

高等学校化学学报 ›› 2016, Vol. 37 ›› Issue (8): 1460.doi: 10.7503/cjcu20160346
收稿日期:2016-05-16
出版日期:2016-07-19
发布日期:2016-07-19
作者简介:联系人简介: 王长生, 男, 博士, 教授, 博士生导师, 主要从事理论与计算化学研究. E-mail:
基金资助:
LI Lei, HUANG Cuiying, JIANG Xiaonan, GAO Xichan, WANG Changsheng*( )
)
Received:2016-05-16
Online:2016-07-19
Published:2016-07-19
Contact:
WANG Changsheng
E-mail:chwangcs@lnnu.edu.cn
Supported by:摘要:
采用MP2/6-31+G(d,p)方法优化得到了22个由精氨酸侧链与碱基尿嘧啶、 胸腺嘧啶、 胞嘧啶、 鸟嘌呤及腺嘌呤形成的氢键复合物的气相稳定结构, 使用包含BSSE校正的MP2/aug-cc-pVTZ方法计算得到了复合物的气相结合能, 通过MP2/6-31+G(d,p)方法和PCM模型优化得到了复合物的水相稳定结构, 采用MP2/aug-cc-pVTZ方法和PCM模型计算得到了复合物的水相结合能. 研究发现, 精氨酸侧链与碱基间的离子氢键作用强度与单体间电荷转移量、 氢键临界点电子密度及二阶作用稳定化能密切相关. 与中性氢键相比, 离子氢键作用具有更显著的共价作用成分. 研究还发现, 精氨酸侧链和碱基间形成的氢键复合物的稳定性次序可以通过氢键受体碱基分子上氧原子和氮原子的质子化反应焓变进行预测, 质子化反应焓变越负, 形成的氢键复合物越稳定.
中图分类号:
TrendMD:
李蕾, 黄翠英, 姜笑楠, 高希婵, 王长生. 精氨酸侧链和核酸碱基间离子氢键作用强度分析. 高等学校化学学报, 2016, 37(8): 1460.
LI Lei,HUANG Cuiying,JIANG Xiaonan,GAO Xichan,WANG Changsheng. Ionic Hydrogen Bonding Between Arginine Side Chain and Nucleic Acid Bases†. Chem. J. Chinese Universities, 2016, 37(8): 1460.
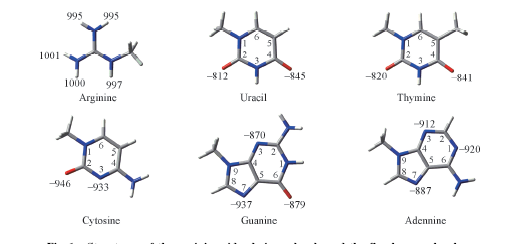
Fig.1 Structures of the arginine side chain molecule and the five base molecules The ΔH(kJ/mol) of the protonation and deprotonation reactions associated with the hydrogen bonding sites obtained at the MP2/aug-cc-pVTZ//MP2/aug-cc-pVDZ level were given in the corresponding sites.
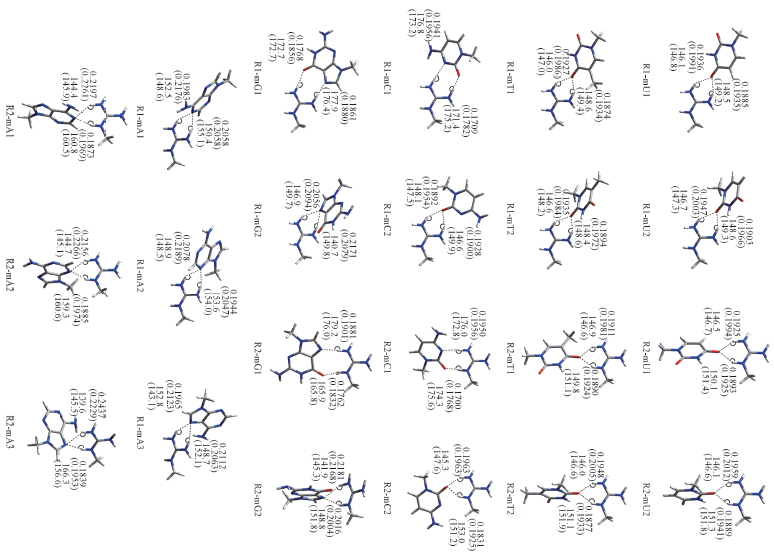
Fig.2 Optimal structures of 22 hydrogen-bonded complexes The hydrogen bond distances[RNH…X (nm, X=N or O)] and bond angles[∠NHX(°)] in gas phase and in water phase are given in the corresponding positions and in parentheses, respectively.
| Complex | Type | RNH…X/nm | Eb/(kJ·mol-1) | ΔE(2) / (kJ·mol-1) | ΣΔE(2) / (kJ·mol-1) | ρc/a.u. | Σρc/a.u. | qt/e |
|---|---|---|---|---|---|---|---|---|
| R1-mU1 | N—H…O | 0.1885 | -104.27(-39.70) | 63.76 | 109.16 | 0.0279 | 0.0522 | 0.0371 |
| N—H…O | 0.1936 | 45.40 | 0.0243 | |||||
| R1-mU2 | N—H…O | 0.1905 | -87.49(-37.91) | 48.91 | 85.14 | 0.0263 | 0.0498 | 0.0333 |
| N—H…O | 0.1947 | 36.23 | 0.0234 | |||||
| R2-mU1 | N—H…O | 0.1893 | -104.01(-40.77) | 59.66 | 111.08 | 0.0266 | 0.0522 | 0.0373 |
| N—H…O | 0.1925 | 51.42 | 0.0256 | |||||
| R2-mU2 | N—H…O | 0.1889 | -87.45(-39.35) | 56.07 | 90.71 | 0.0272 | 0.0501 | 0.0337 |
| N—H…O | 0.1959 | 34.64 | 0.0229 | |||||
| R1-mT1 | N—H…O | 0.1874 | -104.93(-42.06) | 70.58 | 117.48 | 0.0289 | 0.0538 | 0.0385 |
| N—H…O | 0.1927 | 46.90 | 0.0249 | |||||
| R1-mT2 | N—H…O | 0.1894 | -93.51(-39.80) | 51.00 | 89.24 | 0.0270 | 0.0512 | 0.0348 |
| N—H…O | 0.1935 | 38.24 | 0.0241 | |||||
| R2-mT1 | N—H…O | 0.1890 | -105.23(-43.05) | 62.51 | 123.51 | 0.0270 | 0.0539 | 0.0391 |
| N—H…O | 0.1911 | 61.00 | 0.0269 | |||||
| R2-mT2 | N—H…O | 0.1877 | -93.51(-41.03) | 58.41 | 94.81 | 0.0280 | 0.0515 | 0.0353 |
| N—H…O | 0.1948 | 36.40 | 0.0235 | |||||
| R1-mC1 | N—H…O | 0.1709 | -151.08(-62.45) | 156.06 | 245.05 | 0.0428 | 0.0729 | 0.0822 |
| N—H…N | 0.1941 | 88.99 | 0.0300 | |||||
| R1-mC2 | N—H…O | 0.1892 | -144.72(-51.31) | 61.46 | 115.18 | 0.0288 | 0.0546 | 0.0472 |
| N—H…O | 0.1928 | 53.72 | 0.0258 | |||||
| R2-mC1 | N—H…O | 0.1700 | -152.59(-63.58) | 172.05 | 258.32 | 0.0443 | 0.0738 | 0.0858 |
| N—H…N | 0.1950 | 86.27 | 0.0295 | |||||
| R2-mC2 | N—H…O | 0.1831 | -147.11(-55.19) | 96.65 | 137.11 | 0.0353 | 0.0577 | 0.0555 |
| N—H…O | 0.1963 | 40.46 | 0.0224 | |||||
| R1-mG1 | N—H…O | 0.1768 | -158.82(-59.90) | 123.34 | 245.55 | 0.0362 | 0.0716 | 0.0776 |
| N—H…N | 0.1861 | 122.21 | 0.0354 | |||||
| R1-mG2 | N—H…O | 0.2056 | -142.93(-46.98) | 47.03 | 74.90 | 0.0241 | 0.0436 | 0.0387 |
| N—H…O | 0.2171 | 27.87 | 0.0195 | |||||
| R2-mG1 | N—H…O | 0.1762 | -160.96(-61.35) | 133.68 | 249.45 | 0.0375 | 0.0713 | 0.0790 |
| N—H…N | 0.1881 | 115.77 | 0.0338 | |||||
| R2-mG2 | N—H…O | 0.2017 | -144.39(-48.66) | 59.54 | 86.07 | 0.0263 | 0.0454 | 0.0437 |
| N—H…O | 0.2181 | 26.53 | 0.0190 | |||||
| R1-mA1 | N—H…N | 0.1983 | -95.31(-39.73) | 69.37 | 105.90 | 0.0291 | 0.0535 | 0.0515 |
| N—H…N | 0.2058 | 36.53 | 0.0244 | |||||
| R1-mA2 | N—H…N | 0.1944 | -91.63(-40.76) | 75.02 | 110.75 | 0.0310 | 0.0545 | 0.0529 |
| N—H…N | 0.2078 | 35.73 | 0.0235 | |||||
| R1-mA3 | N—H…N | 0.1965 | -90.04(-42.83) | 81.46 | 105.69 | 0.0297 | 0.0507 | 0.0462 |
| N—H…N | 0.2112 | 24.23 | 0.0210 | |||||
| R2-mA1 | N—H…N | 0.1873 | -96.11(-43.43) | 116.69 | 134.39 | 0.0370 | 0.0555 | 0.0587 |
| N—H…N | 0.2197 | 17.70 | 0.0185 | |||||
| R2-mA2 | N—H…N | 0.1885 | -91.59(-41.37) | 103.68 | 127.53 | 0.0353 | 0.0554 | 0.0574 |
| N—H…N | 0.2156 | 23.85 | 0.0200 | |||||
| R2-mA3 | N—H…N | 0.1839 | -90.25(-43.52) | 138.49 | 140.37 | 0.0382 | 0.0493 | 0.0563 |
| N—H…N | 0.2437 | 1.88 | 0.0111 |
Table 1 Hydrogen bond distances(RNH…X), binding energies(Eb), the second-order stabilization energies(ΔE(2)), the electron densities(ρc) at the hydrogen bond critical points, and the charge transfer(qt) between two molecules for the gas phase structures*
| Complex | Type | RNH…X/nm | Eb/(kJ·mol-1) | ΔE(2) / (kJ·mol-1) | ΣΔE(2) / (kJ·mol-1) | ρc/a.u. | Σρc/a.u. | qt/e |
|---|---|---|---|---|---|---|---|---|
| R1-mU1 | N—H…O | 0.1885 | -104.27(-39.70) | 63.76 | 109.16 | 0.0279 | 0.0522 | 0.0371 |
| N—H…O | 0.1936 | 45.40 | 0.0243 | |||||
| R1-mU2 | N—H…O | 0.1905 | -87.49(-37.91) | 48.91 | 85.14 | 0.0263 | 0.0498 | 0.0333 |
| N—H…O | 0.1947 | 36.23 | 0.0234 | |||||
| R2-mU1 | N—H…O | 0.1893 | -104.01(-40.77) | 59.66 | 111.08 | 0.0266 | 0.0522 | 0.0373 |
| N—H…O | 0.1925 | 51.42 | 0.0256 | |||||
| R2-mU2 | N—H…O | 0.1889 | -87.45(-39.35) | 56.07 | 90.71 | 0.0272 | 0.0501 | 0.0337 |
| N—H…O | 0.1959 | 34.64 | 0.0229 | |||||
| R1-mT1 | N—H…O | 0.1874 | -104.93(-42.06) | 70.58 | 117.48 | 0.0289 | 0.0538 | 0.0385 |
| N—H…O | 0.1927 | 46.90 | 0.0249 | |||||
| R1-mT2 | N—H…O | 0.1894 | -93.51(-39.80) | 51.00 | 89.24 | 0.0270 | 0.0512 | 0.0348 |
| N—H…O | 0.1935 | 38.24 | 0.0241 | |||||
| R2-mT1 | N—H…O | 0.1890 | -105.23(-43.05) | 62.51 | 123.51 | 0.0270 | 0.0539 | 0.0391 |
| N—H…O | 0.1911 | 61.00 | 0.0269 | |||||
| R2-mT2 | N—H…O | 0.1877 | -93.51(-41.03) | 58.41 | 94.81 | 0.0280 | 0.0515 | 0.0353 |
| N—H…O | 0.1948 | 36.40 | 0.0235 | |||||
| R1-mC1 | N—H…O | 0.1709 | -151.08(-62.45) | 156.06 | 245.05 | 0.0428 | 0.0729 | 0.0822 |
| N—H…N | 0.1941 | 88.99 | 0.0300 | |||||
| R1-mC2 | N—H…O | 0.1892 | -144.72(-51.31) | 61.46 | 115.18 | 0.0288 | 0.0546 | 0.0472 |
| N—H…O | 0.1928 | 53.72 | 0.0258 | |||||
| R2-mC1 | N—H…O | 0.1700 | -152.59(-63.58) | 172.05 | 258.32 | 0.0443 | 0.0738 | 0.0858 |
| N—H…N | 0.1950 | 86.27 | 0.0295 | |||||
| R2-mC2 | N—H…O | 0.1831 | -147.11(-55.19) | 96.65 | 137.11 | 0.0353 | 0.0577 | 0.0555 |
| N—H…O | 0.1963 | 40.46 | 0.0224 | |||||
| R1-mG1 | N—H…O | 0.1768 | -158.82(-59.90) | 123.34 | 245.55 | 0.0362 | 0.0716 | 0.0776 |
| N—H…N | 0.1861 | 122.21 | 0.0354 | |||||
| R1-mG2 | N—H…O | 0.2056 | -142.93(-46.98) | 47.03 | 74.90 | 0.0241 | 0.0436 | 0.0387 |
| N—H…O | 0.2171 | 27.87 | 0.0195 | |||||
| R2-mG1 | N—H…O | 0.1762 | -160.96(-61.35) | 133.68 | 249.45 | 0.0375 | 0.0713 | 0.0790 |
| N—H…N | 0.1881 | 115.77 | 0.0338 | |||||
| R2-mG2 | N—H…O | 0.2017 | -144.39(-48.66) | 59.54 | 86.07 | 0.0263 | 0.0454 | 0.0437 |
| N—H…O | 0.2181 | 26.53 | 0.0190 | |||||
| R1-mA1 | N—H…N | 0.1983 | -95.31(-39.73) | 69.37 | 105.90 | 0.0291 | 0.0535 | 0.0515 |
| N—H…N | 0.2058 | 36.53 | 0.0244 | |||||
| R1-mA2 | N—H…N | 0.1944 | -91.63(-40.76) | 75.02 | 110.75 | 0.0310 | 0.0545 | 0.0529 |
| N—H…N | 0.2078 | 35.73 | 0.0235 | |||||
| R1-mA3 | N—H…N | 0.1965 | -90.04(-42.83) | 81.46 | 105.69 | 0.0297 | 0.0507 | 0.0462 |
| N—H…N | 0.2112 | 24.23 | 0.0210 | |||||
| R2-mA1 | N—H…N | 0.1873 | -96.11(-43.43) | 116.69 | 134.39 | 0.0370 | 0.0555 | 0.0587 |
| N—H…N | 0.2197 | 17.70 | 0.0185 | |||||
| R2-mA2 | N—H…N | 0.1885 | -91.59(-41.37) | 103.68 | 127.53 | 0.0353 | 0.0554 | 0.0574 |
| N—H…N | 0.2156 | 23.85 | 0.0200 | |||||
| R2-mA3 | N—H…N | 0.1839 | -90.25(-43.52) | 138.49 | 140.37 | 0.0382 | 0.0493 | 0.0563 |
| N—H…N | 0.2437 | 1.88 | 0.0111 |
| [1] |
Meot-Ner, M. , Chem. Rev., 2005, 105( 1), 213- 284
doi: 10.1002/chin.200515284 URL pmid: 15729772 |
| [2] |
Meot-Ner, M. , Chem. Rev., 2012, 112( 1), 22- 103
doi: 10.1021/cr200430n URL pmid: 22873941 |
| [3] | Vá, zquez M. E. , Caamañ, o A. M. , Mascareñ, as J. L. , Chem. Soc. Rev., 2003, 32( 6), 338- 349 |
| [4] |
Shulman-Peleg, A. , Shatsky, M. , Nussinov, R. , Wolfson H., J. , J. Mol. Biol., 2008, 379( 2), 299- 316
doi: 10.1016/j.jmb.2008.03.043 URL pmid: 18452949 |
| [5] |
Antson A., A. , Dodson E., J. , Dodson, G. , Greaves R., B. , Chen X., P. , Gollnick, P. , Nature, 1999, 401, 235- 242
doi: 10.1038/45730 URL pmid: 10499579 |
| [6] |
Gu, J. , Wang, J. , Leszczynski, J. , J. Phys. Chem. B, 2006, 110( 27), 13590- 13596
doi: 10.1021/jp061360x URL pmid: 16821886 |
| [7] |
Wang, P. , Zhang J. Z., H. , J. Phys. Chem. B, 2010, 114( 40), 12958- 129640
doi: 10.1021/jp1030224 URL pmid: 20860351 |
| [8] |
Li, Y. , Jiang X., N. , Wang C., S. , J. Comput. Chem., 2011, 32( 5), 953- 966
doi: 10.1002/jcc.21680 URL pmid: 20949514 |
| [9] | Li, Y. , Wang C., S. , J. Comput. Chem., 2011, 32( 13), 2765- 2773 |
| [10] | Huang C., Y. , Li, Y. , Wang C., S. , Sci. China Chem., 2013, 56( 2), 238- 248 |
| [11] |
刘朋, 李书实, 王长生. 高等学校化学学报, 2014, 35( 1), 154- 160
doi: 10.7503/cjcu20130707 |
|
Liu, P. , Li S., S. , Wang C., S. , Chem. J. Chinese Universities, 2014, 35( 1), 154- 160
doi: 10.7503/cjcu20130707 |
|
| [12] |
Seeman N., C. , Rosenberg J., M. , Rich, A. , Proc. Natl. Acad. Sci. USA, 1976, 73( 3), 804- 808
doi: 10.1073/pnas.73.3.804 URL pmid: 1062791 |
| [13] |
Lehmann M., S. , Verbist J., J. , Hamilton W., C. , Koetzle T., F. , J. Chem. Soc. Perk Trans. II, 1973, 133-137
doi: 10.1039/p29730000133 URL |
| [14] | Luscombe N., M. , Laskowski R., A. , Thornton J., M. , Nucl. Acids Res., 2001, 29( 13), 2860- 2874 |
| [15] |
Cheng A., C. , Chen W., W. , Fuhrmann C., N. , Frankel A., D. , J. Mol. Biol., 2003, 327( 4), 781- 796
doi: 10.1016/S0022-2836(03)00091-3 URL pmid: 12654263 |
| [16] |
Rozas, I. , Alkortab, I. , Elguerob, J. , Org. Biomol. Chem., 2005, 3( 2), 366- 371
doi: 10.1007/s11224-005-1089-9 URL |
| [17] |
Lejeune, D. , Delsaux, N. , Charloteaux, B. , Thomas, A. , Brasseur, R. , Proteins:, Struct. , Funct., Bioinform., 2005, 61( 2), 258- 271
doi: 10.1002/prot.20607 URL pmid: 16121397 |
| [18] | Hao J., J. , Li S., S. , Jiang X., N. , Li X., L. , Wang C., S. , Theor. Chem. Acc., 2014, 133, 1516- 1527 |
| [19] |
Scheiner, S. , J. Phys. Chem. B, 2005, 109( 33), 16132- 16141
doi: 10.1021/jp053416d URL pmid: 16853050 |
| [20] |
Scheiner, S. , J. Phys. Chem. B, 2006, 110( 37), 18670- 18679
doi: 10.1021/jp063225q URL pmid: 16970498 |
| [21] |
Hao J., J. , Wang C., S. , RSC Adv., 2015, 5( 9), 6452- 6461
doi: 10.1039/c4ra16359a URL |
| [22] |
Riley K., E. , Hobza, P. , J. Phys. Chem. A, 2007, 111( 33), 8257- 8263
doi: 10.1021/jp073358r URL pmid: 17649987 |
| [23] |
Li S., S. , Huang C., Y. , Hao J., J. , Wang C., S. , J. Comput. Chem., 2014, 35( 6), 415- 426
doi: 10.1002/jcc.23473 URL pmid: 24497309 |
| [24] |
Nagy P., I. , Erhardt P., W. , J. Phys. Chem. A, 2008, 112( 18), 4342- 4354
doi: 10.1021/jp7108847 URL pmid: 18373368 |
| [25] |
Hanus M. ,
doi: 10.1016/0304-3835(86)90172-2 URL pmid: 3004720 |
| [26] |
Steindal A., H. , Ruud, K. , Frediani, L. , Aidas, K. , Kongsted, J. , J. Phys. Chem. B, 2011, 115( 12), 3027- 3037
doi: 10.1021/jp1101913 URL pmid: 21391548 |
| [27] | Bader R. F., W. , Chem. Rev., 1991, 91( 5), 893- 926 |
| [28] |
Reed A., E. , Weinhold, F. , J. Chem. Phys., 1983, 78( 6), 4066- 4073
doi: 10.1063/1.445134 URL |
| [29] | Frisch M., J. , Trucks G., W. , Schlegel H., B. , Scuseria G., E. , Robb M., A. , Cheeseman J., R. , Scalmani, G. , Barone, V. , Mennucci, B. , Petersson G., A. , Nakatsuji, H. , Caricato, M. , Li, X. , Hratchian H., P. , Izmaylov A., F. , Bloino, J. , Zheng, G. , Sonnenberg J., L. , Hada, M. , Ehara, M. , Toyota, K. , Fukuda, R. , Hasegawa, J. , Ishida, M. , Nakajima, T. , Honda, Y. , Kitao, O. , Nakai, H. , Vreven, T. , Montgomery J. A., Jr. , Peralta J., E. , Ogliaor, F. , Bearpark, M. , Heyd J., J. , Brothers, E. , Kudin K., N. , Staroverov V., N. , Keith, T. , Kobayashi, R. , Normand, J. , Raghavachari, K. , Rendell, A. , Burant J., C. , Iyengar S., S. , Tomasi, J. , Cossi, M. , Rega, N. , Millam J., M. , Klene, M. , Knox J., E. , Cross J., B. , Bakken, V. , Adamo, C. , Jaramillo, J. , Gomperts, R. , Stratmann R., E. , Yazyev, O. , Austin A., J. , Cammi, R. , Pomelli, C. , Ochterski J., W. , Martin R., L. , Morokuma, K. , Zakrzewski V., G. , Voth G., A. , Salvador, P. , Dannenberg J., J. , Dapprich, S. , Daniels A., D. , Farkas, O. , Foresman J., B. , Ortiz J., V. , Cioslowski, J. , Fox D., J. , Gaussian, 09 , Revision D., 01 , Gaussian, Inc. , Wallingford, CT, 2013 |
| [30] |
Biegler-Kö, nig F. , Schö, nbohm J. , Bayles, D. , J. Comput. Chem., 2001, 22( 5), 545- 559
doi: 10.1002/1096-987X(20010415)22:5<545::AID-JCC1027>3.0.CO;2-Y URL |
| [31] |
张敏, 郑艳萍, 姜笑楠, 王长生. 物理化学学报, 2010, 26( 3), 735- 739
doi: 10.3866/PKU.WHXB20100235 |
|
Zhang, M. , Zheng Y., P. , Jiang X., N. , Wang C., S. , Acta Phys.-Chim. Sin., 2010, 26( 3), 735- 739
doi: 10.3866/PKU.WHXB20100235 |
|
| [32] |
Caramori G., F. , Galembeck S., E. , J. Phys. Chem. A, 2007, 111( 9), 1705- 1712
doi: 10.1021/jp066863h URL pmid: 17295458 |
| [33] |
Zhou P., P. , Qiu W., Y. , Chem. Phys. Chem., 2009, 10( 11), 1847- 1858
doi: 10.1021/jp9035452 URL pmid: 19715282 |
| [34] |
Sun, L. , Cukier R., I. , Bu, Y. , J. Phys. Chem. B, 2007, 111( 7), 1802- 1808
doi: 10.1021/jp063645f URL pmid: 17266349 |
| [35] |
刘畅, 于歌, 黄翠英, 王长生. 化学学报, 2015, 73( 4), 357- 365
doi: 10.6023/A14120869 |
|
Liu, C. , Yu, G. , Huang C., Y. , Wang C., S. , Acta Chim. Sinica, 2015, 73( 4), 357- 365
doi: 10.6023/A14120869 |
| [1] | 刘冰彤, 庄永亮. 肽钙螯合物VGLPNSR-Ca的结构表征及在Caco-2单细胞层的促钙吸收性能[J]. 高等学校化学学报, 2019, 40(8): 1643. |
| [2] | 刘海春, 卢帅, 张艳敏, 周伟能, 尹凌枫, 朱露, 赵珺楠, 陆涛, 陈亚东. 分子动力学模拟研究Fedratinib-JAK2/JAK3选择性[J]. 高等学校化学学报, 2018, 39(7): 1540. |
| [3] | 李金星, 邢晓凤, 齐中囡, 艾洪奇. 3种改性小分子对不同Aβ42纤维结构稳定性的影响机制研究[J]. 高等学校化学学报, 2018, 39(10): 2230. |
| [4] | 李蕾, 李书实, 王长生. 带电组氨酸侧链与DNA碱基间非键作用强度的理论研究[J]. 高等学校化学学报, 2017, 38(1): 56. |
| [5] | 孙小丽, 霍瑞萍, 步宇翔, 李吉来. 氢气吸附的密度泛函理论方法的基准研究[J]. 高等学校化学学报, 2015, 36(8): 1570. |
| [6] | 王晓雯, 李爽, 姜笑楠, 王长生. 槲皮素与腺嘌呤氢键作用的最佳位点[J]. 高等学校化学学报, 2015, 36(5): 932. |
| [7] | 刘翠, 张千慧, 宫利东, 卢丽男, 杨忠志. Fapy-G对碱基氢键复合物影响的理论研究[J]. 高等学校化学学报, 2014, 35(12): 2645. |
| [8] | 刘朋, 李书实, 王长生. 取代基对腺嘌呤与胸腺嘧啶氢键复合物体系结合能的影响[J]. 高等学校化学学报, 2014, 35(1): 154. |
| [9] | 张吉 李海斌 吴勇 耿允 段雨爱 廖奕 苏忠民. 染料敏化太阳能电池中具有不同电子给体的吩噻嗪类有机光敏染料的理论研究[J]. 高等学校化学学报, 2011, 32(6): 1343. |
| [10] | 霍红洁 赵东霞 杨忠志. 碱基与氮甲基乙酰胺相互作用的从头算和ABEEMσπ研究[J]. 高等学校化学学报, 2011, 32(12): 2877. |
| [11] | 张文龙, 陈淑玲, 杨忠志. 应用ABEEMσπ/MM模型对重组人纤溶酶原Kringle 1结构域与配体分子的对接计算[J]. 高等学校化学学报, 2010, 31(8): 1630. |
| [12] | 张瑀健, 何振峰, 李国文. 含氮杂冠醚和核酸碱基双亲聚合物的合成及性能[J]. 高等学校化学学报, 2010, 31(7): 1456. |
| [13] | 倪哲明 姚萍 刘晓明 王巧巧 胥倩. 铜锌铝三元水滑石畸变结构和稳定性的理论研究[J]. 高等学校化学学报, 2010, 31(12): 2438. |
| [14] | 冯丰, 于建国, 方维海. DNA和RNA双链稳定性差异的理论研究[J]. 高等学校化学学报, 2009, 30(12): 2445. |
| [15] | 张笑, 何振峰, 李广全, 张瑀健, 陈云霞, 李国文. 含核酸碱基的双亲聚合物纳米球的制备及性能[J]. 高等学校化学学报, 2009, 30(1): 208. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||