

Chem. J. Chinese Universities ›› 2015, Vol. 36 ›› Issue (9): 1743.doi: 10.7503/cjcu20150218
• Physical Chemistry • Previous Articles Next Articles
CHEN Rongfang, XIA Wensheng*( ), WAN Huilin*(
), WAN Huilin*( )
)
Received:2015-03-23
Online:2015-09-10
Published:2015-08-21
Contact:
XIA Wensheng,WAN Huilin
E-mail:wsxia@xmu.edu.cn;hlwan@xmu.edu.cn
Supported by:CLC Number:
TrendMD:
CHEN Rongfang, XIA Wensheng, WAN Huilin. Density Functional Theory Studies on the C—H Bond Activation of Methane by(CeO2)m(m=1—3)†[J]. Chem. J. Chinese Universities, 2015, 36(9): 1743.
| Reaction | Eb/(kJ·mol-1) | ||
|---|---|---|---|
| This work | Predicted by others | Experiment | |
| CH4→CH3+H | 432.2 | 432.2[ | 438.1[ |
| CeO2→CeO+O | 597.9 | 598.3[ | 646.0±19.2[ |
Table 1 Comparison of the predicted bond energies at the level of B3LYP/SDD+TZVP
| Reaction | Eb/(kJ·mol-1) | ||
|---|---|---|---|
| This work | Predicted by others | Experiment | |
| CH4→CH3+H | 432.2 | 432.2[ | 438.1[ |
| CeO2→CeO+O | 597.9 | 598.3[ | 646.0±19.2[ |
| Species | ΔE/(kJ·mol-1) | Species | ΔE/(kJ·mol-1) | ||||
|---|---|---|---|---|---|---|---|
| B3LYP | B3PW91 | CCSD(T) | B3LYP | B3PW91 | CCSD(T) | ||
| CeO2+CH4 | 0 | 0 | 0 | TS | 133.1 | 128.4 | 124.7 |
| CeO2…CH4 | -5.9 | -3.3 | -23.0 | Product | 43.5 | 50.2 | 21.8 |
Table 2 Relative energies of various compounds in the reaction of CeO2 + CH4 predicted at the level of B3LYP/SDD+TZVP, B3PW91/SDD+TZVP and CCSD(T)//B3LYP/ SDD+TZVP
| Species | ΔE/(kJ·mol-1) | Species | ΔE/(kJ·mol-1) | ||||
|---|---|---|---|---|---|---|---|
| B3LYP | B3PW91 | CCSD(T) | B3LYP | B3PW91 | CCSD(T) | ||
| CeO2+CH4 | 0 | 0 | 0 | TS | 133.1 | 128.4 | 124.7 |
| CeO2…CH4 | -5.9 | -3.3 | -23.0 | Product | 43.5 | 50.2 | 21.8 |
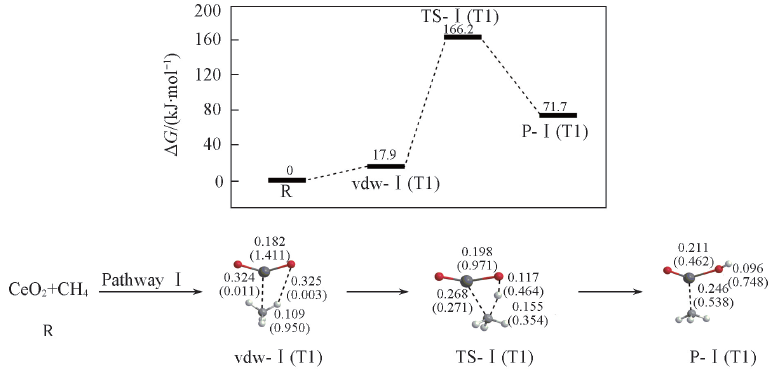
Fig.2 Potential energy surface(at 298 K) and optimized geometries of the product(P), van der Waals(vdw) complex, and transition state(TS) of CH4 activations on the CeO2 cluster at the B3LYP/SDD+TZVP levelThe Wiberg bond orders are included in parentheses. Bond lengths are in nm.
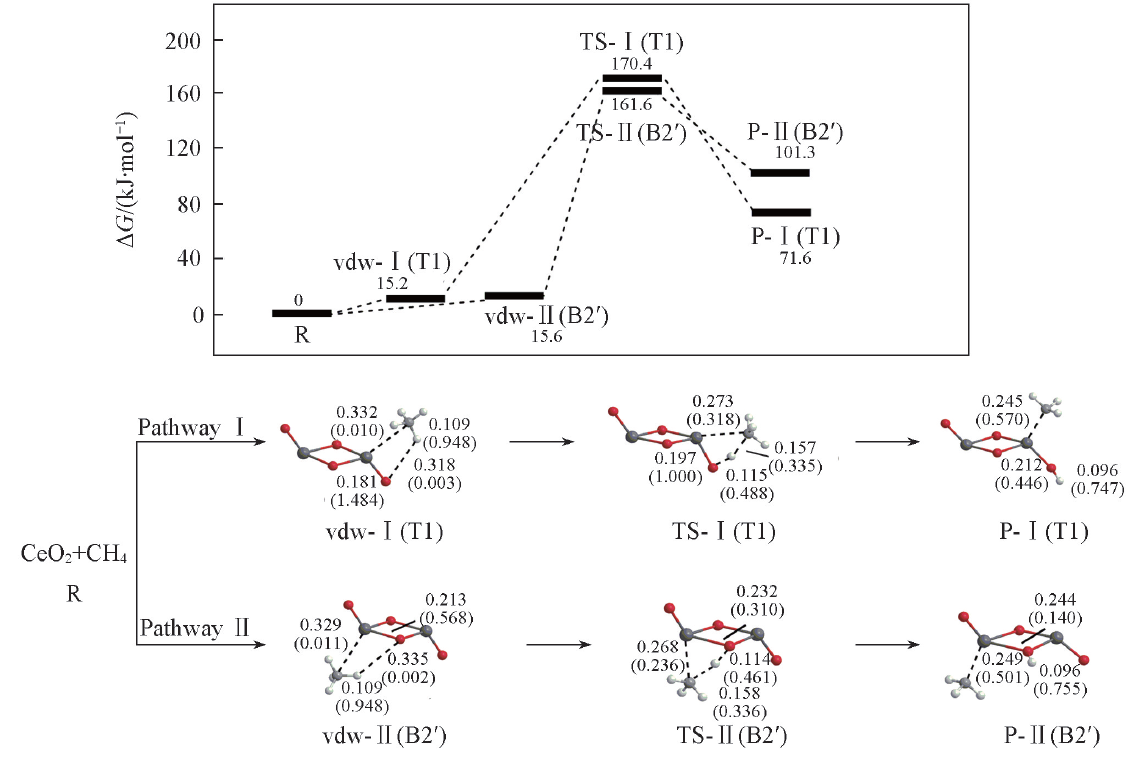
Fig.3 Potential energy surfaces(at 298 K) and optimized geometries of the products(P), van der Waals(vdw) complexes, and transition states(TS) of CH4 activations on the Ce2O4 cluster at the B3LYP/SDD+TZVP levelThe Wiberg bond orders are included in parentheses. Bond lengths are in nm.
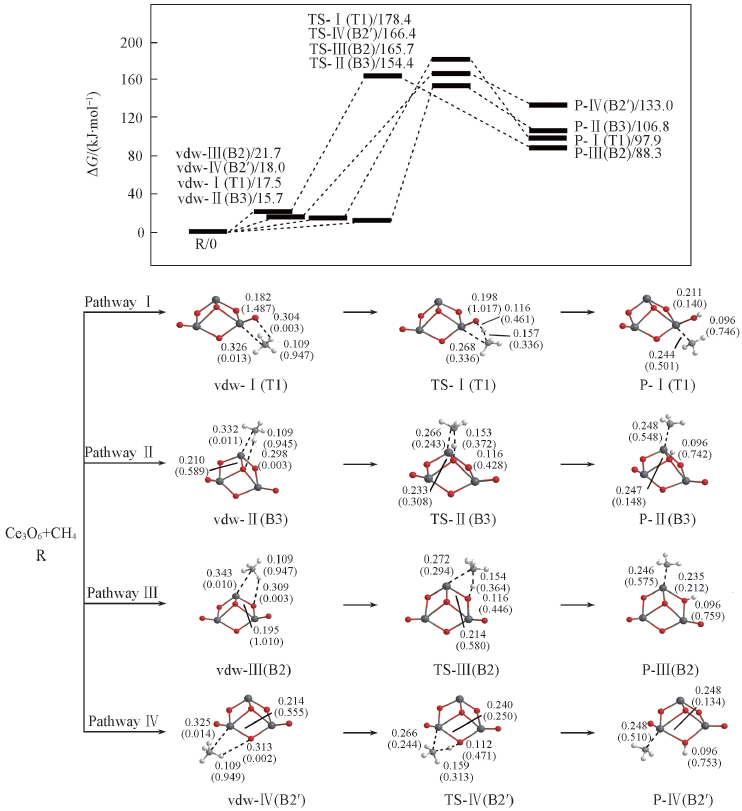
Fig.4 Potential energy surfaces(at 298 K) and optimized geometries of the products(P), van der Waals(vdw) complexes, and transition states(TS) of CH4 activations on the Ce3O6 cluster at the B3LYP/SDD+TZVP levelThe Wiberg bond orders are included in parentheses. Bond lengths are in nm.
| Cluster | C—H activation pathway | q/e(at active sites in clusters w/o CH4) | q/e | νimg/cm-1 (TS) | ΔGvdw/ (kJ· mol-1) | ΔG*/ (kJ· mol-1) | ΔGr/ (kJ· mol-1) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ce(free clusters) | O(free clusters) | Ce (TS) | O (TS) | Cluster (TS) | CH4 (TS) | ||||||
| CeO2(A) | Ⅰ(T1) | 2.266 | -1.133 | 2.372 | -1.087 | 0.190 | -0.190 | 1295i | 17.9 | 166.2 | 71.7 |
| Ce2O4(B) | Ⅰ(T1) | 2.485 | -1.113 | 2.533 | -1.064 | 0.168 | -0.168 | 1214i | 15.2 | 170.4 | 71.6 |
| Ⅱ(B2') | 2.485 | -1.372 | 2.534 | -1.301 | 0.182 | -0.182 | 1292i | 15.6 | 161.6 | 101.3 | |
| Ce3O6(C) | Ⅰ(T1) | 2.549 | -1.092 | 2.560 | -1.048 | 0.149 | -0.149 | 1295i | 17.5 | 178.4 | 97.9 |
| Ⅱ(B3) | 2.494 | -1.434 | 2.526 | -1.354 | 0.159 | -0.159 | 1176i | 15.7 | 154.4 | 106.8 | |
| Ⅲ(B2) | 2.494 | -1.303 | 2.525 | -1.262 | 0.145 | -0.145 | 764i | 21.7 | 165.7 | 88.3 | |
| Ⅳ(B2') | 2.549 | -1.367 | 2.587 | -1.313 | 0.177 | -0.177 | 1258i | 18.0 | 166.4 | 133.0 | |
Table 3 Predicted reaction energetics(at 298 K) for methane on the(CeO2)m(m=1—3) clusters, imaging frequency(νimg) of transition states TS, and NBO charge population analysis on the clusters and CH4 species in TS and the Ce and O atoms interacted with/without CH4, at the level of B3LYP/SDD+TZVP*
| Cluster | C—H activation pathway | q/e(at active sites in clusters w/o CH4) | q/e | νimg/cm-1 (TS) | ΔGvdw/ (kJ· mol-1) | ΔG*/ (kJ· mol-1) | ΔGr/ (kJ· mol-1) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ce(free clusters) | O(free clusters) | Ce (TS) | O (TS) | Cluster (TS) | CH4 (TS) | ||||||
| CeO2(A) | Ⅰ(T1) | 2.266 | -1.133 | 2.372 | -1.087 | 0.190 | -0.190 | 1295i | 17.9 | 166.2 | 71.7 |
| Ce2O4(B) | Ⅰ(T1) | 2.485 | -1.113 | 2.533 | -1.064 | 0.168 | -0.168 | 1214i | 15.2 | 170.4 | 71.6 |
| Ⅱ(B2') | 2.485 | -1.372 | 2.534 | -1.301 | 0.182 | -0.182 | 1292i | 15.6 | 161.6 | 101.3 | |
| Ce3O6(C) | Ⅰ(T1) | 2.549 | -1.092 | 2.560 | -1.048 | 0.149 | -0.149 | 1295i | 17.5 | 178.4 | 97.9 |
| Ⅱ(B3) | 2.494 | -1.434 | 2.526 | -1.354 | 0.159 | -0.159 | 1176i | 15.7 | 154.4 | 106.8 | |
| Ⅲ(B2) | 2.494 | -1.303 | 2.525 | -1.262 | 0.145 | -0.145 | 764i | 21.7 | 165.7 | 88.3 | |
| Ⅳ(B2') | 2.549 | -1.367 | 2.587 | -1.313 | 0.177 | -0.177 | 1258i | 18.0 | 166.4 | 133.0 | |
| Species | Ggas/a.u. | Gsolv/a.u. | Δ | ΔGgas/(kJ·mol-1) | ΔGsolv/(kJ·mol-1) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CH4 | -40.509685 | -40.562719 | -139.2 | |||||||
| CeO2(A) | -625.769905 | -625.766078 | 10.0 | |||||||
| CeO2(A)+CH4 | -666.279590 | -666.328797 | -129.2 | 0 | 0 | |||||
| vdw-Ⅰ(T1) | -666.272785 | -666.332007 | -155.5 | 17.9 | -8.4 | |||||
| TS-Ⅰ(T1) | -666.216277 | -666.277172 | -159.9 | 166.2 | 135.5 | |||||
| P-Ⅰ(T1) | -666.252288 | -666.306037 | -141.1 | 71.7 | 59.8 | |||||
| Ce2O4(B) | -1251.615246 | -1251.626692 | -30.1 | |||||||
| Ce2O4(B)+CH4 | -1292.124931 | -1292.189411 | -169.3 | 0 | 0 | |||||
| vdw-Ⅰ(T1) | -1292.119134 | -1292.187597 | -179.7 | 15.2 | 4.8 | |||||
| vdw-Ⅱ(B2') | -1292.118978 | -1292.187314 | -179.4 | 15.6 | 5.5 | |||||
| TS-Ⅰ(T1) | -1292.060019 | -1292.132049 | -189.1 | 170.4 | 150.6 | |||||
| TS-Ⅱ(B2') | -1292.063375 | -1292.134880 | -187.7 | 161.6 | 143.2 | |||||
| P-Ⅰ(T1) | -1292.097646 | -1292.153706 | -147.2 | 71.6 | 93.7 | |||||
| P-Ⅱ(B2') | -1292.086332 | -1292.148290 | -162.7 | 101.3 | 107.9 | |||||
| Ce3O6(C) | -1877.475699 | -1877.492891 | -45.1 | |||||||
| Ce3O6(C)+CH4 | -1917.985384 | -1918.055610 | -184.3 | 0 | 0 | |||||
| vdw-Ⅰ(T1) | -1917.978710 | -1918.055626 | -201.9 | 17.5 | -0.1 | |||||
| vdw-Ⅱ(B3) | -1917.979392 | -1918.053792 | -195.3 | 15.7 | 4.7 | |||||
| vdw-Ⅲ(B2) | -1917.977104 | -1918.056477 | -208.4 | 21.7 | -2.4 | |||||
| vdw-Ⅳ(B2') | -1917.978539 | -1918.055939 | -203.2 | 18.0 | -0.9 | |||||
| TS-Ⅰ(T1) | -1917.917443 | -1917.999296 | -214.9 | 178.4 | 147.8 | |||||
| TS-Ⅱ(B3) | -1917.926594 | -1918.000597 | -194.3 | 154.4 | 144.4 | |||||
| TS-Ⅲ(B2) | -1917.922263 | -1917.998085 | -199.1 | 165.7 | 150.9 | |||||
| TS-Ⅳ(B2') | -1917.922012 | -1918.001456 | -208.6 | 166.4 | 142.1 | |||||
| P-Ⅰ(T1) | -1917.948078 | -1918.026277 | -205.3 | 97.9 | 76.9 | |||||
| P-Ⅱ(B3) | -1917.944706 | -1918.016064 | -187.4 | 106.8 | 103.7 | |||||
| P-Ⅲ(B2) | -1917.951740 | -1918.016930 | -171.2 | 88.3 | 101.4 | |||||
| P-Ⅳ(B2') | -1917.934711 | -1918.012010 | -202.9 | 133.0 | 114.4 | |||||
Table 4 Free energies(G), solvation free energies(ΔGsolv) and relative free energies(ΔG for van der Waals complex formation, for the C—H activation barrier, for the reaction, relative to CH4+cluster) in the gas phase and in the solvent(water) at the level of B3LYP/SDD+TZVP
| Species | Ggas/a.u. | Gsolv/a.u. | Δ | ΔGgas/(kJ·mol-1) | ΔGsolv/(kJ·mol-1) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CH4 | -40.509685 | -40.562719 | -139.2 | |||||||
| CeO2(A) | -625.769905 | -625.766078 | 10.0 | |||||||
| CeO2(A)+CH4 | -666.279590 | -666.328797 | -129.2 | 0 | 0 | |||||
| vdw-Ⅰ(T1) | -666.272785 | -666.332007 | -155.5 | 17.9 | -8.4 | |||||
| TS-Ⅰ(T1) | -666.216277 | -666.277172 | -159.9 | 166.2 | 135.5 | |||||
| P-Ⅰ(T1) | -666.252288 | -666.306037 | -141.1 | 71.7 | 59.8 | |||||
| Ce2O4(B) | -1251.615246 | -1251.626692 | -30.1 | |||||||
| Ce2O4(B)+CH4 | -1292.124931 | -1292.189411 | -169.3 | 0 | 0 | |||||
| vdw-Ⅰ(T1) | -1292.119134 | -1292.187597 | -179.7 | 15.2 | 4.8 | |||||
| vdw-Ⅱ(B2') | -1292.118978 | -1292.187314 | -179.4 | 15.6 | 5.5 | |||||
| TS-Ⅰ(T1) | -1292.060019 | -1292.132049 | -189.1 | 170.4 | 150.6 | |||||
| TS-Ⅱ(B2') | -1292.063375 | -1292.134880 | -187.7 | 161.6 | 143.2 | |||||
| P-Ⅰ(T1) | -1292.097646 | -1292.153706 | -147.2 | 71.6 | 93.7 | |||||
| P-Ⅱ(B2') | -1292.086332 | -1292.148290 | -162.7 | 101.3 | 107.9 | |||||
| Ce3O6(C) | -1877.475699 | -1877.492891 | -45.1 | |||||||
| Ce3O6(C)+CH4 | -1917.985384 | -1918.055610 | -184.3 | 0 | 0 | |||||
| vdw-Ⅰ(T1) | -1917.978710 | -1918.055626 | -201.9 | 17.5 | -0.1 | |||||
| vdw-Ⅱ(B3) | -1917.979392 | -1918.053792 | -195.3 | 15.7 | 4.7 | |||||
| vdw-Ⅲ(B2) | -1917.977104 | -1918.056477 | -208.4 | 21.7 | -2.4 | |||||
| vdw-Ⅳ(B2') | -1917.978539 | -1918.055939 | -203.2 | 18.0 | -0.9 | |||||
| TS-Ⅰ(T1) | -1917.917443 | -1917.999296 | -214.9 | 178.4 | 147.8 | |||||
| TS-Ⅱ(B3) | -1917.926594 | -1918.000597 | -194.3 | 154.4 | 144.4 | |||||
| TS-Ⅲ(B2) | -1917.922263 | -1917.998085 | -199.1 | 165.7 | 150.9 | |||||
| TS-Ⅳ(B2') | -1917.922012 | -1918.001456 | -208.6 | 166.4 | 142.1 | |||||
| P-Ⅰ(T1) | -1917.948078 | -1918.026277 | -205.3 | 97.9 | 76.9 | |||||
| P-Ⅱ(B3) | -1917.944706 | -1918.016064 | -187.4 | 106.8 | 103.7 | |||||
| P-Ⅲ(B2) | -1917.951740 | -1918.016930 | -171.2 | 88.3 | 101.4 | |||||
| P-Ⅳ(B2') | -1917.934711 | -1918.012010 | -202.9 | 133.0 | 114.4 | |||||
| [1] | Rodriguez J. A., Ma S., Liu P., Hrbek J., Evans J., Perez M., Science, 2007, 318, 1757—1760 |
| [2] | Kaspar J., Fornasiero P., Graziani M., Catal. Today, 1999, 50, 285—298 |
| [3] | Zhang H. L., Ren L. H., Lu A. H., Li W. C., Chin. J. Catal., 2012, 33, 1125—1132 |
| (张慧丽, 任丽会, 陆安慧, 李文翠. 催化学报, 2012, 33, 1125—1132) | |
| [4] | Zhang B., Li D., Wang X. Y., Catal. Today, 2010, 158, 348—353 |
| [5] | Cargnello M., Jaen J. J. D., Garrido J. C. H., Bakhmutsky K., Montini T., Gamez J. J. C., Gorte R. J., Fornasiero P., Science, 2012, 337, 713—717 |
| [6] | Gennard S., Cora F., Catlow C. R. A., J. Phys. Chem. B, 1999, 103, 10158—10170 |
| [7] | Skorodumova N. V., Simak S. I., Lundqvist B. I., Abrikosov I. A., Johansson B., Phys. Rev. Lett., 2002, 89(16), 166601-1—166601-4 |
| [8] | Skorodumova N. V., Baudin M., Hermansson K., Phys. Rev. B, 2004, 69(7), 075401-1—075401-8 |
| [9] | Chen H. L., Peng W. T., Ho J. J., Hsieh H. M., Chem. Phys., 2008, 348, 161—168 |
| [10] | Chen H. T., Choi Y., Liu M., Lin M. C., J. Phys. Chem. C, 2007, 111, 11117—11122 |
| [11] | Chen H. L., Liu S. H., Ho J. J., J. Phys. Chem. B, 2006, 110, 14816—14823 |
| [12] | Wu X. N., Zhao Y. X., Xue W., Wang Z. C., He S. G., Ding X. L., Phys. Chem. Chem. Phys., 2010, 12, 3984—3997 |
| [13] | Frisch M.J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Montgomery J. A., Vreven T., Kudin K. N., Burant J. C., Millam J. M., Iyengar S. S., Tomasi J., Barone V., Mennucci B., Cossi M., Scalmani G., Rega N., Petersson G. A., Nakatsuji H., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Klene M., Li X., Knox J. E., Hratchian H. P., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Ayala P. Y., Morokuma K., Voth G. A., Salvador P., Dannenberg J. J., Zakrzewski V. G., Dapprich S., Daniels A. D., Strain M. C., Farkas O., Malick D. K., Rabuck A. D., Raghavachari K., Foresman J. B., Ortiz J. V., Cui Q., Baboul A. G., Clifford S., Cioslowski J., Stefanov B. B., Liu G., Liashenko A., Piskorz P., Komaromi I., Martin R. L., Fox D. J., Keith T., Al-Laham M. A., Peng C. Y., Nanayakkara A., Challacombe M., Gill P. M. W., Johnson B., Chen W., Wong M. W., Gonzalez C., Pople J. A., Gaussian 03, Revision B. 04, Wallingford CT, Gaussian Inc., 2004 |
| [14] | Lee C. T., Yang W. T., Parr R. G., Phys. Rev. B, 1988, 37(2), 785—789 |
| [15] | Becke A. D., J. Chem. Phys., 1993, 98, 5648—5652 |
| [16] | Golden D. M., Denson S. W., Chem. Rev., 1969, 69, 125—137 |
| [17] | Kordis J., Gingerich K. A., J. Chem. Phys., 1977, 66, 483—491 |
| [18] | Harris N., Shaik S., Schroder D., Schwarz H., Helv. Chim. Acta, 1999, 82, 1784—1797 |
| [19] | Hwang D. Y., Mebel A. M., J. Phys. Chem. A, 2002, 106, 12072—12083 |
| [20] | Wang Y. G., Yang X. F., Hu L. H., Li Y. D., Li J., Chin. J. Catal., 2014, 35, 462—467 |
| (王阳刚, 杨水峰, 胡林华, 李亚栋, 李隽. 催化学报, 2014, 35, 462—467) | |
| [21] | Meng L., Lin J. J., Pu Z. Y., Luo L. F., Jia A. P., Huang W. X., Luo M. F., Lu J. Q., Appl. Catal. B: Environ., 2012, 119, 117—122 |
| [22] | Zuo Y., Huang X. S., Li L. P., Li G. S., J. Mater. Chem. A, 2013, 1(2), 374—380 |
| [23] | Ding X. L., Wu X. N., Zhao Y. X., He S. G., Acc. Chem. Res., 2012, 45, 382—390 |
| [24] | Fu G., Chen Z. N., Xu X., Wan H. L., J. Phys. Chem. A, 2008, 112(4), 717—721 |
| [1] | HE Hongrui, XIA Wensheng, ZHANG Qinghong, WAN Huilin. Density-functional Theoretical Study on the Interaction of Indium Oxyhydroxide Clusters with Carbon Dioxide and Methane [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220196. |
| [2] | ZHOU Leilei, CHENG Haiyang, ZHAO Fengyu. Research Progress of CO2 Hydrogenation over Pd-based Heterogeneous Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220279. |
| [3] | ZHOU Zixuan, YANG Haiyan, SUN Yuhan, GAO Peng. Recent Progress in Heterogeneous Catalysts for the Hydrogenation of Carbon Dioxide to Methanol [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220235. |
| [4] | YANG Dan, LIU Xu, DAI Yihu, ZHU Yan, YANG Yanhui. Research Progress in Electrocatalytic CO2 Reduction Reaction over Gold Clusters [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220198. |
| [5] | PENG Kuilin, LI Guilin, JIANG Chongyang, ZENG Shaojuan, ZHANG Xiangping. Research Progress for the Role of Electrolytes in the CO2 Electrochemical Reduction [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220238. |
| [6] | CHANG Yunfei, LIAO Mingyi, WEN Jiaming. Reduction Performance and Mechanism of Liquid Terminated-carboxyl Fluoroelastomers Using NaBH4/MCl x Reduction System [J]. Chem. J. Chinese Universities, 2022, 43(6): 20210835. |
| [7] | REN Nana, XUE Jie, WANG Zhifan, YAO Xiaoxia, WANG Fan. Effects of Thermodynamic Data on Combustion Characters of 1,3-Butadiene [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220151. |
| [8] | GAO Zhiwei, LI Junwei, SHI Sai, FU Qiang, JIA Junru, AN Hailong. Analysis of Gating Characteristics of TRPM8 Channel Based on Molecular Dynamics [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220080. |
| [9] | WONG Honho, LU Qiuyang, SUN Mingzi, HUANG Bolong. Rational Design of Graphdiyne-based Atomic Electrocatalysts: DFT and Self-validated Machine Learning [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220042. |
| [10] | ZHANG Shiyu, HE Runhe, LI Yongbing, WEI Shijun, ZHANG Xingxiang. Fabrication of Lithium-sulfur Battery Cathode with Radiation Crosslinked Low Molecular Weight of Polyacrylonitrile and the Mechanism of Sulfur Storage [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210632. |
| [11] | BI Gening, XIAO Xiaohua, LI Gongke. Development and Validation of Multiple Physical Fields Coupling Model for Microwave-assisted Extraction [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210739. |
| [12] | SUN Cuihong, LYU Liqiang, LIU Ying, WANG Yan, YANG Jing, ZHANG Shaowen. Mechanism and Kinetics on the Reaction of Isopropyl Nitrate with Cl, OH and NO3 Radicals [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210591. |
| [13] | CHANG Sihui, CHEN Tao, ZHAO Liming, QIU Yongjun. Thermal Degradation Mechanism of Bio-based Polybutylactam Plasticized by Ionic Liquids [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220353. |
| [14] | ZHANG Mi, TIAN Yafeng, GAO Keli, HOU Hua, WANG Baoshan. Molecular Dynamics Simulation of the Physicochemical Properties of Trifluoromethanesulfonyl Fluoride Dielectrics [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220424. |
| [15] | WEN Zhiguo, QIAO Zaiyin, TIAN Chong, MAXIM Borzov, NIE Wanli. Catalytic Activity and Reaction Mechanism of FLPs for the Reduction of Enamine [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220555. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||