

Chem. J. Chinese Universities ›› 2020, Vol. 41 ›› Issue (10): 2216.doi: 10.7503/cjcu20200519
• Article • Previous Articles Next Articles
CHEN Xiangmeng, ZHANG Yaqi, LIANG Hao, CHEN Bin, OUYANG Jiasheng, HE Xiaobo, QIAN Xu, PU Xiaoyun, PAN Bendu, QIU Liqin( )
)
Received:2020-08-03
Online:2020-10-10
Published:2020-09-14
Contact:
QIU Liqin
E-mail:qiuliqin@mail.sysu.edu.cn
Supported by:CLC Number:
TrendMD:
CHEN Xiangmeng, ZHANG Yaqi, LIANG Hao, CHEN Bin, OUYANG Jiasheng, HE Xiaobo, QIAN Xu, PU Xiaoyun, PAN Bendu, QIU Liqin. Iridium-catalyzed Allylation of Morita-Baylis-Hillman Acetates with Indolinone Compounds†[J]. Chem. J. Chinese Universities, 2020, 41(10): 2216.
| Compound | R1 | R2 | R3 | Yieldb(%) | HRMS[M+H]+ |
|---|---|---|---|---|---|
| 3a | H | H | Ph | 98% | 488.2219 |
| 3b | H | H | 2?NO2Ph | 93% | 533.2057 |
| 3c | H | H | 3,4?Cl2Ph | 91% | 556.1433 |
| 3d | H | H | 3?BrPh | 93% | 566.1335 |
| 3e | H | H | 3?ClPh | 94% | 522.1822 |
| 3f | H | H | 3?FPh | 92% | 506.2127 |
| 3g | H | H | 4?CF3Ph | 92% | 556.2082 |
| 3h | H | H | 4?CNPh | 93% | 513.2169 |
| 3i | H | H | 3?MePh | 96% | 502.2371 |
| 3j | H | H | 2?Thiofuran | 94% | 494.1783 |
| 3k | H | 3,5?(OMe)2 | Ph | 96% | 548.2436 |
| 3l | H | 4?OMe | Ph | 98% | 518.2320 |
| 3m | 5?Cl | H | Ph | 95% | 522.1831 |
| 3n | 5?F | H | Ph | 95% | 506.2131 |
| 3o | 5?OMe | H | Ph | 97% | 518.2330 |
| 3p | 5?Me | H | Ph | 96% | 502.2368 |
| 3q | 6?F | H | Ph | 90% | 506.2114 |
| 3r | 7?F | H | Ph | 84% | 506.2134 |
| Compound | R1 | R2 | R3 | Yieldb(%) | HRMS[M+H]+ |
|---|---|---|---|---|---|
| 3a | H | H | Ph | 98% | 488.2219 |
| 3b | H | H | 2?NO2Ph | 93% | 533.2057 |
| 3c | H | H | 3,4?Cl2Ph | 91% | 556.1433 |
| 3d | H | H | 3?BrPh | 93% | 566.1335 |
| 3e | H | H | 3?ClPh | 94% | 522.1822 |
| 3f | H | H | 3?FPh | 92% | 506.2127 |
| 3g | H | H | 4?CF3Ph | 92% | 556.2082 |
| 3h | H | H | 4?CNPh | 93% | 513.2169 |
| 3i | H | H | 3?MePh | 96% | 502.2371 |
| 3j | H | H | 2?Thiofuran | 94% | 494.1783 |
| 3k | H | 3,5?(OMe)2 | Ph | 96% | 548.2436 |
| 3l | H | 4?OMe | Ph | 98% | 518.2320 |
| 3m | 5?Cl | H | Ph | 95% | 522.1831 |
| 3n | 5?F | H | Ph | 95% | 506.2131 |
| 3o | 5?OMe | H | Ph | 97% | 518.2330 |
| 3p | 5?Me | H | Ph | 96% | 502.2368 |
| 3q | 6?F | H | Ph | 90% | 506.2114 |
| 3r | 7?F | H | Ph | 84% | 506.2134 |
| Compound | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(101 MHz, CDCl3), δ |
|---|---|---|
3a | 7.47(m, 3H, PhH), 7.35—7.21(m, 12H, PhH), 7.20—7.08(m, 5H, PhH), 6.96—6.70(m, 2H, PhH, CH), 4.97—4.78(m, 2H, Ph—CH2), 3.89(t, J=7.0 Hz, 2H, CH2), 3.87—3.78(m, 2H, CH2), 1.22—1.06(m, 3H, CH3) | 177.83, 168.67, 143.09, 140.58, 140.02, 135.97, 135.27, 130.20, 129.93, 128.84, 128.70, 128.65, 128.40, 128.37, 128.15, 127.89, 127.44, 127.28, 127.21, 126.94, 126.85, 122.01, 109.09, 60.82, 56.06, 43.90, 34.91, 14.06 |
| 3b | 8.19—8.16(m, 1H, PhH), 7.87(s, 1H, PhH), 7.67—7.63(m, 2H, PhH), 7.31—7.23(m, 8H, PhH, Ph—CH), 7.21—7.13(m, 6H, PhH), 7.00—6.96(m, 1H), 6.71(d, J=8 Hz, 1H, PhH), 4.99—4.66(dd, J=116, 16 Hz, 2H, BnH2), 4.01—3.83(m, 2H), 3.75—3.60(dd, J=48, 12 Hz, 2H), 1.11(t, J=7.1 Hz, 3H) | 177.41, 167.99, 146.91, 143.01, 139.06, 138.82, 135.92, 133.76, 132.44, 130.78, 129.83, 129.39, 128.93, 128.64, 128.59, 128.33, 128.29, 127.40, 127.20, 127.16, 126.92, 126.85, 125.34, 121.98, 109.38, 61.07, 56.29, 43.64, 33.92, 14.01 |
| 3c | 7.49—7.43(m, 2H, PhH), 7.35—7.25(m, 11H, PhH), 7.22—7.13(m, 3H, PhH), 7.01—6.94(m, 2H, PhH), 6.93—6.86(m, 1H, PhH), 6.89—6.73(m, 1H, PhH), 4.91—4.80(q, J=16 Hz, 2H, PhH), 3.97—3.86(m, 2H, CH2), 3.74(s, 2H, CH2),1.15(t, J=7.1 Hz, 3H, CH3) | 177.46, 168.24, 143.05, 139.26, 137.93, 135.92, 135.28, 132.51, 132.08, 131.91, 130.51, 130.28, 129.74, 128.70, 128.45, 128.36, 127.72, 127.58, 127.48, 127.26, 127.20, 126.85, 122.05, 109.24, 61.11, 56.01, 43.89, 34.88, 14.01 |
| 3d | 7.49—7.42(m, 2H, PhH), 7.41—7.35(m, 2H, PhH), 7.34—7.22(m, 8H, PhH, CH), 7.22—7.16(m, 1H, PhH), 7.16—7.10(m, 2H, PhH), 7.07(d, J=8.1 Hz, 1H, PhH), 7.03(dd, J=9.9, 2.8 Hz, 1H), 6.99— 6.93(m, 3H, PhH ), 6.72(d, J=7.8 Hz, 1H, PhH), 4.91—4.79(m, 2H, BnH), 3.97—3.87(m, 2H, CH2), 3.77(s, 2H), 1.15(t, J=7.1 Hz, 3H, CH3) | 177.53, 168.33, 143.04, 139.45, 138.88, 137.38, 135.93, 131.54, 131.50, 130.97, 129.83, 129.76, 128.67, 128.40, 128.26, 127.48, 127.39, 127.21, 127.03, 126.83, 122.44, 122.06, 109.20, 61.01, 56.01, 43.91, 34.86, 14.02 |
| 3e | 7.47—7.44(m, 2H, PhH), 7,38(s, 1H, PhH), 7.30—7.13(m, 10H, PhH), 6.99—6.95(m, 5H, PhH), 6.93(d, J=1.9 Hz, 1H, PhH), 4.91—4.79(m, 2H, BnH2), 4.01—3.84(m, 2H, CH2), 3.84—3.74(m, 2H, CH2), 1.15(t, J=7.1 Hz, 3H, CH3) | 177.58, 168.38, 143.07, 139.50, 139.01, 137.11, 135.95, 134.25, 131.48, 129.81, 129.59, 128.68, 128.42, 128.27, 128.09, 127.50, 127.40, 127.22, 126.85, 126.63, 122.07, 109.20, 61.02, 56.03, 43.90, 34.87, 14.02 |
| 3f | 7.46—7.40(m, 3H, PhH), 7.30—7.25(m, 9H, PhH, CH), 7.22—7.13(m, 3H), 6.98—6.94(m, 2H, PhH), 6.88(d, J=7.7 Hz, 1H, PhH), 6.72—6.69(m, 2H, PhH), 4.85(s, 2H, BnH), 3.95—3.90(m, 2H, CH2), 3.79(q, J=13.6 Hz, 2H, CH2), 1.14(t, J=7.1 Hz, 3H, CH3) | 177.58, 168.41, 163.78, 161.33, 143.08, 139.61, 139.16, 137.48, 137.40, 135.91, 131.35, 129.89, 129.83, 129.80, 128.65, 128.41, 128.24, 127.48, 127.37, 127.18, 126.93, 126.85, 124.37, 124.34, 122.03, 115.67, 115.45, 115.08, 114.87, 109.16, 60.98, 56.03, 43.88, 34.84, 14.01 |
| 3g | 7.49—7.42(m, 5H, PhH), 7.32—7.26(m, 9H, PhH, CH), 7.19—7.10(m, 5H, PhH), 6.97—6.93(m, 1H, PhH), 6.74—6.72(m, 1H, PhH), 4.86(dd, J=42.4, 15.8 Hz, 2H, BnH), 3.97—3.86(m, 2H, CH2), 3.77(s, 2H, CH2), 1.15(t, J=7.1 Hz, 3H, CH3) | 177.53, 168.32, 143.01, 139.35, 138.93, 138.86, 135.89, 132.12, 129.77, 128.82, 128.68, 128.44, 128.33, 127.58, 127.44, 127.25, 127.17, 126.83, 125.28, 125.25, 122.13, 109.21, 77.38, 77.06, 76.74, 61.10, 55.99, 43.89, 34.84, 14.00 |
| 3h | 7.52—7.50(m, 2H, PhH), 7.43—7.39(m, 3H, PhH), 7.32—7.27(m, 8H, PhH), 7.19—7.16(m, 3H, PhH), 7.12—7.02(m, 3H, PhH), 6.99—6.96(m, 1H, PhH), 6.75—6.73(m, 1H), 4.85(dd, J=52, 16 Hz, 2H, BnH), 3.92—3.88(m, 2H, CH2), 3.9—3.71(m, 2H, CH2), 1.14(t, J=7.1 Hz, 3H, CH3) | 177.35, 168.13, 143.06, 140.01, 139.25, 138.28, 135.88, 132.78, 132.04, 131.67, 129.63, 129.20, 128.77, 128.69, 128.61, 128.50, 128.43, 127.64, 127.51, 127.25, 127.08, 126.91, 126.75, 122.13, 118.65, 111.49, 109.23, 61.19, 56.02, 43.86, 34.74, 13.99 |
| 3i | 7.49—7.47(m, 3H, PhH), 7.31—7.26(m, 8H, PhH, CH), 7.17—7.16(m, 3H, PhH), 7.09—6.94(m, 2H), 6.83(s, 1H, PhH), 6.73—6.71(m, 1H, PhH), 4.87(q, J=15.8 Hz, 2H, BnH), 3.93—3.86(m, 2H, CH2), 3.83(s, 2H, CH2), 2.23(s, 3H, CH3), 1.13(t, J=7.1 Hz, 3H, CH3) | 177.99, 168.65, 143.14, 140.77, 140.10, 137.89, 136.02, 135.19, 130.06, 129.98, 129.49, 128.94, 128.65, 128.36, 128.25, 128.08, 127.43, 127.30, 127.26, 127.23, 127.21, 127.00, 125.92, 121.98, 109.07, 60.76, 55.95, 43.93, 35.31, 21.32, 14.06 |
| Compound | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(101 MHz, CDCl3), δ |
3j | 7.60—7.59(m, 3H, PhH), 7.35—7.26(m, 11H, PhH), 7.16—7.12(m, 1H, PhH), 6.99—6.91(m, 2H),, 6.71(d, J=7.7 Hz, 1H, CH), 4.97—4.81(m, 2H, BnH), 3.92(s, 2H, CH2), 3.90—3.79(m, 2H, CH2), 1.09(t, J=7.1 Hz, 3H, CH3) | 178.08, 168.44, 143.14, 140.08, 137.95, 136.00, 133.37, 132.20, 129.81, 128.71, 128.60, 128.38, 128.22, 127.43, 127.40, 127.34, 127.11, 126.97, 126.43, 121.84, 109.08, 77.38, 77.06, 76.74, 60.72, 55.88, 44.01, 36.70, 14.05 |
| 3k | 7.47(s, 1H, PhH), 7.32—7.28(m, 8H, PhH), 7.19—7.09(m, 4H, PhH), 7.04—6.97(m, 2H), 6.95—6.88(m, 4H, PhH), 6.71—6.69(m, 1H), 4.93—4.80(m, 2H, CH2), 3.91—3.83(m, 2H, PhH), 3.84(d, J=8.3 Hz, 2H), 2.25(s, 6H, CH3), 1.14(t, J=7.1 Hz, 3H, CH3) | 177.97, 168.72, 143.02, 140.44, 140.28, 137.80, 136.11, 135.36, 130.58, 130.28, 128.98, 128.91, 128.60, 128.34, 128.13, 127.94, 127.42, 127.31, 126.73, 124.81, 121.99, 108.92, 60.77, 56.14, 43.88, 34.47, 21.51, 14.05 |
| 3l | 7.47(s, 1H, PhH), 7.37—7.26(m, 10H), 7.15—7.10 (m, 4H, PhH), 6.96—6.92(m, 1H, PhH), 6.81—6.79 (m, 2H, PhH), 6.69(d, J=7.7 Hz, 1H, PhH), 4.92—4.78(m, 2H, CH2), 3.91—3.88(q, J=4 Hz, 2H, CH2), 3.79(s, 3H, CH3), 3.78(s, 2H, CH2), 1.13(t, J=7.1 Hz, 3H, CH3) | 178.07, 168.69, 158.72, 143.04, 140.51, 136.00, 135.33, 131.85, 130.25, 130.11, 128.81, 128.63, 128.39, 128.33, 128.08, 127.40, 127.17, 126.86, 121.95, 113.69, 109.07, 60.80, 55.37, 55.25, 43.84, 35.05, 14.03 |
| 3m | 7.52(s, 1H, PhH), 7.39—7.28(m, 12H), 7.24—7.12(m, 3H, PhH), 7.00(s, 1H, PhH), 6.59—6.57(d, J=8 Hz, 1H), 4.81(dd, J=48, 16 Hz, 2H, CH2), 4.07—3.96(m, 2H, CH2), 3.86(dd, J=44, 12 Hz, 2H, CH2), 1.23(t, J=7.1 Hz, 3H, CH3) | 177.24, 168.53, 141.66, 141.19, 139.49, 135.49, 135.13, 131.97, 129.64, 128.74, 128.68, 128.60, 128.49, 128.31, 128.19, 127.63, 127.54, 127.43, 127.17, 126.98, 110.00, 61.06, 56.56, 44.01, 34.30, 14.11 |
| 3n | 7.52(s, 1H, PhH), 7.40—7.42(m, 2H, PhH), 7.32—7.25(m, 12H, PhH), 7.15—7.12(m, 2H, PhH), 6.87—6.77(m, 2H, PhH), 6.64—6.54(m, 1H, PhH), 4.83(dd, J=36, 16 Hz, 2H, BnH), 4.06—3.95(m, 2H, CH2), 3.87(dd, J=40, 12 Hz, 2H, CH2), 1.21(t, J=7.1 Hz, 3H, CH3) | 177.46, 168.52, 159.84, 157.45, 141.03, 139.61, 139.03, 135.65, 135.17, 131.88, 131.80, 129.75, 128.77, 128.72, 128.70, 128.66, 128.56, 128.46, 128.44, 128.42, 128.40, 128.27, 127.58, 127.51, 127.19, 127.15, 127.01, 114.79, 114.65, 114.54, 114.42, 109.54, 109.46, 60.99, 56.67, 44.07, 34.49, 14.09 |
| 3o | 7.49(s, 1H, PhH), 7.47—7.49(m, 2H, PhH), 7.31—7.25(m, 12H, PhH, CH), 7.13—7.11(m, 2H, PhH), 6.73—6.67(m, 2H), 6.58(d, J=6 Hz, 1H), 4.83(q, J=12 Hz, 2H, PhH), 4.97—3.94(m, 2H, CH2), 3.86(q, J=12 Hz, 2H, CH2), 3.69(s, 3H, CH3), 1.17(t, J=7.1 Hz, 3H, CH3) | 177.50, 168.73, 155.36, 140.58, 139.98, 136.59, 136.08, 135.31, 131.29, 130.21, 128.77, 128.64, 128.42, 128.37, 128.10, 127.43, 127.30, 127.23, 113.89, 112.94, 109.42, 60.87, 56.62, 55.61, 43.99, 34.73, 14.06 |
| 3p | 7.47—7.42(m, 3H, PhH) 7.29—7.26(m, 11H, PhH), 6.95(t, J=10.0 Hz, 1H), 7.12—7.10(m, 2H, PhH), 6.86—6.94(m, 2H, PhH), 6.57(d, J=8 Hz, 1H, PhH), 4.83(dd, J=36, 16 Hz, 2H, CH | 177.63, 168.75, 140.68, 140.57, 140.20, 136.10, 135.39, 131.33, 130.20, 130.15, 128.78, 128.61, 128.48, 128.38, 128.33, 128.03, 127.49, 127.38, 127.21, 108.82, 60.82, 56.34, 43.90, 34.54, 21.18, 14.07 |
| 3q | 7.50(s, 1H, PhH), 7.43—7.40(m, 2H, PhH), 7.33—7.26(m, 11H, PhH), 7.14—7.13(d, J=4 Mz, 2H, PhH), 7.04—7.00(m, 1H, PhH), 6.66—6.60(m, 1H, PhH), 6.44—6.41(m, 1H, PhH), 4.89—4.73(m, 2H, CH2), 3.98—3.78(m, 4H, CH2, CH2), 1.17(t, J=7.1 Hz, 3H, CH3) | 178.11, 168.56, 164.06, 161.62, 144.67, 144.56, 140.72, 139.92, 135.42, 135.19, 129.97, 128.77, 128.50, 128.43, 128.26, 127.67, 127.44, 127.20, 127.06, 108.33, 108.11, 97.81, 97.54, 60.93, 55.76, 44.09, 34.81, 14.08 |
| 3r | 7.51(s, 1H, PhH), 7.43—7.26(m, 14H), 7.14—7.13(d, J=4 Mz, 2H), 6.63—6.61(m, 1H), 6.41—6.44(m, 1H), 4.82(dd, J=28, 16 Mz, 2H, CH2), 3.98—3.78(m, 4H, CH2, CH2), 1.17(t, J=7.1 Hz, CH3) | 177.55, 168.50, 148.55, 146.12, 140.90, 139.72, 137.28, 135.20, 133.10, 133.08, 129.78, 128.82, 128.49, 128.42, 128.25, 127.47, 127.34, 127.06, 122.79, 122.76, 122.55, 122.49, 116.27, 116.08, 60.89, 56.38, 45.44, 34.76, 14.07 |
| Compound | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(101 MHz, CDCl3), δ |
|---|---|---|
3a | 7.47(m, 3H, PhH), 7.35—7.21(m, 12H, PhH), 7.20—7.08(m, 5H, PhH), 6.96—6.70(m, 2H, PhH, CH), 4.97—4.78(m, 2H, Ph—CH2), 3.89(t, J=7.0 Hz, 2H, CH2), 3.87—3.78(m, 2H, CH2), 1.22—1.06(m, 3H, CH3) | 177.83, 168.67, 143.09, 140.58, 140.02, 135.97, 135.27, 130.20, 129.93, 128.84, 128.70, 128.65, 128.40, 128.37, 128.15, 127.89, 127.44, 127.28, 127.21, 126.94, 126.85, 122.01, 109.09, 60.82, 56.06, 43.90, 34.91, 14.06 |
| 3b | 8.19—8.16(m, 1H, PhH), 7.87(s, 1H, PhH), 7.67—7.63(m, 2H, PhH), 7.31—7.23(m, 8H, PhH, Ph—CH), 7.21—7.13(m, 6H, PhH), 7.00—6.96(m, 1H), 6.71(d, J=8 Hz, 1H, PhH), 4.99—4.66(dd, J=116, 16 Hz, 2H, BnH2), 4.01—3.83(m, 2H), 3.75—3.60(dd, J=48, 12 Hz, 2H), 1.11(t, J=7.1 Hz, 3H) | 177.41, 167.99, 146.91, 143.01, 139.06, 138.82, 135.92, 133.76, 132.44, 130.78, 129.83, 129.39, 128.93, 128.64, 128.59, 128.33, 128.29, 127.40, 127.20, 127.16, 126.92, 126.85, 125.34, 121.98, 109.38, 61.07, 56.29, 43.64, 33.92, 14.01 |
| 3c | 7.49—7.43(m, 2H, PhH), 7.35—7.25(m, 11H, PhH), 7.22—7.13(m, 3H, PhH), 7.01—6.94(m, 2H, PhH), 6.93—6.86(m, 1H, PhH), 6.89—6.73(m, 1H, PhH), 4.91—4.80(q, J=16 Hz, 2H, PhH), 3.97—3.86(m, 2H, CH2), 3.74(s, 2H, CH2),1.15(t, J=7.1 Hz, 3H, CH3) | 177.46, 168.24, 143.05, 139.26, 137.93, 135.92, 135.28, 132.51, 132.08, 131.91, 130.51, 130.28, 129.74, 128.70, 128.45, 128.36, 127.72, 127.58, 127.48, 127.26, 127.20, 126.85, 122.05, 109.24, 61.11, 56.01, 43.89, 34.88, 14.01 |
| 3d | 7.49—7.42(m, 2H, PhH), 7.41—7.35(m, 2H, PhH), 7.34—7.22(m, 8H, PhH, CH), 7.22—7.16(m, 1H, PhH), 7.16—7.10(m, 2H, PhH), 7.07(d, J=8.1 Hz, 1H, PhH), 7.03(dd, J=9.9, 2.8 Hz, 1H), 6.99— 6.93(m, 3H, PhH ), 6.72(d, J=7.8 Hz, 1H, PhH), 4.91—4.79(m, 2H, BnH), 3.97—3.87(m, 2H, CH2), 3.77(s, 2H), 1.15(t, J=7.1 Hz, 3H, CH3) | 177.53, 168.33, 143.04, 139.45, 138.88, 137.38, 135.93, 131.54, 131.50, 130.97, 129.83, 129.76, 128.67, 128.40, 128.26, 127.48, 127.39, 127.21, 127.03, 126.83, 122.44, 122.06, 109.20, 61.01, 56.01, 43.91, 34.86, 14.02 |
| 3e | 7.47—7.44(m, 2H, PhH), 7,38(s, 1H, PhH), 7.30—7.13(m, 10H, PhH), 6.99—6.95(m, 5H, PhH), 6.93(d, J=1.9 Hz, 1H, PhH), 4.91—4.79(m, 2H, BnH2), 4.01—3.84(m, 2H, CH2), 3.84—3.74(m, 2H, CH2), 1.15(t, J=7.1 Hz, 3H, CH3) | 177.58, 168.38, 143.07, 139.50, 139.01, 137.11, 135.95, 134.25, 131.48, 129.81, 129.59, 128.68, 128.42, 128.27, 128.09, 127.50, 127.40, 127.22, 126.85, 126.63, 122.07, 109.20, 61.02, 56.03, 43.90, 34.87, 14.02 |
| 3f | 7.46—7.40(m, 3H, PhH), 7.30—7.25(m, 9H, PhH, CH), 7.22—7.13(m, 3H), 6.98—6.94(m, 2H, PhH), 6.88(d, J=7.7 Hz, 1H, PhH), 6.72—6.69(m, 2H, PhH), 4.85(s, 2H, BnH), 3.95—3.90(m, 2H, CH2), 3.79(q, J=13.6 Hz, 2H, CH2), 1.14(t, J=7.1 Hz, 3H, CH3) | 177.58, 168.41, 163.78, 161.33, 143.08, 139.61, 139.16, 137.48, 137.40, 135.91, 131.35, 129.89, 129.83, 129.80, 128.65, 128.41, 128.24, 127.48, 127.37, 127.18, 126.93, 126.85, 124.37, 124.34, 122.03, 115.67, 115.45, 115.08, 114.87, 109.16, 60.98, 56.03, 43.88, 34.84, 14.01 |
| 3g | 7.49—7.42(m, 5H, PhH), 7.32—7.26(m, 9H, PhH, CH), 7.19—7.10(m, 5H, PhH), 6.97—6.93(m, 1H, PhH), 6.74—6.72(m, 1H, PhH), 4.86(dd, J=42.4, 15.8 Hz, 2H, BnH), 3.97—3.86(m, 2H, CH2), 3.77(s, 2H, CH2), 1.15(t, J=7.1 Hz, 3H, CH3) | 177.53, 168.32, 143.01, 139.35, 138.93, 138.86, 135.89, 132.12, 129.77, 128.82, 128.68, 128.44, 128.33, 127.58, 127.44, 127.25, 127.17, 126.83, 125.28, 125.25, 122.13, 109.21, 77.38, 77.06, 76.74, 61.10, 55.99, 43.89, 34.84, 14.00 |
| 3h | 7.52—7.50(m, 2H, PhH), 7.43—7.39(m, 3H, PhH), 7.32—7.27(m, 8H, PhH), 7.19—7.16(m, 3H, PhH), 7.12—7.02(m, 3H, PhH), 6.99—6.96(m, 1H, PhH), 6.75—6.73(m, 1H), 4.85(dd, J=52, 16 Hz, 2H, BnH), 3.92—3.88(m, 2H, CH2), 3.9—3.71(m, 2H, CH2), 1.14(t, J=7.1 Hz, 3H, CH3) | 177.35, 168.13, 143.06, 140.01, 139.25, 138.28, 135.88, 132.78, 132.04, 131.67, 129.63, 129.20, 128.77, 128.69, 128.61, 128.50, 128.43, 127.64, 127.51, 127.25, 127.08, 126.91, 126.75, 122.13, 118.65, 111.49, 109.23, 61.19, 56.02, 43.86, 34.74, 13.99 |
| 3i | 7.49—7.47(m, 3H, PhH), 7.31—7.26(m, 8H, PhH, CH), 7.17—7.16(m, 3H, PhH), 7.09—6.94(m, 2H), 6.83(s, 1H, PhH), 6.73—6.71(m, 1H, PhH), 4.87(q, J=15.8 Hz, 2H, BnH), 3.93—3.86(m, 2H, CH2), 3.83(s, 2H, CH2), 2.23(s, 3H, CH3), 1.13(t, J=7.1 Hz, 3H, CH3) | 177.99, 168.65, 143.14, 140.77, 140.10, 137.89, 136.02, 135.19, 130.06, 129.98, 129.49, 128.94, 128.65, 128.36, 128.25, 128.08, 127.43, 127.30, 127.26, 127.23, 127.21, 127.00, 125.92, 121.98, 109.07, 60.76, 55.95, 43.93, 35.31, 21.32, 14.06 |
| Compound | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(101 MHz, CDCl3), δ |
3j | 7.60—7.59(m, 3H, PhH), 7.35—7.26(m, 11H, PhH), 7.16—7.12(m, 1H, PhH), 6.99—6.91(m, 2H),, 6.71(d, J=7.7 Hz, 1H, CH), 4.97—4.81(m, 2H, BnH), 3.92(s, 2H, CH2), 3.90—3.79(m, 2H, CH2), 1.09(t, J=7.1 Hz, 3H, CH3) | 178.08, 168.44, 143.14, 140.08, 137.95, 136.00, 133.37, 132.20, 129.81, 128.71, 128.60, 128.38, 128.22, 127.43, 127.40, 127.34, 127.11, 126.97, 126.43, 121.84, 109.08, 77.38, 77.06, 76.74, 60.72, 55.88, 44.01, 36.70, 14.05 |
| 3k | 7.47(s, 1H, PhH), 7.32—7.28(m, 8H, PhH), 7.19—7.09(m, 4H, PhH), 7.04—6.97(m, 2H), 6.95—6.88(m, 4H, PhH), 6.71—6.69(m, 1H), 4.93—4.80(m, 2H, CH2), 3.91—3.83(m, 2H, PhH), 3.84(d, J=8.3 Hz, 2H), 2.25(s, 6H, CH3), 1.14(t, J=7.1 Hz, 3H, CH3) | 177.97, 168.72, 143.02, 140.44, 140.28, 137.80, 136.11, 135.36, 130.58, 130.28, 128.98, 128.91, 128.60, 128.34, 128.13, 127.94, 127.42, 127.31, 126.73, 124.81, 121.99, 108.92, 60.77, 56.14, 43.88, 34.47, 21.51, 14.05 |
| 3l | 7.47(s, 1H, PhH), 7.37—7.26(m, 10H), 7.15—7.10 (m, 4H, PhH), 6.96—6.92(m, 1H, PhH), 6.81—6.79 (m, 2H, PhH), 6.69(d, J=7.7 Hz, 1H, PhH), 4.92—4.78(m, 2H, CH2), 3.91—3.88(q, J=4 Hz, 2H, CH2), 3.79(s, 3H, CH3), 3.78(s, 2H, CH2), 1.13(t, J=7.1 Hz, 3H, CH3) | 178.07, 168.69, 158.72, 143.04, 140.51, 136.00, 135.33, 131.85, 130.25, 130.11, 128.81, 128.63, 128.39, 128.33, 128.08, 127.40, 127.17, 126.86, 121.95, 113.69, 109.07, 60.80, 55.37, 55.25, 43.84, 35.05, 14.03 |
| 3m | 7.52(s, 1H, PhH), 7.39—7.28(m, 12H), 7.24—7.12(m, 3H, PhH), 7.00(s, 1H, PhH), 6.59—6.57(d, J=8 Hz, 1H), 4.81(dd, J=48, 16 Hz, 2H, CH2), 4.07—3.96(m, 2H, CH2), 3.86(dd, J=44, 12 Hz, 2H, CH2), 1.23(t, J=7.1 Hz, 3H, CH3) | 177.24, 168.53, 141.66, 141.19, 139.49, 135.49, 135.13, 131.97, 129.64, 128.74, 128.68, 128.60, 128.49, 128.31, 128.19, 127.63, 127.54, 127.43, 127.17, 126.98, 110.00, 61.06, 56.56, 44.01, 34.30, 14.11 |
| 3n | 7.52(s, 1H, PhH), 7.40—7.42(m, 2H, PhH), 7.32—7.25(m, 12H, PhH), 7.15—7.12(m, 2H, PhH), 6.87—6.77(m, 2H, PhH), 6.64—6.54(m, 1H, PhH), 4.83(dd, J=36, 16 Hz, 2H, BnH), 4.06—3.95(m, 2H, CH2), 3.87(dd, J=40, 12 Hz, 2H, CH2), 1.21(t, J=7.1 Hz, 3H, CH3) | 177.46, 168.52, 159.84, 157.45, 141.03, 139.61, 139.03, 135.65, 135.17, 131.88, 131.80, 129.75, 128.77, 128.72, 128.70, 128.66, 128.56, 128.46, 128.44, 128.42, 128.40, 128.27, 127.58, 127.51, 127.19, 127.15, 127.01, 114.79, 114.65, 114.54, 114.42, 109.54, 109.46, 60.99, 56.67, 44.07, 34.49, 14.09 |
| 3o | 7.49(s, 1H, PhH), 7.47—7.49(m, 2H, PhH), 7.31—7.25(m, 12H, PhH, CH), 7.13—7.11(m, 2H, PhH), 6.73—6.67(m, 2H), 6.58(d, J=6 Hz, 1H), 4.83(q, J=12 Hz, 2H, PhH), 4.97—3.94(m, 2H, CH2), 3.86(q, J=12 Hz, 2H, CH2), 3.69(s, 3H, CH3), 1.17(t, J=7.1 Hz, 3H, CH3) | 177.50, 168.73, 155.36, 140.58, 139.98, 136.59, 136.08, 135.31, 131.29, 130.21, 128.77, 128.64, 128.42, 128.37, 128.10, 127.43, 127.30, 127.23, 113.89, 112.94, 109.42, 60.87, 56.62, 55.61, 43.99, 34.73, 14.06 |
| 3p | 7.47—7.42(m, 3H, PhH) 7.29—7.26(m, 11H, PhH), 6.95(t, J=10.0 Hz, 1H), 7.12—7.10(m, 2H, PhH), 6.86—6.94(m, 2H, PhH), 6.57(d, J=8 Hz, 1H, PhH), 4.83(dd, J=36, 16 Hz, 2H, CH | 177.63, 168.75, 140.68, 140.57, 140.20, 136.10, 135.39, 131.33, 130.20, 130.15, 128.78, 128.61, 128.48, 128.38, 128.33, 128.03, 127.49, 127.38, 127.21, 108.82, 60.82, 56.34, 43.90, 34.54, 21.18, 14.07 |
| 3q | 7.50(s, 1H, PhH), 7.43—7.40(m, 2H, PhH), 7.33—7.26(m, 11H, PhH), 7.14—7.13(d, J=4 Mz, 2H, PhH), 7.04—7.00(m, 1H, PhH), 6.66—6.60(m, 1H, PhH), 6.44—6.41(m, 1H, PhH), 4.89—4.73(m, 2H, CH2), 3.98—3.78(m, 4H, CH2, CH2), 1.17(t, J=7.1 Hz, 3H, CH3) | 178.11, 168.56, 164.06, 161.62, 144.67, 144.56, 140.72, 139.92, 135.42, 135.19, 129.97, 128.77, 128.50, 128.43, 128.26, 127.67, 127.44, 127.20, 127.06, 108.33, 108.11, 97.81, 97.54, 60.93, 55.76, 44.09, 34.81, 14.08 |
| 3r | 7.51(s, 1H, PhH), 7.43—7.26(m, 14H), 7.14—7.13(d, J=4 Mz, 2H), 6.63—6.61(m, 1H), 6.41—6.44(m, 1H), 4.82(dd, J=28, 16 Mz, 2H, CH2), 3.98—3.78(m, 4H, CH2, CH2), 1.17(t, J=7.1 Hz, CH3) | 177.55, 168.50, 148.55, 146.12, 140.90, 139.72, 137.28, 135.20, 133.10, 133.08, 129.78, 128.82, 128.49, 128.42, 128.25, 127.47, 127.34, 127.06, 122.79, 122.76, 122.55, 122.49, 116.27, 116.08, 60.89, 56.38, 45.44, 34.76, 14.07 |
| Entry | Ligand | Yieldb(%) | Entry | Ligand | Yieldb(%) | |
|---|---|---|---|---|---|---|
| 1 | 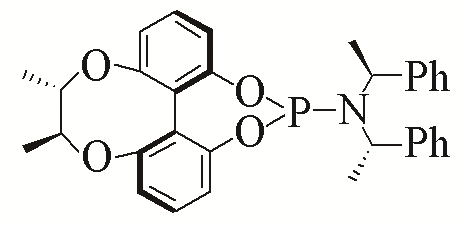 | 94% | 10 | 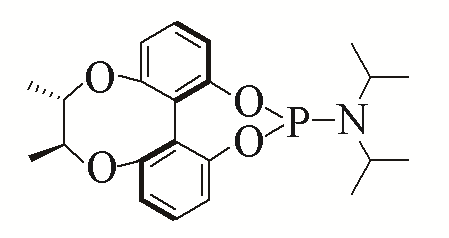 | 96% | |
| L1 | L10 | |||||
| 2 | 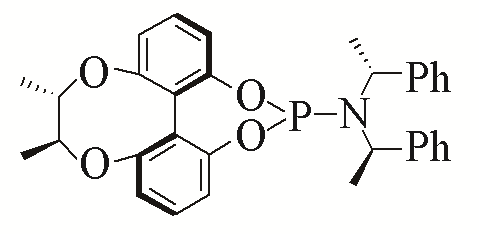 | 93% | 11 | 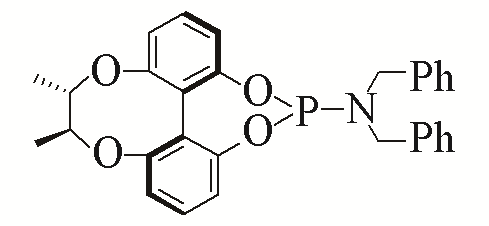 | 97% | |
| L2 | L11 | |||||
| 3 | 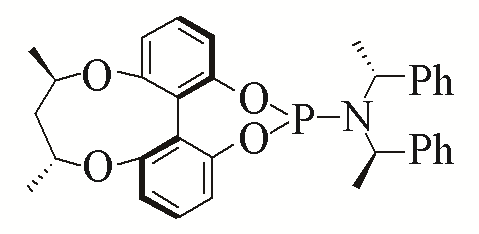 | 95% | 12 | 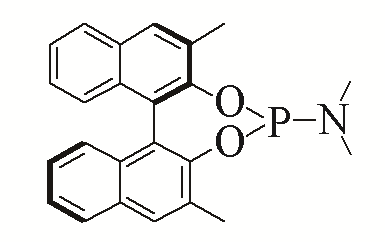 | 95% | |
| L3 | L12 | |||||
| 4 | 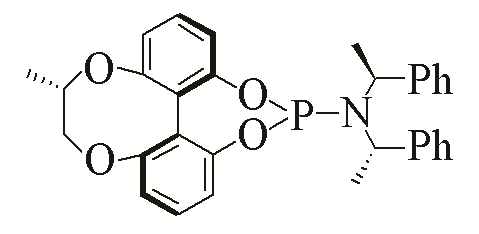 | 96% | 13 | 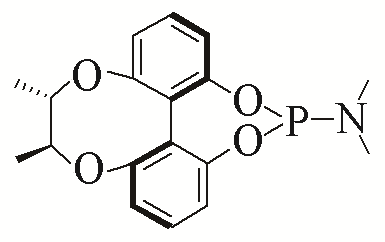 | 95% | |
| L4 | L13 | |||||
| 5 | 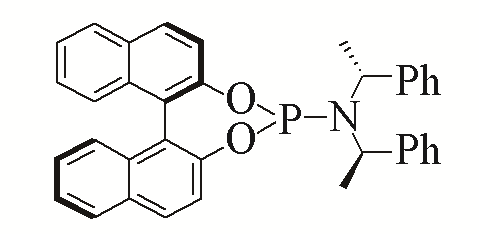 | 95% | 14 | 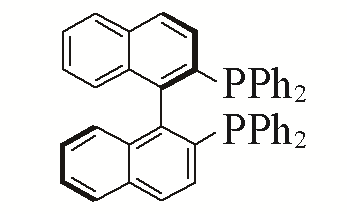 | 97% | |
| L5 | L14 | |||||
| 6 | 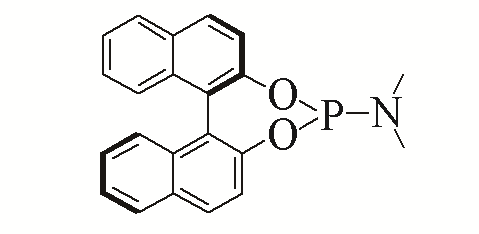 | 98% | 15 | 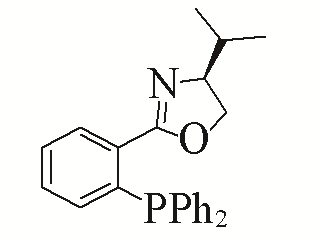 | 93% | |
| L6 | L15 | |||||
| 7 | 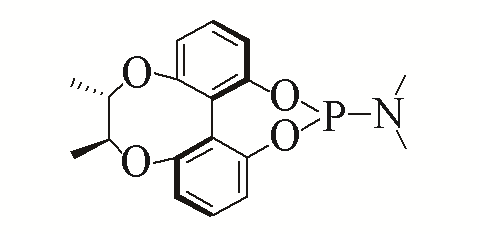 | 92% | 16 | 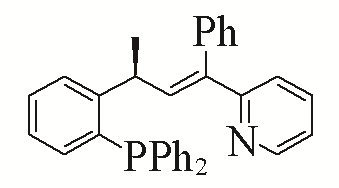 | 91% | |
| L7 | L16 | |||||
| 8 | 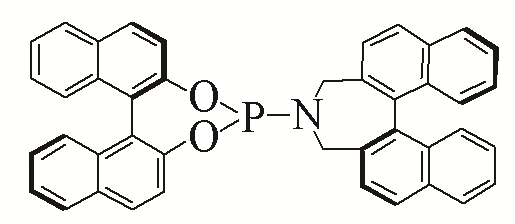 | 90% | 17 | 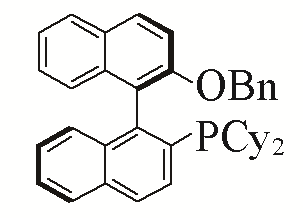 | 93% | |
| L8 | L17 | |||||
| 9 | 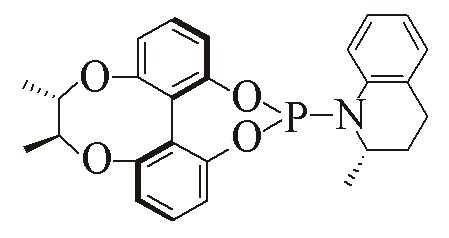 | 94% | ||||
| L9 | ||||||
| Entry | Ligand | Yieldb(%) | Entry | Ligand | Yieldb(%) | |
|---|---|---|---|---|---|---|
| 1 |  | 94% | 10 |  | 96% | |
| L1 | L10 | |||||
| 2 |  | 93% | 11 |  | 97% | |
| L2 | L11 | |||||
| 3 |  | 95% | 12 |  | 95% | |
| L3 | L12 | |||||
| 4 |  | 96% | 13 |  | 95% | |
| L4 | L13 | |||||
| 5 |  | 95% | 14 |  | 97% | |
| L5 | L14 | |||||
| 6 |  | 98% | 15 |  | 93% | |
| L6 | L15 | |||||
| 7 |  | 92% | 16 |  | 91% | |
| L7 | L16 | |||||
| 8 |  | 90% | 17 |  | 93% | |
| L8 | L17 | |||||
| 9 |  | 94% | ||||
| L9 | ||||||
| 1 | Cui H., Feng X., Peng J., Lei J., Jiang K., Chen Y. H., Angew. Chem. Int. Ed., 2009, 48(31), 5737—5740 |
| 2 | Sun W. S., Ma X. Z., Hong L., Wang R., J. Org. Chem., 2011, 76(19), 7826—7833 |
| 3 | Li Y., Liang. F., Li Q., Xu Y. H., Wang Q., Jiang L., Org. Lett., 2011, 13(22), 6082—6085 |
| 4 | Ramachandran P. V., Madhi S., Bland⁃Berry L., Reddy M. R., J. O’Donnell M., J. Am. Chem. Soc., 2005, 127(39), 13450—13451 |
| 5 | Zhang S. J., Cui H., Jiang K., Li R., Ding Z. Y., Chen Y. C., Eur. J. Org. Chem., 2009, (33),5804—5809 |
| 6 | Cui H. L, Peng J., Feng X., Du W., Jiang K., Chen Y. C., Eur. J. Org. Chem., 2009, (15), 1574—1577 |
| 7 | Jiang K., Peng J., Cui H. L, Chen Y. C., Chem. Commun., 2009, (26),3955—3957 |
| 8 | Yang W., Wei X. L., Pan Y. H., Lee R., Zhu B., Liu. H. J., Yan L., Huang K. W., Jiang Z. Y., Tan C. H., Chem. Eur. J.,2011, 17(29), 8066—8070 |
| 9 | Furukawa T., Kawazoe J., Zhang W., Nishimine T., Tokunaga E., Matsumoto T., Shiro M., Shibata N., Angew. Chem. Int. Ed., 2011, 50(41), 9684—9688 |
| 10 | Companyó X., Valero G., Ceban V., Calvet T., Mercé F. B., Moyano A., Rios R., Org. Biomol. Chem., 2011, 9(23), 7986—7989 |
| 11 | Cui H. L., Huang J. R., Lei J., Wang Z. F., Chen S., Wu L., Chen Y. C., Org. Lett., 2010, 12(1), 4—7 |
| 12 | Peng J., Huang X., Cui H. L., Chen Y. C., Org. Lett., 2010, 12(9), 1924—1927 |
| 13 | Hong L., Sun W. S., Liu C. X., Zhao D. P., Wang R., Chem. Commun., 2010, 46(16), 2856—2858 |
| 14 | Chen G. Y., Zhong F. R., Lu Y. X., Org. Lett., 2011, 13(22), 6070—6073 |
| 15 | Jiang Y. Q., Shi Y. G., Shi M., J. Am. Chem. Soc., 2008, 130(23), 7202—7203 |
| 16 | Deng H. P., Shi M., Eur. J. Org. Chem., 2012,(1),183—187 |
| 17 | Zhong F. R., Chen G. Y., Han X. Y., Yao W. J., Lu Y. X., Org. Lett.,2012, 14(14), 3764—3767 |
| 18 | Deng H. P., Wei Y., Shi M., Adv. Synth. Catal., 2012, 354(5), 783—789 |
| 19 | Wang Y., Liu L., Zhang T., Zhong N. J., Wang D., Chen Y. J., J. Org. Chem., 2012, 77(8), 4143—4147 |
| 20 | Yamashita Y., Gopalarathnam A., Hartwig J. F., J. Am. Chem. Soc., 2007, 129(24), 7508—7509 |
| 21 | Ueno S., Hartwig J. F., Angew. Chem. Int. Ed., 2008, 47(10), 1928—1931 |
| 22 | Defieber C., Ariger M. A., Moriel P., Carreira E. M., Angew. Chem. Int. Ed., 2007, 46(17), 3139—3143 |
| 23 | Kato M., Nakamura T., Ogata K., Fukuzawa S. I., Eur. J. Org. Chem.,2009,(30),5232—5238 |
| 24 | Ye K. Y., He H., Liu W. B., Dai L. X., Helmchen G., You S. L., J. Am. Chem. Soc.,2011, 133(46), 19006—19014 |
| 25 | Yang X. F., Yu W. H., Ding C. H., Ding Q. P., Wan S. L., Hou X. L., Dai L. X., Wang P. J., J. Org. Chem., 2013, 78(13), 6503—6509 |
| 26 | Zhang X., Yang Z. P., Huang L., You S. L., Angew. Chem. Int. Ed., 2015, 54(6), 1873—1876 |
| 27 | Wang X. M., Meng F. Y., Wang Y., Han Z. B., Chen Y. Z., Liu L., Wang Z., Ding K. L., Angew. Chem. Int. Ed.,2012, 51(37), 9276—9282 |
| 28 | Wang X. M., Guo P. H., Han Z. B., Wang X. B., Wang Z., Ding K. L., J. Am. Chem. Soc.,2014, 136(1), 405—411 |
| 29 | Wang X. B., Wang X. M., Han Z. B., Wang Z., Ding K. L., Angew. Chem. Int. Ed., 2017, 56(4), 1116—1119 |
| 30 | Trost B. M., Xie J., Sieber J. D., J. Am. Chem. Soc.,2011, 133(50), 20611—20622 |
| 31 | He R., Liu P., Huo X., Zhang W. B., Org. Lett., 2017, 19(20), 5513—5516 |
| 32 | Zhuang Y., He Y., Zhou Z., Xia W., Cheng C., Wang M., Chen B., Zhou Z., Pang J., Qiu L., J. Org. Chem., 2015, 80(14), 6968—6975 |
| 33 | Jiang X., Chen X., Li Y., Liang H., Zhang Y., He X., Chen B., Chan W. T. K., Chan A. S. C., Qiu L., Org. Lett., 2019, 21(3), 608—613 |
| [1] | ZHOU Yonghui, HUANG Rujun, YAN Jianyang, LI Yajun, QIU Huanhuan, YANG Jinxuan, ZHENG Youxuan. Synthesis and Electroluminescence Properties of Two Iridium(Ⅲ) Complexes with Nitrogen Heterocycle Structures [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210415. |
| [2] | HU Chuanchuan, PANG Jingxiang, HE Chuangchuang, LI Wei, SUN Shutao. Sc(OTf)3 Catalyzed 1,6-Conjugate Allylation of δ-CN p-QMs: Synthesis of Allyl Substituted Diarylacetonitrile Compounds [J]. Chem. J. Chinese Universities, 2021, 42(9): 2805. |
| [3] | ZHAO Ke, HONG Zhi, ZHANG Liming. Applications of Bifunctional Biaryl-2-ylphosphine Ligands in Asymmetric Gold Catalysis [J]. Chem. J. Chinese Universities, 2021, 42(8): 2324. |
| [4] | FU Zhinan, TAN Yunlong, XIAO Guyu, YAN Deyue. Synthesis and Properties of Sulfonated Poly(phthalazinone ether phosphine oxide)s with Perfluorobiphenyl Moieties for Proton Exchange Membranes [J]. Chem. J. Chinese Universities, 2021, 42(8): 2635. |
| [5] | ZHANG Huidong, GU Panpan, ZHANG Fang, DU Mingxu, YE Kaiqi, LIU Yu. Design and Electroluminescence Properties of Narrow-spectrum Phosphorescent Complexes [J]. Chem. J. Chinese Universities, 2021, 42(12): 3571. |
| [6] | CHEN Qiuhong, YE Yanchun, REN Mengran, WANG Kaimin, TANG Huaijun, WANG Zhengliang, ZHOU Qiang. Synthesis of an Orange-red-emitting Cationic Iridium(III) Complex Containing a Triphenylamine-triazole Bipolar Unit and Its Application in LEDs [J]. Chem. J. Chinese Universities, 2020, 41(12): 2717. |
| [7] | WANG Jinyu, LIU Chunli, CHEN Yanhui. Synthesis of Aminophosphine Ruthenium Carbene Complex and Its Application in Olefins Metathesis Reaction [J]. Chem. J. Chinese Universities, 2020, 41(12): 2766. |
| [8] | ZHANG Shuxin, FENG Yu, FAN Qinghua. Progress of Transition Metal⁃catalyzed Asymmetric Hydrogenation in China† [J]. Chem. J. Chinese Universities, 2020, 41(10): 2107. |
| [9] | SO Chauming, YUEN Onying, KWONG Fukyee, CHEN Chihchiang, PAI Chengchao, SUN Raymond Waiyin. Application of CM-Phos Ligand in Palladium-catalyzed Cross-coupling Reactions† [J]. Chem. J. Chinese Universities, 2020, 41(10): 2185. |
| [10] | WEI Liangchen, HU Weikang, ZHOU Shixiong, SHU Jun, ZHOU Huidong, HU Xucheng, JIANG Yi, TONG Bihai, ZHANG Qianfeng. Iridium Complex Containing Phosphite and Bipyridine Carboxylate Ligands and Their Aggregation Induced Enhanced Emission and Electroluminescent Properties† [J]. Chem. J. Chinese Universities, 2018, 39(7): 1371. |
| [11] | SUN Riyong,CHEN Zeyu,YE Yanchun,CHEN Mingxian,TANG Huaijun,WANG Kaimin,WANG Zhengliang. Bis(2-phenylpyridine)(2,2'-bithiazole)iridium(Ⅲ) Hexafluorophosphate: Synthesis and Application in Neutral/Warm White Light-emitting Diodes† [J]. Chem. J. Chinese Universities, 2018, 39(5): 869. |
| [12] | MA Xiaoyu,LIANG Jie,YE Ling,FAN Yong,XU Jianing,YE Kaiqi. Synthesis and Electroluminescence Characterization of Mixed-C^N Ligand Tris-cyclometalated Ir(Ⅲ) Complexes† [J]. Chem. J. Chinese Universities, 2018, 39(10): 2129. |
| [13] | PAN Shuai, LI Zhanhong, CHEN Yang, ZHAO Xueling, CHEN Cheng, ZHU Zhigang. Glucose Biosensor Based on Rebuilding the Surface of the Spiral-type Pt-Ir Electrode† [J]. Chem. J. Chinese Universities, 2017, 38(7): 1163. |
| [14] | WANG Rui, WANG Tingyong, XU Haibo. Preparation and Electrocatalytic Properties of Metal Oxide-coated Titanium Anodes Used in Low-temperature Seawater† [J]. Chem. J. Chinese Universities, 2016, 37(4): 701. |
| [15] | LIU Jia, YAN Li, JIANG Miao, DING Yunjie. Effect of Metal Particle Size on the Performance of Tethered-phosphine Modified Rh/SiO2 in Hydroformylation† [J]. Chem. J. Chinese Universities, 2016, 37(1): 114. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||