剂的合成及生物活性评价[J]. 高等学校化学学报, 2012, 33(06): 1226. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||