

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (12): 2574.doi: 10.7503/cjcu20190346
• Physical Chemistry • Previous Articles Next Articles
Yuzhen DUAN,Jinyu ZHU,Junming GUO( ),Mingwu XIANG(
),Mingwu XIANG( ),Xiaofang LIU,Hongli BAI,Changwei SU
),Xiaofang LIU,Hongli BAI,Changwei SU
Received:2019-06-19
Online:2019-12-04
Published:2019-12-04
Contact:
Junming GUO,Mingwu XIANG
E-mail:guojunming@tsinghua.org.cn;xmwbboy@163.com
Supported by:CLC Number:
TrendMD:
Yuzhen DUAN,Jinyu ZHU,Junming GUO,Mingwu XIANG,Xiaofang LIU,Hongli BAI,Changwei SU. Synthesis and Electrochemical Properties of Spinel Lithium Manganese Cathode Material LiNi0.01Co0.03Mn1.96O4 †[J]. Chem. J. Chinese Universities, 2019, 40(12): 2574.
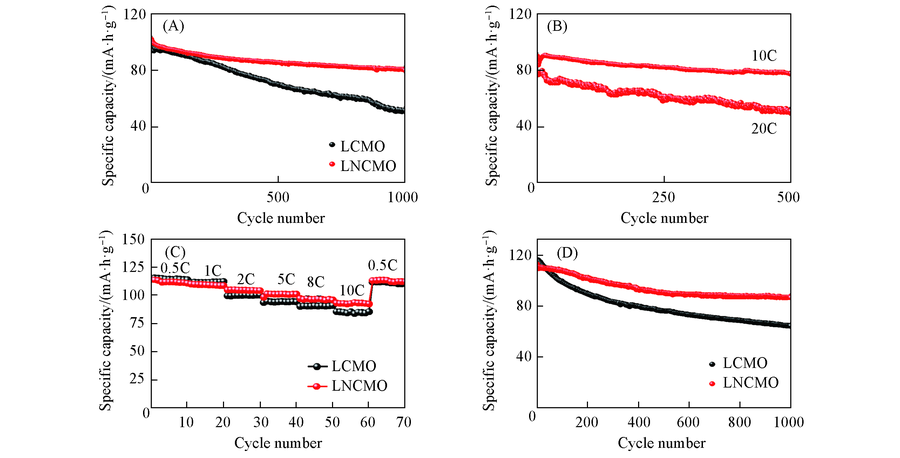
Fig.8 Cycle performance of LCMO and LNCMO samples at 5C under 25 ℃(A), cycle performance of LNCMO sample at 10C and 20C under 25 ℃(B), rate performance of LCMO and LNCMO samples(C) and high-temperature cycle performance of LCMO and LNCMO samples at 1C under 55 ℃(D)
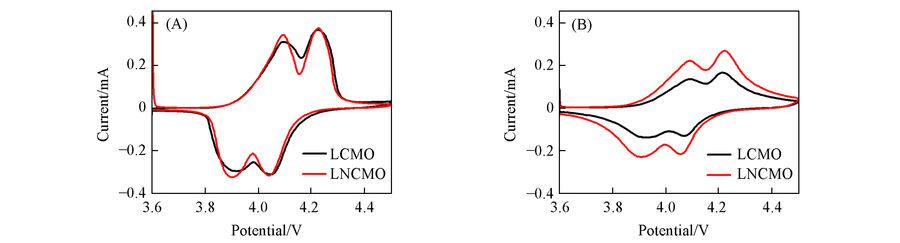
Fig.9 CV curves associated with LCMO, LNCMO at the first(A) and after 1000 cycles(B) ranging from 3.6 V to 4.5 V(vs. Li/Li+) at a scan rate of 0.15 mV/s
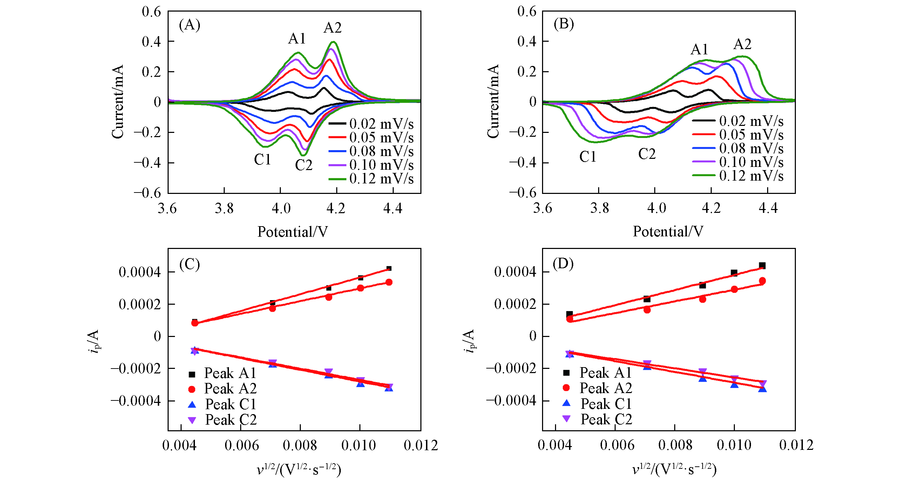
Fig.10 CV curves of LNCMO(A) and LCMO(B) electrodes ranging from 3.6 V to 4.5 V(vs. Li/Li+) at different scan rates and ip-υ1/2 plots of LNCMO(C) and LCMO(D)
| Redox peak | 1011/(cm2·s-1) | |
|---|---|---|
| LCMO | LNCMO | |
| A1 | 2.87 | 4.77 |
| A2 | 2.45 | 3.55 |
| C1 | 2.62 | 4.40 |
| C2 | 1.22 | 3.21 |
| Redox peak | 1011/(cm2·s-1) | |
|---|---|---|
| LCMO | LNCMO | |
| A1 | 2.87 | 4.77 |
| A2 | 2.45 | 3.55 |
| C1 | 2.62 | 4.40 |
| C2 | 1.22 | 3.21 |
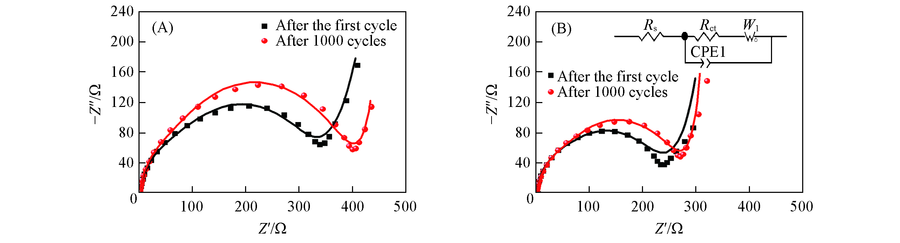
Fig.11 Nyquist plots of LCMO(A) and LNCMO(B) electrodes after the first cycle, and after 1000 cycles at 1C and 25 ℃ Inset shows the equivalent circuit.
| [1] |
Chen S., He T., Su Y. F., Lu Y., Bao L. Y., Chen L., Zhang Q. Y., Wan J. G., Chen R. J., Wu F ., ACS Appl. Mater. Interfaces, 2017,9(35), 29732— 29743
doi: 10.1021/acsami.7b08006 URL |
| [2] |
Zhan C., Wu T. P., Lu J., Amine K ., Energy Environ. Sci., 2018,11(2), 243— 257
doi: 10.1039/C7EE03122J URL |
| [3] |
Zhao H. Y., Li F., Liu X. Q., Xiong W. Q., Chen B., Shao H. L., Que D. Y., Zhang Z., Wu Y ., Electrochimica Acta, 2015,166, 124— 133
doi: 10.1016/j.electacta.2015.03.040 URL |
| [4] | Feng J. J., Xu R. Q., Tang Z. Y., Ai H. Q ., Chem. J. Chinese Universities, 2007,28(8), 1532— 1536 |
| ( 冯季军, 徐荣琪, 唐致远, 艾洪奇 . 高等学校化学学报, 2007,28(8), 1532— 1536) | |
| [5] |
Fergus J. W ., J. Power Sources, 2010,195(4), 939— 954
doi: 10.1016/j.jpowsour.2009.08.089 URL |
| [6] |
Han C. G., Zhu C. Y., Saito G., Akiyama T ., Electrochimica Acta, 2016,209, 225— 234
doi: 10.1016/j.electacta.2016.05.075 URL |
| [7] |
Lin B. H., Yin Q., Hu H. R., Lu F. J., Xia H ., J. Solid State Chem., 2014,209, 23— 28
doi: 10.1016/j.jssc.2013.10.016 URL |
| [8] |
Xu G. J., Liu Z. H., Zhang C. J., Cui G. L., Chen L. Q ., J. Mater. Chem. A, 2015,3(8), 4092— 4123
doi: 10.1039/C4TA06264G URL |
| [9] |
Fang D. L., Li J. C., Liu X., Huang P. F., Xu T. R., Qian M. C., Zheng C. H ., J. Alloy Compd., 2015,640, 82— 89
doi: 10.1016/j.jallcom.2015.03.243 URL |
| [10] | Guo G. H., Chen S., Liu F. F., Zhang L. Y ., New Chem. Mater., 2013,41(10), 169— 171 |
| ( 郭光辉, 陈珊, 刘芳芳, 张利玉 . 化工新型材料, 2013,41(10), 169— 171) | |
| [11] | Sun H. B., Zhu D., Chen Y. G ., J. Func Mater., 2011,5(42), 955— 958 |
| ( 孙怀兵, 朱丁, 陈云贵 . 功能材料学报, 2011,5(42), 955— 958) | |
| [12] |
Sun H. D., He S. C., Xia B. B., Fang G. Q., Liu W. W., Chou G. C., Zhang Q., Kaneko S., Zheng J. W., Li D. C ., Chem. J. Chinese Universities, 2013,34(5), 1059— 1066
doi: 10.7503/cjcu20120832 URL |
|
( 孙洪丹, 贺诗词, 夏丙波, 方国清, 刘伟伟, 仇光超, 张茜, 金子信悟, 郑军伟, 李德成 . 高等学校化学学报, 2013,34(5), 1059— 1066)
doi: 10.7503/cjcu20120832 URL |
|
| [13] |
Suryakala K., Marikkannu K. R., Kalaignan G. P., Vasudevan T ., J. Solid State Electrochem., 2007,11(12), 1671— 1677
doi: 10.1007/s10008-007-0325-1 URL |
| [14] |
Wei Q., Wang X., Yang X., Ju B., Hu B., Shu H., Wen W., Zhou M., Song Y., Wu H., Hu H ., J. Mater. Chem. A, 2013,1(12), 4010— 4016
doi: 10.1039/c3ta01698f URL |
| [15] |
Iqbal A., Iqbal Y., Khan A. M., Ahmed S ., Ionics, 2017,23(8), 1— 9
doi: 10.1007/s11581-016-1864-1 URL |
| [16] |
Zhang H., Xu Y., Liu D., Zhang X., Zhao C ., Electrochimica Acta, 2014,125(12), 225— 231
doi: 10.1016/j.electacta.2014.01.102 URL |
| [17] |
Han D. W., Ryu W. H., Kim W. K., Eom J. Y., Kwon H. S ., J. Phys. Chem. C, 2013,117(10), 4913— 4919
doi: 10.1021/jp310011m URL |
| [18] |
Amarilla J. M., Petrov K., Picó F., Avdeev G., Rojo J. M., Rojas R. M ., J. Power Sources, 2009,191(2), 591— 600
doi: 10.1016/j.jpowsour.2009.02.026 URL |
| [19] |
Chen M., Chen P., Yang F., Song H., Liao S ., Electrochimica Acta, 2016,206, 356— 365
doi: 10.1016/j.electacta.2016.04.148 URL |
| [20] |
Wang G. G., Wang J. M., Mao W. Q., Liu L. Q., Zhang J. Q., Cao C. N ., Acta Phys-Chim Sin., 2005,21(11), 1285— 1290
doi: 10.3866/PKU.WHXB20051118 URL |
|
( 王国光, 王建明, 毛文曲, 刘立清, 张鉴清, 曹楚南 . 物理化学学报, 2005,21(11), 1285— 1290)
doi: 10.3866/PKU.WHXB20051118 URL |
|
| [21] | Tong J., Hu G. R., ., Chinese Battery Ind., 2012,17(5), 267— 271 |
| ( 佟健, 胡国荣 . 电池工业, 2012,17(5), 267— 271) | |
| [22] | Zheng Z., Wu Z. G., Xiang W., Hua W. B., Guo X. D ., Chem. J. Chinese Universities, 2017,38(8), 1458— 1464 |
| ( 郑卓, 吴振国, 向伟, 滑纬博, 郭孝东 . 高等学校化学学报, 2017,38(8), 1458— 1464) | |
| [23] |
Huang S. S., Wu H., Chen P. H., Guo Y., Nie B., Chen B. J., Liu H., Zhang Y ., Mater. Chem. A, 2015,3(7), 3633— 3640
doi: 10.1039/C4TA06522K URL |
| [24] |
Kim J. S., Kim K. S., Cho W., Shin W. H., Kanno R., Choi J. W ., Nano Lett., 2012,12(12), 6358— 6365
doi: 10.1021/nl303619s URL pmid: 23145851 |
| [25] |
Yang C. X., Deng Y. F., Gao M., Yang X. F., Qin X. S., Chen G. H ., Electrochimica Acta, 2017,225, 198— 206
doi: 10.1016/j.electacta.2016.12.096 URL |
| [26] |
Jiang C., Tang Z., Wang S., Zhang Z. T ., J. Power Sources, 2017,357, 144— 148
doi: 10.1016/j.jpowsour.2017.04.079 URL |
| [27] |
Lee S. X. Y. L. T, Hu H. S., Xia Y. G., Xiao F., Liu Z. P ., Chin. Sci. Bull., 2013,58(32), 3350— 3356
doi: 10.1360/972013-805 URL |
|
( 赛喜雅勒图, 胡华胜, 夏永高, 肖锋, 刘兆平 . 科学通报, 2013,58(32), 3350— 3356)
doi: 10.1360/972013-805 URL |
|
| [28] |
Guo D., Wei X., Chang Z. R., Tang H. W., Li B., Shang G. E., Chang K., Yuan X., Wang H. J ., J. Alloys Compd., 2015,632, 222— 228
doi: 10.1016/j.jallcom.2015.01.182 URL |
| [29] |
Duan Y. Z., Guo J. M., Xiang M. W., Zhu J. Y., Su C. W., Bai H. L., Liu X. F., Bai W ., Solid State Ionics, 2018,326, 100— 109
doi: 10.1016/j.ssi.2018.09.014 URL |
| [30] |
Liu J. T., Li G., Yu Y., Bai H. L., Shao M. M., Guo J. M., Su C. W., Liu X. F., Bai W ., J. Alloy Compd., 2017,728, 1315— 1318
doi: 10.1016/j.jallcom.2017.09.098 URL |
| [31] |
Quan K., Zhao Y., An X., Liu J. M., Dong Y. Z., Chen L ., Electrochimica Acta, 2010,55(5), 1575— 1581
doi: 10.1016/j.electacta.2009.10.028 URL |
| [32] |
Ferencz Z., Baan K., Oszko A., Konya Z., Kecskes T., Erdohelyi A ., Catalysis Today, 2013,228, 123— 130
doi: 10.1016/j.cattod.2013.11.014 URL |
| [33] |
Qu Y., Mo Y., Jia X., Zhang L., Du B. D., Lu Y., Li D., Cheng Y ., J. Alloy Compd., 2019,788, 810— 818
doi: 10.1016/j.jallcom.2019.02.285 URL |
| [34] | Li X. Q., Wang J., Liu Z. S ., Chinese J. Power Sources, 2017,41(7), 987— 988 |
| ( 李小庆, 王晶, 刘正实 . 电源技术, 2017,41(7), 987— 988) | |
| [35] |
Liu J. T., Li G., Bai H. L., Shao M. M., Su C. W., Guo J. M., Liu X. F., Bai W ., Solid State Ionics, 2017,307(11), 79— 89
doi: 10.1016/j.ssi.2017.04.014 URL |
| [36] |
Wang Y. Z., Shao X., Xu H. Y., Xie M., Deng S. X., Wang H ., J. Power Sources, 2013,226(6), 140— 148
doi: 10.1016/j.jpowsour.2012.10.077 URL |
| [37] |
Wang J. L., Li Z. H., Yang J., Tang J. J., Yu J. J., Nie W. B., Lei G. T., Xiao Q. Z ., Electrochimica Acta, 2012,75(4), 115— 122
doi: 10.1016/j.electacta.2012.04.136 URL |
| [38] |
Tang S. B., Lai M. O., Lu L ., Mater. Chem. Phys., 2008,111(1), 149— 153
doi: 10.1016/j.matchemphys.2008.03.041 URL |
| [39] |
Wang C. Y., Lu S. G., Kan S. R., Pang J., Jin W.R., Zhang X. J ., J. Power Sources, 2009,189(1), 607— 610
doi: 10.1016/j.jpowsour.2008.09.104 URL |
| [1] | LI Huiyang, ZHU Siying, LI Sha, ZHANG Qiaobao, ZHAO Jinbao, ZHANG Li. Influencing Factors and Promotion Strategies of the First-cycle Coulombic Efficiency of Silicon Suboxide Anodes in Lithium-ion Batteries [J]. Chem. J. Chinese Universities, 2021, 42(8): 2342. |
| [2] | LIU Tiefeng, ZHANG Ben, SHENG Ouwei, NAI Jianwei, WANG Yao, LIU Yujing, TAO Xinyong. Research Progress of the Binders for the Silicon Anode [J]. Chem. J. Chinese Universities, 2021, 42(5): 1446. |
| [3] | WANG Renheng, XIAO Zhe, LI Yan, SUN Yiling, FAN Shuting, ZHENG Junchao, QIAN Zhengfang, HE Zhenjiang. Synthesis of Li2FeP2O7 Cathode Material at Different Temperatures and Its Electrochemical Performance for Lithium Ion Batteries [J]. Chem. J. Chinese Universities, 2021, 42(4): 1299. |
| [4] | HAN Muyao, ZHAO Lina, SUN Jie. Advances in Silicon and Silicon-based Anode Materials [J]. Chem. J. Chinese Universities, 2021, 42(12): 3547. |
| [5] | ZHANG Chenyang,WEN Yuehua,ZHAO Pengcheng,CHENG Jie,QIU Jingyi,SUN Yanzhi. Effect of Organic Carbon Source on Performance of LiTi2(PO4)3/C Composite Electrodes in Aqueous Solutions † [J]. Chem. J. Chinese Universities, 2020, 41(6): 1352. |
| [6] | JI Tianyi, LIU Xiaoxu, ZHAO Jiupeng, LI Yao. Synthesis and Lithium-storage Characteristics of Three-dimensional Cross-linked Graphene Nanofibers † [J]. Chem. J. Chinese Universities, 2020, 41(4): 821. |
| [7] | FANG Liang,DING Xiaoli,SONG Yun,LIU Dongming,LI Yongtao,ZHANG Qingan. Effect of Morphological Tuning on Electrochemical Performance of Perovskite LaCoO3 Anodes† [J]. Chem. J. Chinese Universities, 2019, 40(7): 1456. |
| [8] | LI Xiangnan,YU Mingming,FAN Yong,WANG Qiuxian,ZHANG Huishuang,YANG Shuting. Study on Electrochemical Performances of N-doped P/C Composite as Anode Material of Lithium Ion Batteries † [J]. Chem. J. Chinese Universities, 2019, 40(11): 2360. |
| [9] | WU Wei, LIU Yuchun, ZHU Guancun, AN Jiayu, DOU Guangpeng, WANG Yuyan, LIU Jing, SUN Donglan, GUO Yeping. Application of Polyethylene Separator Modified by Methyl Acrylic Polymer in Lithium Ion Battery † [J]. Chem. J. Chinese Universities, 2019, 40(11): 2332. |
| [10] | LI Yi, LI Chun, YU Kaifeng. Preparation, Characterization and Electrochemical Properties of Mesoporous Biomass Carbon Derived from Corn Stalk† [J]. Chem. J. Chinese Universities, 2018, 39(4): 607. |
| [11] | SUN Bing,JIANG Shang,WANG Runwei,NI Ling,QIU Shilun,ZHANG Zongtao. Preparation and Application of High Performance Lithium Titanate/reduced Graphene Oxide Nanocomposites for Lithium Batteries† [J]. Chem. J. Chinese Universities, 2018, 39(12): 2767. |
| [12] | AI Shujuan, ZONG Chengxing, WU Wei, FENG Jingjing, JIN Can, FU Fengzhi, LIU Jing, SUN Donglan, ZHENG Qin, GUO Yeping. Effect of Derivatives of Glycerol Sulfite as Electrolyte Additive on Electrochemical Performance of Lithium Ion Battery† [J]. Chem. J. Chinese Universities, 2018, 39(11): 2520. |
| [13] | WANG Kun, HUANG Mengyi, ZHANG Xiaosong, HUANG Junjie, DENG Xiang, LIU Changlu. Preparation and Electrochemical Performance of LiNi1/3Co1/3Mn1/3O2@C Composite† [J]. Chem. J. Chinese Universities, 2018, 39(1): 141. |
| [14] | XU Dan, XIAO Shanshan, WU Pan, PAN Ying, CHEN Lihua, SU Baolian, XUE Ming, QIU Shilun. Synthesis of Organodiphosphonate MOFs-derived Ni2P/C Composite† [J]. Chem. J. Chinese Universities, 2018, 39(1): 19. |
| [15] | ZHANG Dong, LI Tingting, QIU Hailong, WEI Yingjin, WANG Chunzhong, CHEN Gang, YUE Huijuan. Preparation and Characterization of N-doped Li2FeSiO4/C Cathode Materials for Lithium Ion Batteries [J]. Chem. J. Chinese Universities, 2017, 38(9): 1633. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||