

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (7): 1490.doi: 10.7503/cjcu20180098
• Physical Chemistry • Previous Articles Next Articles
WU Juan, WANG Hongqiang, LIU Xiaoyun, SHI Zhiyuan, QIU Yongqing*( )
)
Received:2018-02-02
Online:2018-07-10
Published:2018-06-19
Contact:
QIU Yongqing
E-mail:qiuyq466@nenu.edu.cn
CLC Number:
TrendMD:
WU Juan, WANG Hongqiang, LIU Xiaoyun, SHI Zhiyuan, QIU Yongqing. Theoretical Study on the Second-order Nonlinear Optical Properties of D-A-D(D') o-Carborane Triads†[J]. Chem. J. Chinese Universities, 2018, 39(7): 1490.
| Molecule | d(C1—C2)/nm | d(C3—C4)/nm | d(C4—C5)/nm | Molecule | d(C1—C2)/nm | d(C3—C4)/nm | d(C4—C5)/nm |
|---|---|---|---|---|---|---|---|
| 1 | 0.177(0.173)[ | 0.121(0.119)[ | 0.142(0.144)[ | 5 | 0.178(0.174)[ | 0.121(0.120)[ | 0.142(0.144)[ |
| 2 | 0.177 | 0.121 | 0.142 | 6 | 0.177 | 0.121 | 0.142 |
| 3 | 0.178(0.173)[ | 0.121(0.119)[ | 0.142(0.145)[ | 7 | 0.179 | 0.121 | 0.142 |
| 4 | 0.178(0.173)[ | 0.121(0.120)[ | 0.142(0.144)[ |
Table 1 Selected bond lengths of molecules 1—7
| Molecule | d(C1—C2)/nm | d(C3—C4)/nm | d(C4—C5)/nm | Molecule | d(C1—C2)/nm | d(C3—C4)/nm | d(C4—C5)/nm |
|---|---|---|---|---|---|---|---|
| 1 | 0.177(0.173)[ | 0.121(0.119)[ | 0.142(0.144)[ | 5 | 0.178(0.174)[ | 0.121(0.120)[ | 0.142(0.144)[ |
| 2 | 0.177 | 0.121 | 0.142 | 6 | 0.177 | 0.121 | 0.142 |
| 3 | 0.178(0.173)[ | 0.121(0.119)[ | 0.142(0.145)[ | 7 | 0.179 | 0.121 | 0.142 |
| 4 | 0.178(0.173)[ | 0.121(0.120)[ | 0.142(0.144)[ |
| Molecule | Charge/e | 1030 μx/(C·m) | 1030 μy/(C·m) | 1030 μz/(C·m) | 1030 μtot/(C·m) | |
|---|---|---|---|---|---|---|
| Carborane | Substituent | |||||
| 1 | -0.34 | 0.40 | -0.33 | 2.33 | 0 | 2.33 |
| 2 | -0.34 | 0.42 | 0 | 5.34 | -0.33 | 5.34 |
| 3 | -0.33 | 0.39 | 1.00 | 30.35 | 2.00 | 30.35 |
| 4 | -0.35 | 0.42 | -1.33 | 35.69 | 0.67 | 35.69 |
| 5 | -0.35 | 0.42 | -2.33 | 39.69 | 1.67 | 39.69 |
| 6 | -0.35 | 0.42 | 5.67 | 30.35 | -8.34 | 32.02 |
| 7 | -0.33 | 0.40 | -2.00 | 58.71 | 0.33 | 58.71 |
| 7a | -0.31 | 0.37 | -15.68 | 31.69 | 2.33 | 35.69 |
| 7b | -0.35 | 0.43 | 16.68 | 32.36 | 0.33 | 36.36 |
| 7c | -0.38 | 0.42 | -9.34 | 44.03 | 0.67 | 45.03 |
| 7d | -0.43 | 0.42 | -4.34 | 47.70 | 0 | 48.03 |
| 7e | -0.36 | 0.44 | 4.67 | 49.37 | -5.00 | 49.70 |
| 7f | -0.33 | 0.40 | 12.34 | 48.70 | 1.33 | 50.37 |
Table 2 NBO charges and dipole moments of molecules 1—7 and 7a—7f at the PBE1PBE/6-311++G(d,p) level
| Molecule | Charge/e | 1030 μx/(C·m) | 1030 μy/(C·m) | 1030 μz/(C·m) | 1030 μtot/(C·m) | |
|---|---|---|---|---|---|---|
| Carborane | Substituent | |||||
| 1 | -0.34 | 0.40 | -0.33 | 2.33 | 0 | 2.33 |
| 2 | -0.34 | 0.42 | 0 | 5.34 | -0.33 | 5.34 |
| 3 | -0.33 | 0.39 | 1.00 | 30.35 | 2.00 | 30.35 |
| 4 | -0.35 | 0.42 | -1.33 | 35.69 | 0.67 | 35.69 |
| 5 | -0.35 | 0.42 | -2.33 | 39.69 | 1.67 | 39.69 |
| 6 | -0.35 | 0.42 | 5.67 | 30.35 | -8.34 | 32.02 |
| 7 | -0.33 | 0.40 | -2.00 | 58.71 | 0.33 | 58.71 |
| 7a | -0.31 | 0.37 | -15.68 | 31.69 | 2.33 | 35.69 |
| 7b | -0.35 | 0.43 | 16.68 | 32.36 | 0.33 | 36.36 |
| 7c | -0.38 | 0.42 | -9.34 | 44.03 | 0.67 | 45.03 |
| 7d | -0.43 | 0.42 | -4.34 | 47.70 | 0 | 48.03 |
| 7e | -0.36 | 0.44 | 4.67 | 49.37 | -5.00 | 49.70 |
| 7f | -0.33 | 0.40 | 12.34 | 48.70 | 1.33 | 50.37 |
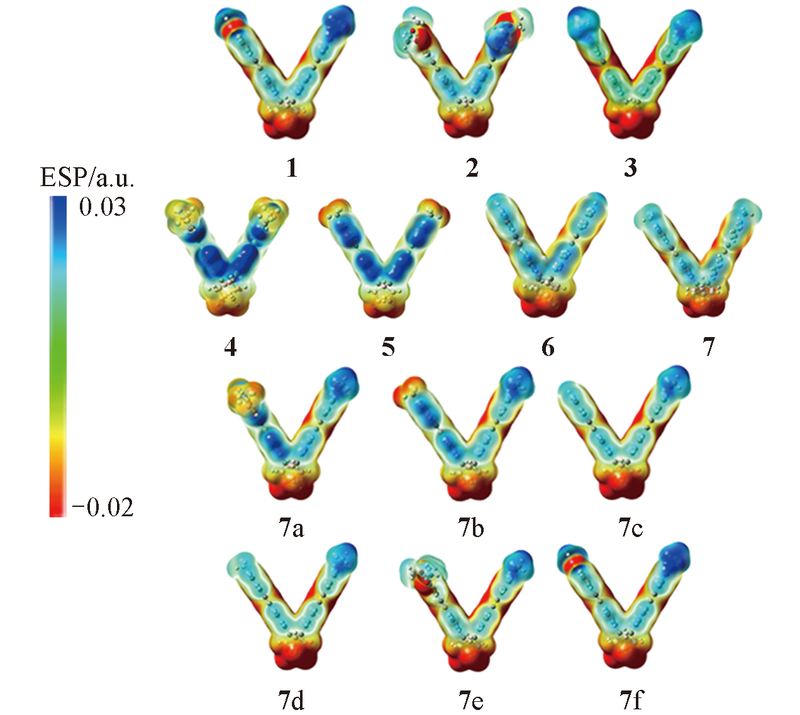
Fig.2 Molecular electrostatic potential distributions of molecules 1—7 and 7a—7f^Red regions indicate negative charge, while blue regions represent positive charge.
| Molecule | Method | βx/a.u. | βy/a.u. | βz/a.u. | βtot/a.u. | Molecule | Method | βx/a.u. | βy/a.u. | βz/a.u. | βtot/a.u. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | B3LYP | -48 | 636 | -53 | 640 | 7 | PBE1PBE | -658 | 57676 | 394 | 57681 |
| PBE1PBE | -54 | 968 | -82 | 973 | 7a | B3LYP | -19321 | 33987 | 2825 | 39197 | |
| 2 | B3LYP | 14 | 3328 | -104 | 3330 | PBE1PBE | -17568 | 31530 | 2588 | 36187 | |
| PBE1PBE | 15 | 3390 | -107 | 3392 | 7b | B3LYP | 19209 | 34941 | 259 | 39874 | |
| 3 | B3LYP | 377 | 10601 | 679 | 10629 | PBE1PBE | 17468 | 32349 | 236 | 36765 | |
| PBE1PBE | 359 | 10013 | 641 | 10040 | 7c | B3LYP | -18110 | 37474 | 773 | 41628 | |
| 4 | B3LYP | -584 | 16883 | 259 | 16895 | PBE1PBE | -16539 | 34693 | 709 | 38440 | |
| PBE1PBE | -550 | 15846 | 243 | 15858 | 7d | B3LYP | -14345 | 40814 | 0 | 43262 | |
| 5 | B3LYP | -1537 | 27273 | 1080 | 27338 | PBE1PBE | -13029 | 37816 | 0 | 39998 | |
| PBE1PBE | -1433 | 25404 | 1005 | 25464 | 7e | B3LYP | 10294 | 46256 | 246 | 47388 | |
| 6 | B3LYP | -1436 | 18177 | -688 | 18247 | PBE1PBE | 9357 | 42817 | 223 | 43828 | |
| PBE1PBE | -1273 | 16999 | -648 | 17059 | 7f | B3LYP | 17164 | 39086 | 149 | 42689 | |
| 7 | B3LYP | -19321 | 33987 | 2825 | 39197 | PBE1PBE | 15654 | 36221 | 137 | 39460 |
Table 3 Static first hyperpolarizabilities of molecules 1—7 and 7a—7f at various levels of theory
| Molecule | Method | βx/a.u. | βy/a.u. | βz/a.u. | βtot/a.u. | Molecule | Method | βx/a.u. | βy/a.u. | βz/a.u. | βtot/a.u. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | B3LYP | -48 | 636 | -53 | 640 | 7 | PBE1PBE | -658 | 57676 | 394 | 57681 |
| PBE1PBE | -54 | 968 | -82 | 973 | 7a | B3LYP | -19321 | 33987 | 2825 | 39197 | |
| 2 | B3LYP | 14 | 3328 | -104 | 3330 | PBE1PBE | -17568 | 31530 | 2588 | 36187 | |
| PBE1PBE | 15 | 3390 | -107 | 3392 | 7b | B3LYP | 19209 | 34941 | 259 | 39874 | |
| 3 | B3LYP | 377 | 10601 | 679 | 10629 | PBE1PBE | 17468 | 32349 | 236 | 36765 | |
| PBE1PBE | 359 | 10013 | 641 | 10040 | 7c | B3LYP | -18110 | 37474 | 773 | 41628 | |
| 4 | B3LYP | -584 | 16883 | 259 | 16895 | PBE1PBE | -16539 | 34693 | 709 | 38440 | |
| PBE1PBE | -550 | 15846 | 243 | 15858 | 7d | B3LYP | -14345 | 40814 | 0 | 43262 | |
| 5 | B3LYP | -1537 | 27273 | 1080 | 27338 | PBE1PBE | -13029 | 37816 | 0 | 39998 | |
| PBE1PBE | -1433 | 25404 | 1005 | 25464 | 7e | B3LYP | 10294 | 46256 | 246 | 47388 | |
| 6 | B3LYP | -1436 | 18177 | -688 | 18247 | PBE1PBE | 9357 | 42817 | 223 | 43828 | |
| PBE1PBE | -1273 | 16999 | -648 | 17059 | 7f | B3LYP | 17164 | 39086 | 149 | 42689 | |
| 7 | B3LYP | -19321 | 33987 | 2825 | 39197 | PBE1PBE | 15654 | 36221 | 137 | 39460 |
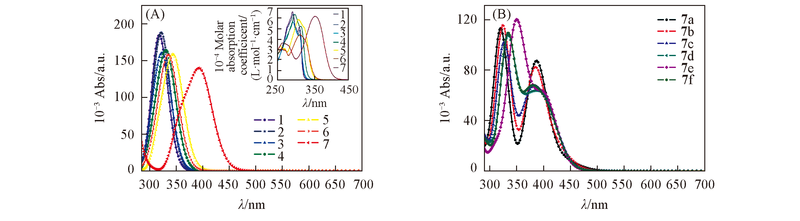
Fig.3 Absorption spectra of compounds 1—7(A) and 7a—7f(B) at the PBE1PBE/6-311++G(d,p) level in THF^nset of (A) reperesents the experimental absorption spectra[29].
| [1] | Nalwa H. S., Appl. Organomet.Chem., 1991, 5(5), 349—377 |
| [2] | Nalwa H. S., Adv. Mater., 1993, 5(5), 341—358 |
| [3] | Taylor J., Caruso J., Newlon A., Englich U., Ruhlandt Senge K., Spencer J. T., Inorg. Chem., 2001, 40(14), 3381—3388 |
| [4] | Zhao X. X., Ma J. P., Dong Y. B., Huang R. Q., Lai T., Cryst. Growth Des., 2007, 7(6), 1058—1068 |
| [5] | Li R. R., Wang H. Q., Wang L., Wu J., Qiu Y. Q., Chem. J. Chinese Universities, 2017, 38(10), 1796—1803 |
| (李荣荣, 王洪强, 王丽, 吴娟, 仇永清.高等学校化学学报,2017, 38(10), 1796—1803) | |
| [6] | Wu K., Chen C., Appl. Phys. A, 1992, 54(3), 209—220 |
| [7] | Verbiest T., Houbrechts S., Kauranen M., Clays K., Persoons A., J. Mater. Chem., 1997, 7(11), 2175—2189 |
| [8] | Fukui H., Shigeta Y., Nakano M., Kubo T., Kamada K., Ohta K., Champagne B., Botek E., J. Phys. Chem. A, 2011, 115(6), 1117—1124 |
| [9] | Kanis D. R., Ratner M. A., Marks T. J., Chem. Rev., 1994, 94(1), 195—242 |
| [10] | Coe B. J., Jones L. A., Harris J. A., Brunschwig B. S., Asselberghs I., Clays K., Persoons A., J. Am. Chem. Soc., 2003, 125(4), 862—863 |
| [11] | Coe B. J., Foxon S. P., Harper E. C., Raftery J., Shaw R., Swanson C. A., Asselberghs I., Clays K., Brunschwig B. S., Fitch A. G., Inorg. Chem., 2009, 48(4), 1370—1379 |
| [12] | Di Bella S., Chem. Soc.Rev., 2001, 30(6), 355—366 |
| [13] | Mendes P. J., Silva T. J. L., Garcia M. H., Ramalho J. P., Carvalho A. P., J. Chem. Inf. Model., 2012, 52(8), 1970—1983 |
| [14] | Coe B. J., Acc. Chem.Res., 2006, 39(6), 383—393 |
| [15] | Wang C. H., Ma N. N., Sun X. X., Sun S. L., Qiu Y. Q., Liu P. J., J. Phys. Chem. A, 2012, 116(43), 10496—10506 |
| [16] | Wang W. Y., Ma N. N., Wang C. H., Zhang M. Y., Sun S. L., Qiu Y. Q., J. Mol. Graph Modell., 2014, 48, 28—35 |
| [17] | Lachman A., Eur. J. Inorg.Chem., 1900, 33(1), 1030—1034 |
| [18] | Wang H. Q., Wang L., Li R. R., Ye J. T., Chen Z. Z., Chen H., Qiu Y. Q., Xie H. M., J. Phys. Chem. A, 2016, 120(46), 9330—9340 |
| [19] | Ma N. N., Li S. J., Yan L. K., Qiu Y. Q., Su Z. M., Dalton Trans., 2014, 43(13), 5069—5075 |
| [20] | Mukherjee S., Thilagar P., Chem. Commun., 2016, 52(6), 1070—1093 |
| [21] | Fang X. Y., Wang W. Y., Wang J., Li X. Q., Song H. J., Qiu Y. Q., Chem. J. Chinese Universities, 2014, 35(11), 2377—2383 |
| ( 方新燕, 王文勇, 王娇, 李晓倩, 宋洪娟, 仇永清.高等学校化学学报,2014, 35(11), 2377—2383) | |
| [22] | Plesek J., Chem.Rev., 1992, 92(2), 269—278 |
| [23] | Reed C. A., Acc. Chem.Res., 1998, 31(3), 133—139 |
| [24] | Reed C. A., Acc. Chem.Res., 2009, 43(1), 121—128 |
| [25] | Allis D. G., Spencer J. T., J. Organomet. Chem., 2000, 614/615, 309—313 |
| [26] | Allis D. G., Spencer J. T., Inorg.Chem., 2001, 40(14), 3373—3380 |
| [27] | Taylor J., Caruso J., Nevolon A., Englich U., Ruhlandt-senge K., Spencer J. T., Inorg. Chem., 2001, 40(14), 3381—3388 |
| [28] | Fang X. Y., Wang L., Zhu C. L., Wang W. Y., Qiu Y. Q., J. Organomet. Chem., 2016, 801, 54—59 |
| [29] | Kokado K., Chujo Y., J. Org. Chem., 2011, 76(1), 316—319 |
| [30] | Furue R., Nishimoto T., Park I. S., Lee J., Yasuda T., Angew. Chem. Int. Ed., 2016, 55(25), 7171—7175 |
| [31] | Frisch M.J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Montgomery J. A. Jr., Vreven T., Kudin K. N., Burant J. C., Millam J. M., Iyengar S. S., Tomasi J., Barone V., Mennucci B., Cossi M., Scalmani G., Rega N., Petersson G. A., Nakatsuji H., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Klene M., Li X., Knox J. E., Hratchian H. P., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Ayala P. Y., Morokuma K., Voth G. A., Salvador P., Dannenberg J. J., Zakrzewski V. G., Dapprich S., Daniels A. D., Strain M. C., Farkas O., Malick D. K., Rabuck A. D., Raghavachari K., Foresman J. B., Ortiz J. V., Cur Q., Baboul A. G., Clifford S., Cioslowski J., Stefanov B. B., Liu G., Liashenko A., Piskorz P., Komaromi I., Martin R. L., Fox D. J., Keith T., Al-Laham M. A., Peng C. Y., Nanayakkara A., Challacombe M., Gill P. M. W., Johnson B., Chen W., Wong M. W., Gonzalez C., Pople J. A., Gaussian 09, Revision D. 01, Gaussian Inc.,Wallingford CT, 2009 |
| [32] | Kurtz H. A., Stewart J. J. P., Dieter K. M., J. Comput. Chem., 1990, 11, 82—87 |
| [1] | CHENG Xiao, K BORA Debajeet, GLANS Per⁃Anders, GUO Jinghua, LUO Yi. An In-depth Theoretical Study of Ligand Field and Charge Transfer Effects on Co2+2pL2,3-edges X-ray Absorption Spectra [J]. Chem. J. Chinese Universities, 2021, 42(7): 2197. |
| [2] | CHANG Hui, YAO Shuangquan, HAN Wenjia, KANG Xiena, ZHANG Li, LI Xinping, ZHANG Zhao. Highly Solvatochromic Terpyridine Compounds for Identification of Butanol Isomers [J]. Chem. J. Chinese Universities, 2021, 42(3): 902. |
| [3] | WANG Linshuo, LI Kunjie, LIU Yumin, ZHAO Ruihong, LI Qing, QIAN Xin, ZHANG Fan, XUE Zhiwei. Theoretical Studies of Triphenyl-s-triazine Groups Regulating Photoelectric Properties of Sensitizing Dyes† [J]. Chem. J. Chinese Universities, 2020, 41(7): 1653. |
| [4] | LIU Chong, LIU Lilai, NIE Jiahui. Synthesis of Carbon Ball Modified g-C3N4 for Improved Photocatalytic Activity† [J]. Chem. J. Chinese Universities, 2018, 39(7): 1511. |
| [5] | LI Xiang,WANG Huiying,WANG Hongqiang,YE Jinting,QIU Yongqing. Theoretical Studies on the Second-order Nonlinear Optical Properties of RuⅡ/Ⅲ Complexes of Bipyridyl† [J]. Chem. J. Chinese Universities, 2018, 39(10): 2221. |
| [6] | CHEN Deli, YANG Pengyong, WU Shengnan, HE Sihui, WANG Fangfang. Ab initio Molecular Dynamics Simulations on the Structures and Stabilities of Pd Clusters Encapsulated UiO-66 Materials† [J]. Chem. J. Chinese Universities, 2017, 38(7): 1210. |
| [7] | LI Rongrong, WANG Hongqiang, WANG Li, WU Juan, QIU Yongqing. Theoretical Study on the Second-order Nonlinear Optical Properties of Diaryl Ammonia(Boron)-π-carborane Ternary Compounds† [J]. Chem. J. Chinese Universities, 2017, 38(10): 1796. |
| [8] | CHEN Jiuju. Theoretical Studies on the of Ambipolar Charge Transport in Terazulene Single Crystal† [J]. Chem. J. Chinese Universities, 2016, 37(1): 121. |
| [9] | LI Shaochen, YU Guangtao, CHEN Wei, ZHOU Zhongjun, HUANG Xuri. Investigation on Structures and Nonlinear Optical Properties of PPV and Its Derivatives Systems with Adsorbing Alkali Metal Atom† [J]. Chem. J. Chinese Universities, 2015, 36(6): 1146. |
| [10] | PANG Ran, JIN Xi, ZHAO Liubin, DING Songyuan, WU Deyin, TIAN Zhongqun. Quantum Chemistry Study of Electrochemical Surface-enhanced Raman Spectroscopy† [J]. Chem. J. Chinese Universities, 2015, 36(11): 2087. |
| [11] | JIANG Xin, QIN Xiaoyu, GONG Mengdi, LI Xiuling, LI Guangzhi, YANG Libin, ZHAO Bing. Improvement of Surface-enhanced Raman Scattering Properties of TiO2 Nanoparticles by Metal Ni Doping† [J]. Chem. J. Chinese Universities, 2014, 35(3): 488. |
| [12] | CHENG Rongmin, LI Na, ZHAN Conghong. Influence of Co-existing Species on Charge Transfer in Dye-sensitized TiO2 Nanocrystalline System† [J]. Chem. J. Chinese Universities, 2014, 35(2): 351. |
| [13] | LI Shaochen, YU Guangtao, CHEN Wei, HUANG Xuri. Investigation on Structures and Nonlinear Optical Properties of Super-short Carbon Nanotube Systems with Surface-adsorbing Lithium Atoms† [J]. Chem. J. Chinese Universities, 2014, 35(11): 2390. |
| [14] | FANG Xinyan, WANG Wenyong, WANG Jiao, LI Xiaoqian, SONG Hongjuan, QIU Yongqing. Theoretical Studies on the Structures and Second-order Nonlinear Optical Properties of 12-Vertex Fluorocarborane Molecules† [J]. Chem. J. Chinese Universities, 2014, 35(11): 2377. |
| [15] | WEN Zhi, KAN Yu-He, YAN Wen-Yan, DING Yan-Yan, WANG Xin-Long. Impact of Core on Structures and Properties of Tetrathiafulvalene Terminated Star-shaped Molecules [J]. Chem. J. Chinese Universities, 2013, 34(6): 1483. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||