

高等学校化学学报 ›› 2015, Vol. 36 ›› Issue (3): 469.doi: 10.7503/cjcu20140907
王美君, 卢俊瑞( ), 辛春伟, 刘金彪, 穆江蓓, 张贺, 张瑞波, 杨旭云, 王宏韫
), 辛春伟, 刘金彪, 穆江蓓, 张贺, 张瑞波, 杨旭云, 王宏韫
收稿日期:2014-10-11
出版日期:2015-03-10
发布日期:2015-01-30
作者简介:联系人简介: 卢俊瑞, 男, 博士, 教授, 博士生导师, 主要从事生物活性化合物分子设计及靶向药物创制研究. E-mail:基金资助:
WANG Meijun, LU Junrui*( ), XIN Chunwei, LIU Jinbiao, MU Jiangbei, ZHANG He, ZHANG Ruibo, YANG Xuyun, WANG Hongyun
), XIN Chunwei, LIU Jinbiao, MU Jiangbei, ZHANG He, ZHANG Ruibo, YANG Xuyun, WANG Hongyun
Received:2014-10-11
Online:2015-03-10
Published:2015-01-30
Contact:
LU Junrui
E-mail:lujunrui@tjut.edu.cn
Supported by:摘要:
以3-芳基/烷基-4-氨基-5-巯基-1,2,4-三唑为原料, 经过环化和糖化反应, 设计合成了10个未见报道的化合物3-芳基/烷基-6-S-2',3',4',6'-四-O-乙酰基-β-D-吡喃葡萄糖基-1,2,4-三唑并[3,4-b]-1,3,4-噻二唑(3a~3j), 其结构经核磁共振波谱、 高分辨质谱和红外光谱确认. 生物活性测试表明, 所有化合物均对大肠杆菌、 金黄色葡萄球菌、 枯草芽孢杆菌和白色念株菌表现出一定的抗菌活性, 其中化合物3c对4种测试菌株的最小抑菌浓度(MIC)最低, 且接近于氟康唑的参照数据, 具有较强的抗菌活性. 利用AutoDock 4.0程序研究了目标化合物3a~3j与大肠杆菌FabⅠ受体蛋白分子的相互作用和结合自由能变化规律.
中图分类号:
TrendMD:
王美君, 卢俊瑞, 辛春伟, 刘金彪, 穆江蓓, 张贺, 张瑞波, 杨旭云, 王宏韫. 新型1,2,4-三唑并[3,4-b]-1,3,4-噻二唑类化合物的合成、 抗菌活性及与FabⅠ作用的分子模拟. 高等学校化学学报, 2015, 36(3): 469.
WANG Meijun, LU Junrui, XIN Chunwei, LIU Jinbiao, MU Jiangbei, ZHANG He, ZHANG Ruibo, YANG Xuyun, WANG Hongyun. Synthesis, Antibacterial Activity and Molecular Simulation with FabⅠ of a Series of Novel 1,2,4-Triazolo[3,4-b]-1,3,4-thiadiazoles†. Chem. J. Chinese Universities, 2015, 36(3): 469.
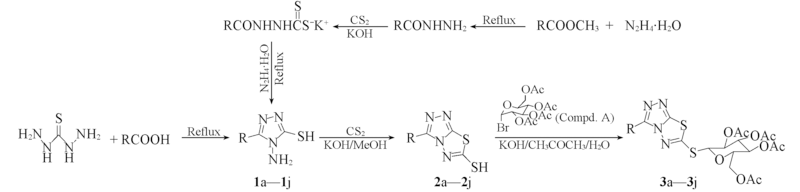
Scheme 1 Syntheses of the target compounds 3a—3j a. R=C6H5; b. R=2-OHC6H5; c. R=4-OHC6H5; d. R=4-CH3C6H5; e. R=4-ClC6H5;f. R=2-ClC6H5; g. R=CH3; h. R=C2H5; i. R=C3H7; j. R=C7H15
| Compd. | m.p./℃ | m.p.(ref.)/℃ | Compd. | m.p./℃ | m.p.(ref.)/℃ |
|---|---|---|---|---|---|
| A | 87.3—88.6 | 88.5[ | 1i | 107.9—109.3 | 108—109[ |
| 1a | 195.9—198.1 | 204—205[ | 2a | 211.7—213.3 | 212—213[ |
| 1b | 175.6—177.3 | 177.1[ | 2c | 167.3—169.1 | 257[ |
| 1c | 267.2—269.6 | >250[ | 2d | 210.3—212.5 | 208[ |
| 1d | 214.3—215.2 | 213[ | 2e | 191.4—193.7 | 134—135[ |
| 1e | 205.7—208.2 | 211—213[ | 2f | 179.3—181.7 | 171—172[ |
| 1f | 147.4—148.6 | 152[ | 2g | 240.7—242.8 | 235[ |
| 1g | 197.3—199.1 | 208—210[ | 2h | 225.3—227.9 | 222[ |
| 1h | 150.6—154.6 | 144[ |
Table 1 Physiochemical data of the intermediates*
| Compd. | m.p./℃ | m.p.(ref.)/℃ | Compd. | m.p./℃ | m.p.(ref.)/℃ |
|---|---|---|---|---|---|
| A | 87.3—88.6 | 88.5[ | 1i | 107.9—109.3 | 108—109[ |
| 1a | 195.9—198.1 | 204—205[ | 2a | 211.7—213.3 | 212—213[ |
| 1b | 175.6—177.3 | 177.1[ | 2c | 167.3—169.1 | 257[ |
| 1c | 267.2—269.6 | >250[ | 2d | 210.3—212.5 | 208[ |
| 1d | 214.3—215.2 | 213[ | 2e | 191.4—193.7 | 134—135[ |
| 1e | 205.7—208.2 | 211—213[ | 2f | 179.3—181.7 | 171—172[ |
| 1f | 147.4—148.6 | 152[ | 2g | 240.7—242.8 | 235[ |
| 1g | 197.3—199.1 | 208—210[ | 2h | 225.3—227.9 | 222[ |
| 1h | 150.6—154.6 | 144[ |
| Compd. | Appearance | Yield(%) | m.p./℃ | IR(KBr), | ESI-TOF-MS ([M+H]+), m/z |
|---|---|---|---|---|---|
| 2b | Pale yellow solid | 81.4 | 150.3—152.5 | 3121, 2134, 1645, 1575, 1476, 1238, 765 | 251.0060 |
| 3a | White solid | 50.3 | 94.4—96.9 | 3131, 2356, 1747, 1635, 1575, 1375, 917, 889, 773, 618 | 565.1069 |
| 3b | White solid | 57.7 | 96.9—98.4 | 3421, 3163, 2360, 1752, 1639, 1582, 1374, 918, 893, 773, 620 | 581.1009 |
| 3c | White solid | 68.2 | 139.8—142.3 | 3441, 3113, 2361, 1752, 1614, 1548, 1380, 917, 893, 776, 619 | 581.1008 |
| 3d | White solid | 68.9 | 92.7—94.8 | 3135, 2361, 1746, 1643, 1554, 1387, 1046, 919, 898, 775, 645, 630 | 570.1223 |
| 3e | White solid | 63.4 | 186.8—189.2 | 3180, 2364, 1745, 1655, 1551, 1385, 921, 896, 774, 617 | 599.0670 |
| 3f | Pale yellow solid | 59.4 | 53.4—55.7 | 3164, 2362, 1745, 1636, 1582, 1376, 919, 891, 779, 619 | 599.0668 |
| 3g | White solid | 54.9 | 155.5—157.2 | 1751, 1655, 1602, 1385, 920, 898, 778, 618 | 503.0900 |
| 3h | White solid | 52.67 | 146.1—148.7 | 1743, 1635, 1378, 923, 897, 778, 619 | 517.1068 |
| 3i | White solid | 54.8 | 109.2—111.7 | 1743, 1636, 1378, 919, 892, 778, 617 | 531.1215 |
| 3j | Pale yellow solid | 51.7 | 76.3—77.7 | 1747, 1636, 1378, 920, 897, 779, 616 | 587.1849 |
Table 2 Physiochemical data, IR and MS data of intermediate 2b and target compounds 3a—3j
| Compd. | Appearance | Yield(%) | m.p./℃ | IR(KBr), | ESI-TOF-MS ([M+H]+), m/z |
|---|---|---|---|---|---|
| 2b | Pale yellow solid | 81.4 | 150.3—152.5 | 3121, 2134, 1645, 1575, 1476, 1238, 765 | 251.0060 |
| 3a | White solid | 50.3 | 94.4—96.9 | 3131, 2356, 1747, 1635, 1575, 1375, 917, 889, 773, 618 | 565.1069 |
| 3b | White solid | 57.7 | 96.9—98.4 | 3421, 3163, 2360, 1752, 1639, 1582, 1374, 918, 893, 773, 620 | 581.1009 |
| 3c | White solid | 68.2 | 139.8—142.3 | 3441, 3113, 2361, 1752, 1614, 1548, 1380, 917, 893, 776, 619 | 581.1008 |
| 3d | White solid | 68.9 | 92.7—94.8 | 3135, 2361, 1746, 1643, 1554, 1387, 1046, 919, 898, 775, 645, 630 | 570.1223 |
| 3e | White solid | 63.4 | 186.8—189.2 | 3180, 2364, 1745, 1655, 1551, 1385, 921, 896, 774, 617 | 599.0670 |
| 3f | Pale yellow solid | 59.4 | 53.4—55.7 | 3164, 2362, 1745, 1636, 1582, 1376, 919, 891, 779, 619 | 599.0668 |
| 3g | White solid | 54.9 | 155.5—157.2 | 1751, 1655, 1602, 1385, 920, 898, 778, 618 | 503.0900 |
| 3h | White solid | 52.67 | 146.1—148.7 | 1743, 1635, 1378, 923, 897, 778, 619 | 517.1068 |
| 3i | White solid | 54.8 | 109.2—111.7 | 1743, 1636, 1378, 919, 892, 778, 617 | 531.1215 |
| 3j | Pale yellow solid | 51.7 | 76.3—77.7 | 1747, 1636, 1378, 920, 897, 779, 616 | 587.1849 |
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(400 MHz, CDCl3), δ |
|---|---|---|
| 2b | 13.8(s, 1H, SH), 11.06(s, 1H, OH), 8.41(d, 1H, J=6.7 Hz, ArH), 7.49(t, 1H, J=6.3 Hz, ArH), 7.12(t, 2H, J=6.3 Hz, ArH) | 157.02, 155.98, 155.32, 134.09, 132.41, 129.14, 128.68, 119.89, 116.93 |
| 3a | 8.32(d, 2H, J=7.12 Hz, ArH), 7.55(m, 3H, ArH) 5.36(m, 1H, H1'), 5.24(m, 2H, H2, H3'), 5.18(t, 1H, | 170.48, 170.16, 169.96, 169.27, 161.47, 154.76, 146.32, 130.62, 128.99, 128.65, 126.44, 125.70, 125.58, 83.73, 73.23, 69.70, 69.51, 67.68, 61.67, 20.68, 20.63, 20.55, 20.51 |
| 3b | 10.97(s, 1H, OH), 7.94(d, 1H, J=7.7 Hz, ArH), 7.36(t, 1H, J=7.6 Hz, ArH), 7.02(d, 1H, J=8.2 Hz, ArH), 6.97(t, 1H, J=7.6 Hz, ArH), 5.65(d, 1H, JH1'-H2'=10.2 Hz, H1'), 5.46(t, 1H, | 170.41, 169.94, 169.73, 169.57, 156.28, 155.14, 153.55, 132.35, 127.93, 120.08, 119.92, 117.22, 112.48, 82.63, 75.26, 73.42, 70.34, 68.43, 62.20, 21.49, 20.91, 20.80, 20.72 |
| 3c | 10.13(s, 1H, OH), 8.04(d, 2H, J=8.7 Hz, ArH), 6.97(d, 2H, J=8.7 Hz, ArH), 5.76(d, 1H, JH1'-H2'=10.0 Hz, H1'), 5.48(t, 1H, JH2'-H1'=JH2'-H3'=9.4 Hz, H2'), 5.14(t, 1H, JH3'-H4'=JH3'-H2'=9.7 Hz, H3'), 5.07(t, 1H, JH4'-H5'=JH4'-H3'=9.7 Hz, H4'), 4.21(m, 3H, H6″, H6', H5'), 2.09, 2.07, 2.02, 1.96(4s, 12H, 4CH3CO) | 170.38, 169.95, 169.75, 169.71, 159.98, 154.62, 145.81, 132.50, 128.17, 124.43, 120.09, 116.49, 116.43, 82.52, 75.65, 72.94, 69.74, 68.17, 62.17, 20.90, 20.83, 20.79, 20.71 |
| 3d | 8.19(d, 2H, J=8.0 Hz, ArH), 7.36(d, 2H, J=8.0 Hz, ArH), 5.36(d, 1H, JH1'-H2'=8.6 Hz, H1'), 5.24(m, 2H, H2', H3'), 5.18(t, 1H, JH4'-H5'=JH4'-H3'=9.7 Hz, H4'), 4.32(dd, 1H, JH6″-H6'=12.5 Hz, JH6″-H5'=5.1 Hz, H6″), 4.22(dd, 1H, JH6'-H6″=11.9 Hz, JH6'-H5'=2.0 Hz, H6'), 3.91(ddd, 1H, JH5'-H4'=10.0 Hz, JH5'-H6″=5.1 Hz, JH5'-H6'=2.0 Hz, H5'), 2.45(s, 3H, CH3), 2.13, 2.08, 2.05, 2.04(4s, 12H, 4CH3CO) | 170.46, 169.94, 169.38, 169.24, 161.06, 154.42, 146.48, 140.91, 129.65, 128.43, 126.34, 122.50, 120.99, 83.79, 73.27, 69.55, 67.71, 66.33, 61.68, 21.54, 20.67, 20.58, 20.49, 10.07 |
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(400 MHz, CDCl3), δ |
| 3e | 8.38(d, 2H, J=8.6 Hz, ArH), 7.64(d, 2H, J=8.6 Hz, ArH), 5.35(d, 1H, JH1'-H2'=9.1 Hz, H1'), 5.26—5.15(m, 3H, H2', H3', H4'), 4.32(dd, 1H, JH6″-H6'=12.6 Hz, JH6″-H5'=5.1 Hz, H6″), 4.24(dd, 1H, JH6'-H6″=12.5 Hz, JH6'-H5'=2.0 Hz, H6'), 3.91(ddd, 1H, JH5'-H4'=10.0 Hz, JH5'-H6″=5.1 Hz, JH5'-H6'=2.1 Hz, H5'), 2.13, 2.08, 2.06, 2.04(4s, 12H, 4CH3CO) | 170.38, 169.97, 169.76, 169.72, 162.70, 155.77, 144.66, 135.54, 129.81, 129.74, 128.43, 128.03, 124.58, 82.52, 75.69, 72.97, 69.73, 68.15, 62.20, 20.90, 20.83, 20.78, 20.70 |
| 3f | 8.43(d, 1H, J=7.6 Hz, ArH), 8.03(d, 1H, J=8.0 Hz, ArH), 7.57(t, 1H, J=7.6 Hz, ArH), 7.50(t, 1H, J=7.5 Hz, ArH), 5.32—5.12(m, 4H, H1', H2', H3', H4'), 4.29(dd, 1H, JH6″-H6'=12.6 Hz, JH6″-H5'=4.8 Hz, H6″), 4.16(dd, 1H, JH6'-H6″=12.6 Hz, JH6'-H5'=1.7 Hz, H6'), 3.86(ddd, 1H, JH5'-H4'=12.0 Hz, JH5'-H6″=4.7 Hz, JH5'-H6'=1.9 Hz, H5'), 2.06, 2.04, 2.03, 2.01(4s, 12H, 4CH3CO) | 170.48, 169.94, 169.24, 168.91, 161.84, 154.69, 145.08, 133.80, 132.09, 130.91, 128.81, 127.09, 124.50, 83.67, 73.23, 69.43, 67.57, 65.54, 61.54, 20.82, 20.69, 20.57, 20.48 |
| 3g | 5.33(m, 1H, H1'), 5.17(m, 3H, H2', H3', H4'), 4.34(dd, 1H, JH6″-H6'=12.6 Hz, JH6″-H5'=4.9 Hz, H6″), 4.23(dd, 1H, JH6'-H6″=12.6 Hz, JH6'-H5'=2.0 Hz, H6'), 3.91(ddd, 1H, JH5'-H4'=10.0 Hz, JH5'-H6″=4.8 Hz, JH5'-H6'=2.1 Hz, H5'), 2.78(s, 1H, CH3), 2.13, 2.12, 2.07, 2.05(4s, 12H, 4CH3CO) | 170.40, 169.94, 169.72, 169.68, 161.41, 153.80, 144.39, 82.60, 75.62, 72.92, 69.79, 68.07, 62.13, 20.99, 20.81, 20.74, 20.69, 10.27 |
| 3h | 5.33(t, 1H, JH1'-H2'=6.8 Hz, H1'), 5.19—5.13(m, 3H, H2', H3', H4'), 4.32(dd, 1H, JH6″-H6'=12.6 Hz, JH6″-H5'=4.9 Hz, H6″), 4.22(dd, 1H, JH6'-H6″=12.5 Hz, JH6'-H5'=1.8 Hz, H6'), 3.91(ddd, 1H, JH5'-H4'=10.0 Hz, JH5'-H6″=4.8 Hz, JH5'-H6'=2.0 Hz, H5'), 3.12(dd, 2H, J1=15.1 Hz, J2=7.6 Hz, CH2), 2.12, 2.10, 2.07, 2.04(4s, 12H, 4CH3CO), 1.46(t, 3H, J=7.6 Hz, CH3) | 170.43, 169.95, 169.22, 169.17, 160.53, 154.24, 140.47, 83.73, 73.24, 73.12, 69.53, 67.60, 61.61, 20.78, 20.68, 20.54, 20.51, 18.75, 11.09 |
| 3i | 5.30(t, 1H, JH1'-H2'=9.0 Hz, H1'), 5.17—5.11(m, 3H, H2', H3', H4'), 4.29(dd, 1H, JH6″-H6=12.7 Hz, JH6″-H5'=4.9 Hz, H6″), 4.18(dd, 1H, JH6'-H6″=12.5 Hz, JH6'-H5'=1.7 Hz, H6'), 3.91(ddd, 1H, JH5'-H4'=10.0 Hz, JH5'-H6″=4.6 Hz, JH5'-H6'=1.9 Hz, H5'), 3.04(t, 2H, J=7.5 Hz, CH2), 2.08, 2.06, 2.03, 2.00(4s, 12H, 4CH3CO), 1.88(m, 2H, CH2), 1.02(t, 3H, J=7.4 Hz, CH3) | 170.44, 169.93, 169.22, 169.16, 160.83, 153.60, 148.04, 83.67, 73.22, 69.51, 67.58, 63.67, 61.57, 29.62, 26.82, 20.74, 20.52, 20.49, 20.21, 13.70 |
| 3j | 5.28(t, 1H, JH1'-H2'=7.0 Hz, H1'), 5.15-5.09(m, 3H, H2', H3', H4'), 4.28(dd, 1H, JH6″-H6'=12.6 Hz, JH6″-H5'=4.9 Hz, H6″), 4.17(dd, 1H, JH6'-H6″=12.5 Hz, JH6'-H5'=1.9 Hz, H6'), 3.91(ddd, 1H, JH5'-H4'=10.0 Hz, JH5'-H6″=4.8 Hz, JH5'-H6'=2.0 Hz, H5'), 3.03(t, 2H, J=7.6 Hz, CH2), 2.07, 2.05, 2.02, 2.00(4s, 12H, 4CH3CO), 1.83(m, 2H, CH2), 1.40—1.19(m, 8H, CH2CH2CH2CH2), 0.84(t, 3H, J=6.7 Hz, CH3) | 170.37, 169.89, 169.18, 169.09, 160.76, 153.55, 148.21, 83.64, 73.22, 69.53, 67.61, 61.57, 58.16, 31.57, 29.00, 28.73, 26.62, 24.89, 22.52, 20.78, 20.67, 20.46, 20.42, 13.98 |
Table 3 1H NMR and 13C NMR data of intermediate 2b and target compounds 3a—3j
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(400 MHz, CDCl3), δ |
|---|---|---|
| 2b | 13.8(s, 1H, SH), 11.06(s, 1H, OH), 8.41(d, 1H, J=6.7 Hz, ArH), 7.49(t, 1H, J=6.3 Hz, ArH), 7.12(t, 2H, J=6.3 Hz, ArH) | 157.02, 155.98, 155.32, 134.09, 132.41, 129.14, 128.68, 119.89, 116.93 |
| 3a | 8.32(d, 2H, J=7.12 Hz, ArH), 7.55(m, 3H, ArH) 5.36(m, 1H, H1'), 5.24(m, 2H, H2, H3'), 5.18(t, 1H, | 170.48, 170.16, 169.96, 169.27, 161.47, 154.76, 146.32, 130.62, 128.99, 128.65, 126.44, 125.70, 125.58, 83.73, 73.23, 69.70, 69.51, 67.68, 61.67, 20.68, 20.63, 20.55, 20.51 |
| 3b | 10.97(s, 1H, OH), 7.94(d, 1H, J=7.7 Hz, ArH), 7.36(t, 1H, J=7.6 Hz, ArH), 7.02(d, 1H, J=8.2 Hz, ArH), 6.97(t, 1H, J=7.6 Hz, ArH), 5.65(d, 1H, JH1'-H2'=10.2 Hz, H1'), 5.46(t, 1H, | 170.41, 169.94, 169.73, 169.57, 156.28, 155.14, 153.55, 132.35, 127.93, 120.08, 119.92, 117.22, 112.48, 82.63, 75.26, 73.42, 70.34, 68.43, 62.20, 21.49, 20.91, 20.80, 20.72 |
| 3c | 10.13(s, 1H, OH), 8.04(d, 2H, J=8.7 Hz, ArH), 6.97(d, 2H, J=8.7 Hz, ArH), 5.76(d, 1H, JH1'-H2'=10.0 Hz, H1'), 5.48(t, 1H, JH2'-H1'=JH2'-H3'=9.4 Hz, H2'), 5.14(t, 1H, JH3'-H4'=JH3'-H2'=9.7 Hz, H3'), 5.07(t, 1H, JH4'-H5'=JH4'-H3'=9.7 Hz, H4'), 4.21(m, 3H, H6″, H6', H5'), 2.09, 2.07, 2.02, 1.96(4s, 12H, 4CH3CO) | 170.38, 169.95, 169.75, 169.71, 159.98, 154.62, 145.81, 132.50, 128.17, 124.43, 120.09, 116.49, 116.43, 82.52, 75.65, 72.94, 69.74, 68.17, 62.17, 20.90, 20.83, 20.79, 20.71 |
| 3d | 8.19(d, 2H, J=8.0 Hz, ArH), 7.36(d, 2H, J=8.0 Hz, ArH), 5.36(d, 1H, JH1'-H2'=8.6 Hz, H1'), 5.24(m, 2H, H2', H3'), 5.18(t, 1H, JH4'-H5'=JH4'-H3'=9.7 Hz, H4'), 4.32(dd, 1H, JH6″-H6'=12.5 Hz, JH6″-H5'=5.1 Hz, H6″), 4.22(dd, 1H, JH6'-H6″=11.9 Hz, JH6'-H5'=2.0 Hz, H6'), 3.91(ddd, 1H, JH5'-H4'=10.0 Hz, JH5'-H6″=5.1 Hz, JH5'-H6'=2.0 Hz, H5'), 2.45(s, 3H, CH3), 2.13, 2.08, 2.05, 2.04(4s, 12H, 4CH3CO) | 170.46, 169.94, 169.38, 169.24, 161.06, 154.42, 146.48, 140.91, 129.65, 128.43, 126.34, 122.50, 120.99, 83.79, 73.27, 69.55, 67.71, 66.33, 61.68, 21.54, 20.67, 20.58, 20.49, 10.07 |
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(400 MHz, CDCl3), δ |
| 3e | 8.38(d, 2H, J=8.6 Hz, ArH), 7.64(d, 2H, J=8.6 Hz, ArH), 5.35(d, 1H, JH1'-H2'=9.1 Hz, H1'), 5.26—5.15(m, 3H, H2', H3', H4'), 4.32(dd, 1H, JH6″-H6'=12.6 Hz, JH6″-H5'=5.1 Hz, H6″), 4.24(dd, 1H, JH6'-H6″=12.5 Hz, JH6'-H5'=2.0 Hz, H6'), 3.91(ddd, 1H, JH5'-H4'=10.0 Hz, JH5'-H6″=5.1 Hz, JH5'-H6'=2.1 Hz, H5'), 2.13, 2.08, 2.06, 2.04(4s, 12H, 4CH3CO) | 170.38, 169.97, 169.76, 169.72, 162.70, 155.77, 144.66, 135.54, 129.81, 129.74, 128.43, 128.03, 124.58, 82.52, 75.69, 72.97, 69.73, 68.15, 62.20, 20.90, 20.83, 20.78, 20.70 |
| 3f | 8.43(d, 1H, J=7.6 Hz, ArH), 8.03(d, 1H, J=8.0 Hz, ArH), 7.57(t, 1H, J=7.6 Hz, ArH), 7.50(t, 1H, J=7.5 Hz, ArH), 5.32—5.12(m, 4H, H1', H2', H3', H4'), 4.29(dd, 1H, JH6″-H6'=12.6 Hz, JH6″-H5'=4.8 Hz, H6″), 4.16(dd, 1H, JH6'-H6″=12.6 Hz, JH6'-H5'=1.7 Hz, H6'), 3.86(ddd, 1H, JH5'-H4'=12.0 Hz, JH5'-H6″=4.7 Hz, JH5'-H6'=1.9 Hz, H5'), 2.06, 2.04, 2.03, 2.01(4s, 12H, 4CH3CO) | 170.48, 169.94, 169.24, 168.91, 161.84, 154.69, 145.08, 133.80, 132.09, 130.91, 128.81, 127.09, 124.50, 83.67, 73.23, 69.43, 67.57, 65.54, 61.54, 20.82, 20.69, 20.57, 20.48 |
| 3g | 5.33(m, 1H, H1'), 5.17(m, 3H, H2', H3', H4'), 4.34(dd, 1H, JH6″-H6'=12.6 Hz, JH6″-H5'=4.9 Hz, H6″), 4.23(dd, 1H, JH6'-H6″=12.6 Hz, JH6'-H5'=2.0 Hz, H6'), 3.91(ddd, 1H, JH5'-H4'=10.0 Hz, JH5'-H6″=4.8 Hz, JH5'-H6'=2.1 Hz, H5'), 2.78(s, 1H, CH3), 2.13, 2.12, 2.07, 2.05(4s, 12H, 4CH3CO) | 170.40, 169.94, 169.72, 169.68, 161.41, 153.80, 144.39, 82.60, 75.62, 72.92, 69.79, 68.07, 62.13, 20.99, 20.81, 20.74, 20.69, 10.27 |
| 3h | 5.33(t, 1H, JH1'-H2'=6.8 Hz, H1'), 5.19—5.13(m, 3H, H2', H3', H4'), 4.32(dd, 1H, JH6″-H6'=12.6 Hz, JH6″-H5'=4.9 Hz, H6″), 4.22(dd, 1H, JH6'-H6″=12.5 Hz, JH6'-H5'=1.8 Hz, H6'), 3.91(ddd, 1H, JH5'-H4'=10.0 Hz, JH5'-H6″=4.8 Hz, JH5'-H6'=2.0 Hz, H5'), 3.12(dd, 2H, J1=15.1 Hz, J2=7.6 Hz, CH2), 2.12, 2.10, 2.07, 2.04(4s, 12H, 4CH3CO), 1.46(t, 3H, J=7.6 Hz, CH3) | 170.43, 169.95, 169.22, 169.17, 160.53, 154.24, 140.47, 83.73, 73.24, 73.12, 69.53, 67.60, 61.61, 20.78, 20.68, 20.54, 20.51, 18.75, 11.09 |
| 3i | 5.30(t, 1H, JH1'-H2'=9.0 Hz, H1'), 5.17—5.11(m, 3H, H2', H3', H4'), 4.29(dd, 1H, JH6″-H6=12.7 Hz, JH6″-H5'=4.9 Hz, H6″), 4.18(dd, 1H, JH6'-H6″=12.5 Hz, JH6'-H5'=1.7 Hz, H6'), 3.91(ddd, 1H, JH5'-H4'=10.0 Hz, JH5'-H6″=4.6 Hz, JH5'-H6'=1.9 Hz, H5'), 3.04(t, 2H, J=7.5 Hz, CH2), 2.08, 2.06, 2.03, 2.00(4s, 12H, 4CH3CO), 1.88(m, 2H, CH2), 1.02(t, 3H, J=7.4 Hz, CH3) | 170.44, 169.93, 169.22, 169.16, 160.83, 153.60, 148.04, 83.67, 73.22, 69.51, 67.58, 63.67, 61.57, 29.62, 26.82, 20.74, 20.52, 20.49, 20.21, 13.70 |
| 3j | 5.28(t, 1H, JH1'-H2'=7.0 Hz, H1'), 5.15-5.09(m, 3H, H2', H3', H4'), 4.28(dd, 1H, JH6″-H6'=12.6 Hz, JH6″-H5'=4.9 Hz, H6″), 4.17(dd, 1H, JH6'-H6″=12.5 Hz, JH6'-H5'=1.9 Hz, H6'), 3.91(ddd, 1H, JH5'-H4'=10.0 Hz, JH5'-H6″=4.8 Hz, JH5'-H6'=2.0 Hz, H5'), 3.03(t, 2H, J=7.6 Hz, CH2), 2.07, 2.05, 2.02, 2.00(4s, 12H, 4CH3CO), 1.83(m, 2H, CH2), 1.40—1.19(m, 8H, CH2CH2CH2CH2), 0.84(t, 3H, J=6.7 Hz, CH3) | 170.37, 169.89, 169.18, 169.09, 160.76, 153.55, 148.21, 83.64, 73.22, 69.53, 67.61, 61.57, 58.16, 31.57, 29.00, 28.73, 26.62, 24.89, 22.52, 20.78, 20.67, 20.46, 20.42, 13.98 |
| Compd. | Minimal inhibitory concentration/(μg·mL-1) | |||
|---|---|---|---|---|
| Bacillus subtilis | Staphylococcus aureus | Escherichia coli | Monilia albican | |
| 3a | 64 | 64 | 128 | 8 |
| 3b | 64 | 32 | 64 | 4 |
| 3c | 32 | 16 | 32 | 4 |
| 3d | 64 | 32 | 64 | 8 |
| 3e | 64 | 32 | 32 | 8 |
| 3f | 64 | 64 | 128 | 4 |
| 3g | 64 | 64 | 64 | 4 |
| 3h | 64 | 64 | 128 | 4 |
| 3i | 128 | 64 | 128 | 16 |
| 3j | 64 | 128 | 64 | 32 |
| Triclosan | 16 | 4 | 2 | 32 |
| Fluconazole | 32 | 16 | 128 | 2 |
Table 4 Antibacterial activity data of compounds 3a—3j
| Compd. | Minimal inhibitory concentration/(μg·mL-1) | |||
|---|---|---|---|---|
| Bacillus subtilis | Staphylococcus aureus | Escherichia coli | Monilia albican | |
| 3a | 64 | 64 | 128 | 8 |
| 3b | 64 | 32 | 64 | 4 |
| 3c | 32 | 16 | 32 | 4 |
| 3d | 64 | 32 | 64 | 8 |
| 3e | 64 | 32 | 32 | 8 |
| 3f | 64 | 64 | 128 | 4 |
| 3g | 64 | 64 | 64 | 4 |
| 3h | 64 | 64 | 128 | 4 |
| 3i | 128 | 64 | 128 | 16 |
| 3j | 64 | 128 | 64 | 32 |
| Triclosan | 16 | 4 | 2 | 32 |
| Fluconazole | 32 | 16 | 128 | 2 |
| [1] | Almajan G. L., Barbuceanu S. F., Bancescu G., Saramet I., Saramet G., Draghici C., European Journal of Medicinal Chemistry,2010, 45(12), 6139—6146 |
| [2] | Husain A., Rashid M., Akhter A., Journal of Pharmacy Research,2011, 4(3), 888—890 |
| [3] | Li Y. D., Mao W. T., Fan Z. J., Li J. J., Fang Z., Ji X. T., Zong G. N., Li F. Y., Chem. Res. Chinese Universities,2014, 30(3), 390—395 |
| [4] | Peng C. Y., Lu J. R., Xin C. W., Li J. F., Ji D., Bao X. R., Chem. J. Chinese Universities,2013, 34(6), 1394—1402 |
| (彭春勇, 卢俊瑞, 辛春伟, 李建发, 戢丹, 鲍秀荣. 高等学校化学学报, 2013, 34(6), 1394—1402) | |
| [5] | Rajesh D. H., Satyanarayana D., Int. J. Pharm. Bio. Sci., 2012, 3(4), 183—192 |
| [6] | Mahendra S., Rajesh K., Indian Journal of Chemistry,2006, 4(5), 1009—1013 |
| [7] | Sadaf J. G., Suroor A. K., Nadeem S., Medicinal Chemistry Letters,2010, 20(16), 4762—4765 |
| [8] | Gita C., Umesh K., Sandhya B., Jagdish K., Journal of Enzyme Inhibition and Medicinal Chemistry,2012, 27(5), 658—665 |
| [9] | Mohd A., Kumar H., Javed S. A., European Journal of Medicinal Chemistry,2008, 43(10), 2056—66 |
| [10] | Zhao J., Xuan L. N., Zhao H. C., Cheng J., Fu X. Y., Li S., Jing F., Liu Y. M., Chen B. Q., Chem. Res. Chinese Universities,2014, 30(5), 764—769 |
| [11] | Ibrahim D. A., European Journal of Medicinal Chemistry, 2009, 44(7), 2776—2781 |
| [12] | Asif H., Mohammad A. N., Mohammad S., Acta Poloniae Pharmaceutica,2009, 66(2), 135—140 |
| [13] | Feng Z. N., Lu J. R., Xin C. W., Li J. F., Bao X. R., Zhang T. T., Chem. J. Chinese Universities,2013, 34(5), 1143—1150 |
| (冯钟念, 卢俊瑞, 辛春伟, 李建发, 鲍秀容, 张彤彤. 高等学校化学学报, 2013, 34(5), 1143—1150) | |
| [14] | Driguez H., Chem.Bio.Chem., 2001, 2(5), 311—318 |
| [15] | Knapp S., Gonzalez S., Myers D. S., Eekinan L. L., Bewley C. A., Org. Lett., 2002, 4(24), 4337—4339 |
| [16] | Jahn M., Marles J., Warren R. A., Withers S. G., Angew. Chem. Int. Ed., 2003, 42(3), 352—354 |
| [17] | Uhrig M. L., Manzano V. E., Varela O., Eur. J. Org. Chem., 2006, 2006(1), 162—168 |
| [18] | Mu M. M., Lu B. W., Lu J. R., Xin C. W., Ji D., Li J. F., Bao X. R., Chinese Journal of Organic Chemistry,2012, 32, 1101—1107 |
| (穆曼曼, 卢博为, 卢俊瑞, 辛春伟, 戢丹, 李建发, 鲍秀荣. 有机化学, 2012, 32, 1101—1107) | |
| [19] | Zhao Q., Lu J. R., Xin C. W., Bao X. R., Gao H. Y., Zhao X., Li S., Rui T. T., Chem. J. Chinese Universities,2011, 32(12), 2806—2811 |
| (赵芡, 卢俊瑞, 辛春伟, 鲍秀荣, 高海燕, 赵旭, 李莎, 芮甜甜. 高等学校化学学报, 2011, 32(12), 2806—2811) | |
| [20] | Peng C. Y., Lu J. R., Xin C. W., Li J. F., Ji D., Bao X. R., Chinese Journal of Organic Chemistry,2013, 33, 383—388 |
| (彭春勇, 卢俊瑞, 辛春伟, 李建发, 戢丹, 鲍秀荣. 有机化学, 2013, 33, 383—388) | |
| [21] | Ai Y. X., Lu J. R., Xin C. W., Mu J. B., Yang X. Y., Zhang H., Acta Phys. Chim. Sin., 2014, 30(3), 559—568 |
| (艾义新, 卢俊瑞, 辛春伟, 穆江蓓, 杨旭云, 张贺. 物理化学学报, 2014, 30(3), 559—568) | |
| [22] | Ling X.L.,Journal of Luoyang Normal University, 2008, (2), 75—77 |
| (凌勋利. 洛阳师范学院学报, 2008, (2), 75—77) | |
| [23] | Karegoudar P., Prasad D. J., Ashok M., Mahalinga M., Poojary B., Holla B. S., European Journal of Medicinal Chemistry,2008, 43, 808—815 |
| [24] | Liu C. Y., Zhao Q. Q., Li J., Chemical Reagents,2001, 23(6), 344—345 |
| (柳翠英, 赵全芹, 李娟. 化学试剂, 2001, 23(6), 344—345) | |
| [25] | Eweiss N. F., Bahajaj A. A., Journal of Heterocyclic Chemistry,1987, 24(4), 1173—1182 |
| [26] | Mohan J., Indian Journal of Chemistry, Section B, 2005, 44B(3), 628—630 |
| [27] | Potts K.T., Huseby R. M., Chemistry & Industry(London, United Kingdom), 1964, 46, 1919—1920 |
| [28] | Ji D., Lu J.R., Lu B. W., Xin C. W., Mu J. B., Li J. F., Peng C. Y., Bao X. R., Bioorganic & Medicinal Chemistry Letters, 2013, 23, 1997—2000 |
| [1] | 胡志飞, 徐鹏, 魏炳成, 俞飚. 苯丙素苷Acteoside,Isoacteoside和Ligupurpuroside J的全合成[J]. 高等学校化学学报, 2020, 41(8): 1708. |
| [2] | 朱铃,王雨辰,赵江源,文孟良,李铭刚,韩秀林. 重组β-木糖苷酶转化人参皂苷Rb3及C-Mx[J]. 高等学校化学学报, 2020, 41(5): 1010. |
| [3] | 毛龙, 刘跃军, 范淑红. 聚吡咯改性层状黏土/聚己内酯抗菌纳米复合材料的制备与性能[J]. 高等学校化学学报, 2019, 40(8): 1726. |
| [4] | 张隽荣, 尤慧梅, 井宇星, 赵佳文, 王韦, 刘文星, 周敏, 江志勇. 柳叶五层龙正丁醇提取部分中的3个新酚性成分及其α-葡萄糖苷酶抑制活性[J]. 高等学校化学学报, 2019, 40(3): 456. |
| [5] | 李成, 王承健, 晋万军, 韩健利, 杨梅芳, 郜茜, 黄琳娟, 王仲孚. 银杏种子糖蛋白的SDS-PAGE分离及N-糖链的质谱分析[J]. 高等学校化学学报, 2019, 40(1): 69. |
| [6] | 贾云静, 史文思, 胡飞柳, 朱华结, 刘莉, 马正月. 漆斑菌中单端孢霉烯化合物及衍生物的细胞毒活性[J]. 高等学校化学学报, 2018, 39(8): 1668. |
| [7] | 刘莉, 马洋洋, 王宽, 贾云静, 李婉, 朱华结. β-咔啉衍生物的抗肿瘤及抗菌活性[J]. 高等学校化学学报, 2018, 39(4): 674. |
| [8] | 刘欣茹, 刘春莹, 徐龙权, 宋建国, 鱼红闪. 人参皂苷β-葡萄糖苷酶基因的毕赤酵母载体构建及生物转化[J]. 高等学校化学学报, 2018, 39(11): 2451. |
| [9] | 张冰洋, 马洋洋, 郭华, 朱华结, 李婉. 三七内生真菌Talaromyces Purpureogenus中2个Drimane倍半萜绝对构型的鉴定[J]. 高等学校化学学报, 2017, 38(6): 1046. |
| [10] | 马洋洋, 蔡卉, 杜敏, 曹飞, 朱华结. 海洋来源真菌Penicillium pinophilum次级代谢产物中Azaphilones类化合物的分离及抗菌活性[J]. 高等学校化学学报, 2017, 38(11): 1963. |
| [11] | 高彤, 蔡思源, 许兰兰, 曹飞, 朱华结. 海洋来源真菌Penicillim grisefulvum中桔霉素类化合物的分离及抗菌活性[J]. 高等学校化学学报, 2016, 37(7): 1282. |
| [12] | 王宏韫, 刘金彪, 卢俊瑞, 应明, 杨旭云, 杨树勋, 马瑶. 5-甲基-1,2,4-三唑-3-硫酮类糖苷化合物的合成及抗菌活性[J]. 高等学校化学学报, 2016, 37(2): 246. |
| [13] | 许春春, 于渤浩, 王红蕾, 李晶, 刘淑莹, 于珊珊. 重组嗜热β-葡萄糖苷酶转化稀有人参皂苷Rd和CK[J]. 高等学校化学学报, 2016, 37(2): 281. |
| [14] | 许兰兰, 赵齐齐, 俞和, 王景晨, 王慧君, 杨芹, 朱华结, 李艳. 分离自木霉菌Trichoderma sp.的新型化合物Trichoderol A及绝对构型的确定[J]. 高等学校化学学报, 2016, 37(11): 1972. |
| [15] | 张瑞波, 卢俊瑞, 刘金彪, 穆江蓓, 杨旭云, 王宏韫, 王美君, 张贺, 张玫. 3-S-(β-D-吡喃葡萄糖基)-1,2,4-三唑的合成及抗菌活性[J]. 高等学校化学学报, 2015, 36(8): 1521. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||