

高等学校化学学报 ›› 2015, Vol. 36 ›› Issue (7): 1254.doi: 10.7503/cjcu20141088
收稿日期:2014-12-12
出版日期:2015-07-10
发布日期:2015-06-03
作者简介:联系人简介: 杨天林, 男, 博士, 教授, 主要从事无机化学方面的研究. E-mail : 基金资助:
YANG Shuilan, SONG Pan, SHE Wenjie, YANG Tianlin*( )
)
Received:2014-12-12
Online:2015-07-10
Published:2015-06-03
Contact:
YANG Tianlin
E-mail:yang_tl@nxu.edu.cn
Supported by:摘要:
在pH=7.3的Tris-HCl缓冲溶液(模拟生理条件)中, 采用荧光光谱、 循环伏安曲线和紫外光谱研究了N-二(苯-二氨基甲酰基)甲基磷酸铕(Ⅲ)配合物[Eu(pic)3L]与牛血清白蛋白(BSA)的相互作用. 实验结果表明: 配合物与BSA可以形成1∶1结合型无荧光复合物Eu(pic)3L-BSA, Eu(pic)3L对 BSA 内源荧光的猝灭类型为静态猝灭. 根据双对数回归方程计算出二者在不同温度下的结合常数K及结合位点数n, 通过热力学参数得出配合物与 BSA 之间以氢键和范德华力为主. 根据Foster的偶极-偶极无辐射能量转移机理可知配合物与BSA之间可能以偶极-偶极无辐射能量转移方式进行能量传递. 分别考察了Fe3+和Cu2+对配合物与BSA结合作用的影响, 推测Fe3+和Cu2+可能在配合物与BSA间起“离子架桥”作用, 使Eu(pic)3L-BSA复合物的稳定性增强. 循环伏安法研究结果表明配合物与BSA相互作用形成无电活性的Eu(pic)3L-BSA复合物, 使得溶液中游离的配合物浓度降低.
中图分类号:
TrendMD:
杨水兰, 宋盼, 佘文洁, 杨天林. 含磷三足体稀土铕(Ⅲ)配合物与牛血清白蛋白的作用机理. 高等学校化学学报, 2015, 36(7): 1254.
YANG Shuilan, SONG Pan, SHE Wenjie, YANG Tianlin. Mechanism of the Interaction Between a Phosphorus-containing Tripod Ligand Europium(Ⅲ) Complex and Bovine Serum Albumin†. Chem. J. Chinese Universities, 2015, 36(7): 1254.
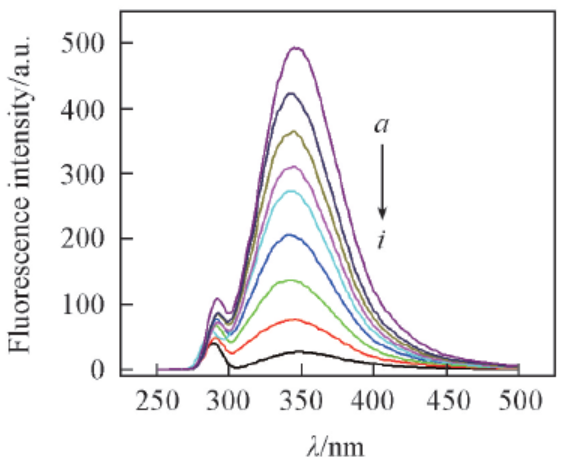
Fig.2 Fluorescence emission spectra of BSA influenced by different concentrations of Eu(pic)3LT=303 K, pH=7.3, λex=280 nm, cBSA=1×10-7 mol/L. 107cEu(pic)3L/(mol·L-1) from a to i: 0, 1.0, 2.0, 3.0, 4.0, 5.0, 6.0, 7.0, 8.0.
| T/K | 10-5 KSV/(L·mol-1) | 10-13Kq/(L·mol-1·s-1) | R2 |
|---|---|---|---|
| 293 | 4.16 | 4.16 | 0.996 |
| 303 | 3.88 | 3.88 | 0.993 |
| 313 | 3.15 | 3.15 | 0.995 |
| 323 | 3.10 | 3.10 | 0.996 |
Table 1 KSV and kq data of Eu(pic)3L complex-BSA system at different temperatures
| T/K | 10-5 KSV/(L·mol-1) | 10-13Kq/(L·mol-1·s-1) | R2 |
|---|---|---|---|
| 293 | 4.16 | 4.16 | 0.996 |
| 303 | 3.88 | 3.88 | 0.993 |
| 313 | 3.15 | 3.15 | 0.995 |
| 323 | 3.10 | 3.10 | 0.996 |
| T/K | Equation | K/(L·mol-1) | R2 | n |
|---|---|---|---|---|
| 293 | lg(F0/F-1)=6.57+1.33lgcQ | 1.24×106 | 0.991 | 1.33 |
| 303 | lg(F0/F-1)=6.39 +1.30lgcQ | 2.88×105 | 0.993 | 1.30 |
| 313 | lg(F0/F-1)=6.14+1.26lgcQ | 1.51×105 | 0.994 | 1.26 |
| 323 | lg(F0/F-1)=5.82+1.21lgcQ | 4.11×104 | 0.994 | 1.21 |
Table 2 Binding constants(K), binding sites(n) and R2 of Eu(pic)3L complex-BSA at different temperatures
| T/K | Equation | K/(L·mol-1) | R2 | n |
|---|---|---|---|---|
| 293 | lg(F0/F-1)=6.57+1.33lgcQ | 1.24×106 | 0.991 | 1.33 |
| 303 | lg(F0/F-1)=6.39 +1.30lgcQ | 2.88×105 | 0.993 | 1.30 |
| 313 | lg(F0/F-1)=6.14+1.26lgcQ | 1.51×105 | 0.994 | 1.26 |
| 323 | lg(F0/F-1)=5.82+1.21lgcQ | 4.11×104 | 0.994 | 1.21 |
| T/K | ΔH/(kJ·mol-1) | ΔS/(J·K-1) | ΔG/(kJ·mol-1) |
|---|---|---|---|
| 293 | -107.76 | -251.13 | -34.19 |
| 303 | -107.76 | -251.12 | -31.67 |
| 313 | -107.76 | -245.14 | -31.03 |
| 323 | -107.76 | -245.29 | -28.53 |
Table 3 Thermodynamic parameters of complex-BSA binding procedure
| T/K | ΔH/(kJ·mol-1) | ΔS/(J·K-1) | ΔG/(kJ·mol-1) |
|---|---|---|---|
| 293 | -107.76 | -251.13 | -34.19 |
| 303 | -107.76 | -251.12 | -31.67 |
| 313 | -107.76 | -245.14 | -31.03 |
| 323 | -107.76 | -245.29 | -28.53 |
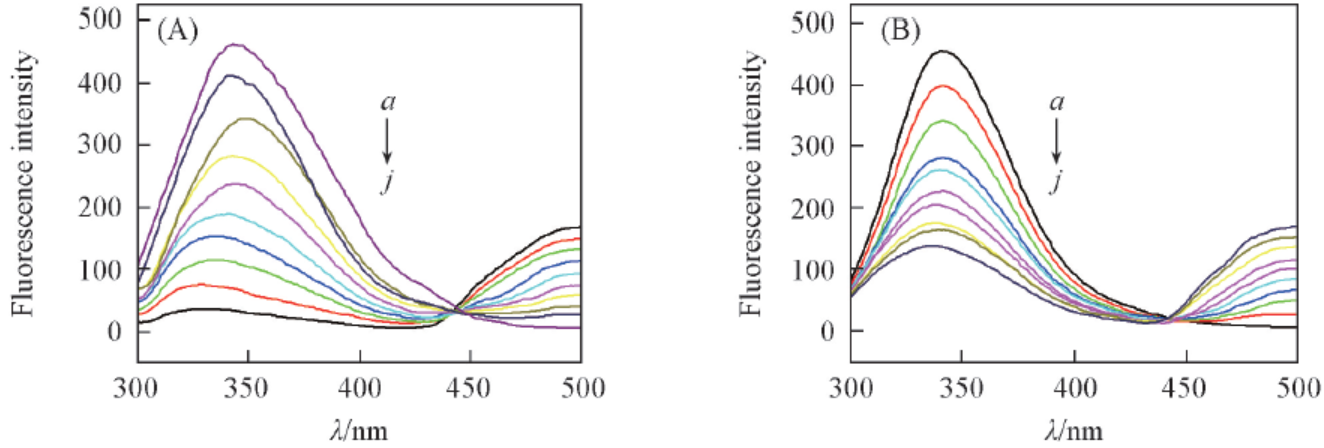
Fig.8 Quenching effect of Eu(pic)3L complex on BSA fluorescence in the presence of Cu2+(A) and Fe3+(B) T=303 K, pH=7.3, λex=280 nm, cCu2+-BSA or cFe3+-BSA=1×10-6 mol/L. 107cEu(pic)3L/(mol·L-1): a. 0; b. 1.0; c. 2.0; d. 3.0; e. 4.0; f. 5.0; g. 6.0; h. 7.0; i. 8.0; j. 9.0.
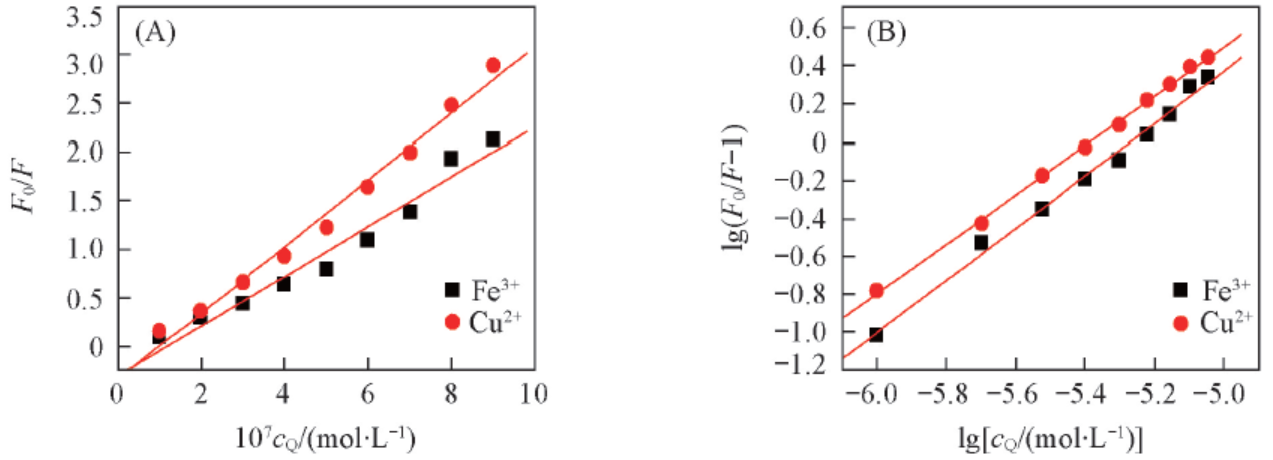
Fig.9 Stern-Volmer curves(A) and double logarithm plots(B) showing the Eu(pic)3L complex quenching effect on BSA fluorescence in the presence of Fe3+ and Cu2+ ions respectively(B)
| System | K/(L·mol-1) | n | R2 |
|---|---|---|---|
| Complex-BSA | 2.88×105 | 1.30 | 0.993 |
| Complex-BSA-Cu2+ | 9.98×106 | 1.31 | 0.992 |
| Complex-BSA-Fe3+ | 1.79×106 | 1.38 | 0.998 |
Table 4 Binding parameters of Eu(pic)3L complex-BSA system in the presence of Fe3+ and Cu2+
| System | K/(L·mol-1) | n | R2 |
|---|---|---|---|
| Complex-BSA | 2.88×105 | 1.30 | 0.993 |
| Complex-BSA-Cu2+ | 9.98×106 | 1.31 | 0.992 |
| Complex-BSA-Fe3+ | 1.79×106 | 1.38 | 0.998 |
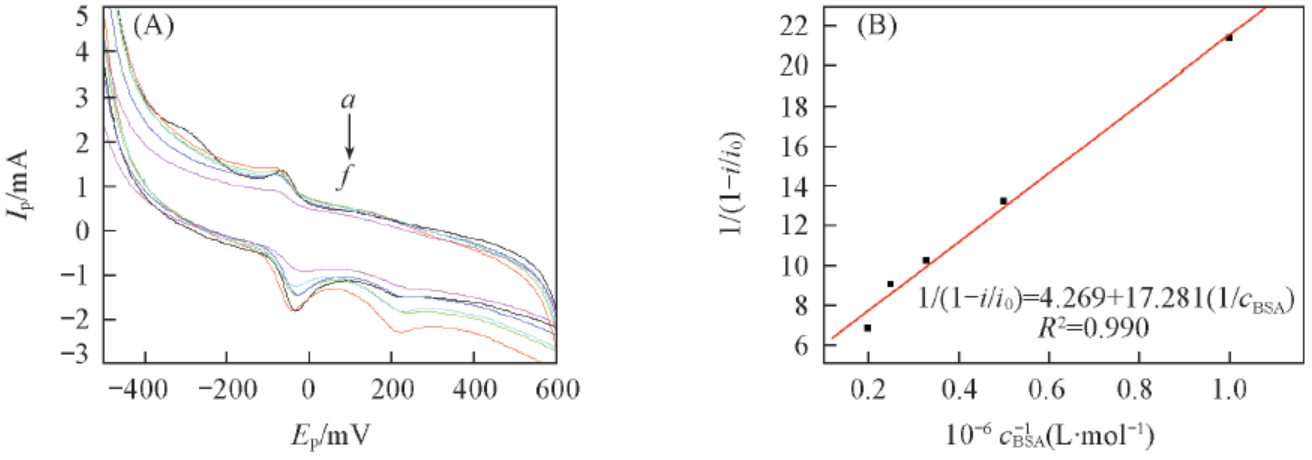
Fig.11 CV curves of Eu(pic)3L complex with DNA(A) and the plot of 1/(1-i/i0) vs 1/cBSA(B)cEu(pic)3L=1×10-5 mol/L. cBSA/(10-6 mol·L-1) form a to f: 0; 1.0; 2.0; 3.0; 4.0; 5.0.
| System | T/K | 10-5Kb/(L·mol-1) | R2 |
|---|---|---|---|
| Complex-BSA | 293 | 1.57 | 0.996 |
| Complex-BSA | 303 | 3.28 | 0.998 |
Table 5 Kb of Eu(pic)3L complex and BSA at different temperatures
| System | T/K | 10-5Kb/(L·mol-1) | R2 |
|---|---|---|---|
| Complex-BSA | 293 | 1.57 | 0.996 |
| Complex-BSA | 303 | 3.28 | 0.998 |
| [1] | Wu M. X., Zhao Z., Zhou J., Wang Y. Q., Animal Husbandry and Feed Science, 2013, 34(3), 10—13 |
| (吴明旭, 赵治, 周晶, 王玉芹. 畜牧与调料科学,2013, 34(3), 10—13) | |
| [2] | Huo L. N., Liu W., Yang T. L., Li X. H., Chinese Rare Earths, 2014, 35(2), 36—43 |
| (霍丽娜, 刘伟, 杨天林, 李晓红. 稀土,2014, 35(2), 36—43) | |
| [3] | Tang S. F., Song J. L., Li X. L., Mao J. G., Cryst. Growth Des., 2007, 7(2), 360—366 |
| [4] | Costantino F., Ienco A., Gentili P. L., Presciutti F., Cryst. Growth Des., 2010, 10(11), 4831—4838 |
| [5] | She W. J., Liu W., Zhang Y., Yang T. L., Journal of the Chinese Society of Rare Earths, 2014, 32(5), 595—603 |
| (佘文洁, 刘伟, 张瑶, 杨天林. 中国稀土学报,2014, 32(5), 595—603) | |
| [6] | Zhao L., Lu J. X., Xiang Z. L., Li J., Xu J. Y., Anal. Sci., 2011, 27(5), 581—585 |
| (赵龙, 卢继新, 项振玲, 李娟, 徐靖源. 分析科学学报,2011, 27(5), 581—585) | |
| [7] | Liu B., Wang J., Wang X., Liu B. M., He L. L., Xu S. K., Spectrochim. Acta Part A, 2010, 77, 1115—1121 |
| [8] | Narla S. N., Pinnamaneni P., Nie H., Li Y., Sun X. L., Biochem. Biophys. Res. Commun., 2013, 44, 562—567 |
| [9] | Xu X. W., Li X. J., Zhan S. H., Li Y. T., Wu Z. Y., Yan C. W., J. Mol. Struct., 2013, 1039, 28—36 |
| [10] | Zhang J., Li W. X., Ao B. Y., Feng S. Y., Xin X. D., Spectrochim. Acta Part A, 2014, 118, 972—980 |
| [11] | Xiao F. J., Gu M. Q., Liang Y., Li L. L., Li Y. J., Spectrochim. Acta Part A, 2014, 118, 1106—1112 |
| [12] | Shao S., Qiu J., Acta Phys.-Chim.Sin, 2009, 25(7), 1342—1346 |
| (邵爽, 邱瑾. 物理化学学报,2009, 25(7), 1342—1346 | |
| [13] | Vicente-Sancheza C., Egidoa J., Sanchez-Gonzaleza P. D., Perez-Barriocanal F., Lopez-Novoa J. M., Morales A. I., Food Chem. Toxicol., 2008, 46(6), 2279—2287 |
| [14] | Bourassa P., Dubeau S., Maharvi G. M., Fauq A. H., Thomas T. J., Tajmir-Riahi H. A., Eur. J. Med. Chem., 2011, 46, 4344—4353 |
| [15] | Yang S. P., Han L. J., Pan Y., Wang D. Q., Zhao C., Wang B., Chem. J. Chinese Universities, 2012, 33(1), 14—21 |
| (杨树平, 韩立军, 潘燕, 王大奇, 赵翠, 王波. 高等学校化学学报,2012, 33(1), 14—21) | |
| [16] | Guo Q., Li L. Z., Dong J. F., Liu H. Y., Xu T., Li J. H., Spectrochim. Acta Part A, 2013, 106, 155—162 |
| [17] | Saha S., Majumdar R., Roy M., Dighe R. R., Chakravarty A. R., Inorg. Chem., 2009, 48, 2652—2662 |
| [18] | Song Y. M., Wang L., Yao X. Q., Chinese J. Inorg. Chem., 2013, 29(1), 81—88 |
| (宋玉民, 王莉, 姚小强. 无机化学学报,2013, 29(1), 81—88) | |
| [19] | Wang Y. R., Liu F. T., Zhang H. Y., Zhang M., Hu P., Journal of Instrumental Analysis, 2012, 31(3), 267—272 |
| (王月荣, 刘馥婷, 章弘扬, 张敏, 胡坪. 分析测试学报,2012, 31(3), 267—272) | |
| [20] | Tang R.R., Tang C. H., Tang C. Q., J. Organomet. Chem., 2011, 696, 2040—2046 |
| [21] | Yan C. N., Zhang H. X., Liu Y., Acta Chim. Sinica, 2005, 18(63), 1727—1732 |
| (颜承农, 张华新, 刘义. 化学学报,2005, 18(63), 1727—1732) | |
| [22] | Belatik A., Hotchandani S., Bariyanga J., Tajmir-Riahi H. A., Eur. J. Med. Chem., 2012, 48, 114—123 |
| [23] | Wang Y. Q., Zhang H. M., Zhang G. C., Tao W. H., Fei Z. H., Liu Z. T., J. Pharm. Biomed. Anal., 2007, 43, 1869—1875 |
| [24] | Tian L. F., Liu Z. F., Hu X. L., Kong L., Liu S. P., Chem. J. Chinese Universities, 2012, 33(1), 59—65 |
| (田伦富, 刘忠芳, 胡小莉, 孔玲, 刘绍璞. 高等学校化学学报,2012, 33(1), 59—65) | |
| [25] | Timasheff S.N., Peeters H., Proteins of Biological Fluids, Pergamon Press, Oxford, 1972, 511—519 |
| [26] | Ross P. D., Subramanian S., Biochemistry, 1981, 20, 3096—3102 |
| [27] | Lin H. B., Zhen L., Lin Y. Q., Zhou Z. H., Chem. J. Chinese Universities, 2013, 34(8), 1818—1825 |
| (林海彬, 郑琳, 林玉琴, 周朝晖. 高等学校化学学报,2013, 34(8), 1818—1825) | |
| [28] | Lin H. B., Zhang M. X., Lin L. L., Zhou Z. H., Chinese J. Inorg. Chem., 2013, 29(11), 2315—2322 |
| (林海彬, 张美鑫, 林凉凉, 周朝晖. 无机化学学报,2013, 29(11), 2315—2322) | |
| [29] | Karami K., Hosseini-Kharat M., Sadeghi-Aliabadi H., Lipkowski J., Mirian M., Eur. J. Med. Chem., 2014, 73, 8—17 |
| [30] | Sun C. F., Guo N., Studies of Trace Elements and Health, 2011, 28(2), 64—66 |
| (孙长峰, 郭娜. 微量元素与健康研究,2011, 28(2), 64—66) | |
| [31] | Fu C. X., Gao Z. H., Physical Testing and Chemical Analysis, Part B: Chem. Anal., 2014, 50(2), 175—179 |
| (付彩霞, 高宗华. 理化检验,化学分册, 2014, 50(2), 175—179) | |
| [32] | Guo M., Wang W., Zhou J. Z., Guo X. Q., Chemical Research and Application, 2008, 20(4), 458—460 |
| (郭明, 王炜, 周建钟, 郭晓勤. 化学研究与应用,2008, 20(4), 458—460) | |
| [33] | Fu L., Wang S. J., Zhang S. Q., Jin H. L., Meng Y. N., J. Anal. Sci., 2014, 3(2), 239—243 |
| (傅丽, 王树军, 张世乾, 靳红利, 孟雅男. 分析科学学报,2014, 3(2), 239—243) | |
| [34] | Kang J. W., Su B. Q., Li Z. F., Wu H. X., Lu X. Q., Chemical Research and Application, 2006, 18(4), 360—365 |
| (康敬万, 苏碧泉, 李志峰, 吴海霞, 卢小泉. 化学研究与应用,2006, 18(4), 360—365) | |
| [35] | Wu H. L., Yuan J. K., Zhang Y. H., Shi F. R., Pan G. L., Kong J., Fan X. Y., Inorg. Chim. Acta, 2013, 404, 13—22 |
| [36] | Bakkialakshmi S., Chandrakala D., Spectrochim. Acta Part A, 2012, 88, 2—9 |
| [1] | 李梦硕, 张静, 刘丹, 朱亚先, 张勇. 芘与人血清白蛋白和牛血清白蛋白结合位点微环境极性的差异[J]. 高等学校化学学报, 2021, 42(3): 731. |
| [2] | 郝元元, 吴琪, 李季, 葛超, 马超盈, 钱勇, 苏志, 刘红科. 芳基锇联吡啶配合物的合成、 结构、 细胞毒性及与DNA/BSA的相互作用[J]. 高等学校化学学报, 2018, 39(4): 614. |
| [3] | 邱家欣, 江奇, 高艺珂, 彭俊棋, 段志虹, 卢晓英. MnO2包覆改性富锂锰基正极材料作用机理的电化学研究[J]. 高等学校化学学报, 2018, 39(10): 2238. |
| [4] | 安鹏姣, 于楠楠, 孙睿声, 隋小芳, 宋玉光. 全硫取代三苯甲基自由基酯基衍生物与牛血清白蛋白的相互作用[J]. 高等学校化学学报, 2017, 38(8): 1354. |
| [5] | 张静, 陈霖锋, 朱亚先, 张勇. 羟丙基-β-环糊精对1-羟基芘与牛血清白蛋白相互作用的影响[J]. 高等学校化学学报, 2017, 38(1): 28. |
| [6] | 张静, 陈薇晓, 张唯, 段滢, 朱玉秀, 朱亚先, 张勇. 荧光各向异性结合同步荧光法研究1-羟基芘与牛血清白蛋白的相互作用[J]. 高等学校化学学报, 2015, 36(8): 1511. |
| [7] | 蒋建宏, 李旭, 肖圣雄, 谷惠文, 李传华, 杨平, 魏得良, 何笃贵, 李爱桃, 李霞, 姚飞虹, 李强国. 2-{[4-氨基-5-(3,4,5-三甲氧基-苄基)-嘧啶-2-亚胺基]-甲基}-6-甲氧基-苯酚与酵母细胞和牛血清白蛋白的相互作用[J]. 高等学校化学学报, 2014, 35(4): 831. |
| [8] | 郝和群, 姚萍. 葡聚糖分子量和接枝度对阿霉素/白蛋白-葡聚糖纳米粒子体外抗肿瘤效果的影响[J]. 高等学校化学学报, 2014, 35(3): 652. |
| [9] | 王满元, 张超, 李静, 李朝霞, 龚慕辛. 青蒿截疟组合物与牛血清白蛋白的相互作用[J]. 高等学校化学学报, 2014, 35(2): 309. |
| [10] | 刘小霞, 邓浩, 王妍媖, 鲁志伟, 曾宪垠, 王显祥, 邹平, 饶含兵. BSA在不同形貌氧化锌材料上的吸附热力学[J]. 高等学校化学学报, 2014, 35(10): 2156. |
| [11] | 林海彬, 郑琳, 林玉琴, 周朝晖. 邻菲啰啉和氨三乙酸钴配合物与牛血清白蛋白相互作用的荧光分析[J]. 高等学校化学学报, 2013, 34(8): 1818. |
| [12] | 杨树平, 韩立军, 潘燕, 王大奇, 王南南, 王婷. 8-或6-(3-氯苯甲酰)香豆素衍生物的合成、表征、生物活性及与牛血清白蛋白的相互作用[J]. 高等学校化学学报, 2013, 34(2): 364. |
| [13] | 黄英, 王娟, 郭改英, 陶朱, 薛赛凤, 祝黔江, 周清娣. 光谱法研究硫鸟嘌呤与七元瓜环及牛血清白蛋白的超分子相互作用[J]. 高等学校化学学报, 2013, 34(2): 375. |
| [14] | 吕鉴泉, 胡芹芹, 丁然, 张霞, 周兴旺. 核壳结构AgInS2@ZnS量子点的合成及荧光性能[J]. 高等学校化学学报, 2013, 34(11): 2478. |
| [15] | 王珊珊, 王雪婷, 郭明明, 于俊生. 配体对CdTe量子点与BSA的选择性相互作用的影响[J]. 高等学校化学学报, 2012, 33(06): 1195. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||