

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (7): 1414.doi: 10.7503/cjcu20140099
• Analytical Chemistry • Previous Articles Next Articles
NIE Guizhen, LI Laisheng*( ), CHENG Biaoping, ZHOU Rendan, ZHANG Hongfu
), CHENG Biaoping, ZHOU Rendan, ZHANG Hongfu
Received:2014-02-08
Online:2014-07-10
Published:2014-06-09
Contact:
LI Laisheng
E-mail:lilaishengcn@163.com
Supported by:CLC Number:
TrendMD:
NIE Guizhen, LI Laisheng, CHENG Biaoping, ZHOU Rendan, ZHANG Hongfu. Enantioseparations of Dihydropyridine Drugs by Sulfobutyl Ether-β-Cyclodextrin Modified Capillary Electrochromatography†[J]. Chem. J. Chinese Universities, 2014, 35(7): 1414.
| pH | t1 | t2 | α | RS |
|---|---|---|---|---|
| 2.5 | 9.03 | 10.14 | 1.46 | 1.85 |
| 3.5 | 7.34 | 7.88 | 1.45 | 1.84 |
| 4.0 | 6.38 | 7.55 | 1.46 | 1.84 |
| 4.5 | 4.22 | 4.63 | 1.18 | 1.24 |
| 6.5 | 3.90 | 3.90 | 1.00 | 0 |
Table 1 Effects of pH values on the separationparameters
| pH | t1 | t2 | α | RS |
|---|---|---|---|---|
| 2.5 | 9.03 | 10.14 | 1.46 | 1.85 |
| 3.5 | 7.34 | 7.88 | 1.45 | 1.84 |
| 4.0 | 6.38 | 7.55 | 1.46 | 1.84 |
| 4.5 | 4.22 | 4.63 | 1.18 | 1.24 |
| 6.5 | 3.90 | 3.90 | 1.00 | 0 |
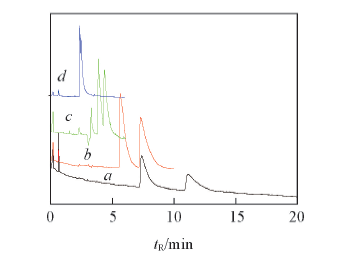
Fig.6 Effect of acetonitrile content on the enantioseparation of amlodipine besylate a. 18%; b. 22%; c. 25%. d. 30%. Conditions: 20 mmol/L NaH2PO4(pH=4.0), 4.0 mmol/L SBE-β-CD, applied voltage 20 kV and temperature 15 ℃, injection: 2 kV×5 s.
| RSD(%) | ||||
|---|---|---|---|---|
| t1 | t2 | A1 | A2 | |
| Intra-day | 1.09 | 1.24 | 4.35 | 4.48 |
| Inter-day | 1.21 | 1.56 | 5.54 | 5.65 |
Table 2 Reproducibilities of amlodipine enantiomers about retention time and peak area(n=5)
| RSD(%) | ||||
|---|---|---|---|---|
| t1 | t2 | A1 | A2 | |
| Intra-day | 1.09 | 1.24 | 4.35 | 4.48 |
| Inter-day | 1.21 | 1.56 | 5.54 | 5.65 |
| Compound | t1/min | t2/min | Rs | N1/(Plates·m-1) | N2/(Plates·m-1) | ACN(%) | V/kV |
|---|---|---|---|---|---|---|---|
| Amlodipine besylate | 6.38 | 7.55 | 1.84 | 22976 | 20054 | 22 | 20 |
| Amlodipine mesylate | 4.64 | 4.93 | 1.75 | 30261 | 29851 | 22 | 25 |
| Nimodipine | 7.04 | 7.87 | 3.62 | 61011 | 60034 | 20 | 25 |
| Nicardipine | 5.33 | 6.03 | 3.52 | 50142 | 49867 | 15 | 25 |
| Manidipine | 10.97 | 11.75 | 1.52 | 37072 | 35721 | 25 | 15 |
| Benidipine | 6.23 | 6.64 | 1.54 | 54853 | 52014 | 10 | 20 |
| Cilnidipine | 7.37 | 8.16 | 1.50 | 13795 | 12876 | 25 | 25 |
| Nitredipine | 11.88 | 12.56 | 1.15 | 25603 | 20230 | 10 | 15 |
| Felodipine | 15.90 | 16.76 | 1.14 | 12065 | 10274 | 18 | 25 |
| Lercanidipine | 5.70 | 6.13 | 0.63 | 11254 | 10235 | 12 | 25 |
| Lacidipine | 29.12 | 29.83 | 0.32 | 10242 | 10112 | 12 | 20 |
Table 3 Enantioseparation results of dihydropyridine drugs*
| Compound | t1/min | t2/min | Rs | N1/(Plates·m-1) | N2/(Plates·m-1) | ACN(%) | V/kV |
|---|---|---|---|---|---|---|---|
| Amlodipine besylate | 6.38 | 7.55 | 1.84 | 22976 | 20054 | 22 | 20 |
| Amlodipine mesylate | 4.64 | 4.93 | 1.75 | 30261 | 29851 | 22 | 25 |
| Nimodipine | 7.04 | 7.87 | 3.62 | 61011 | 60034 | 20 | 25 |
| Nicardipine | 5.33 | 6.03 | 3.52 | 50142 | 49867 | 15 | 25 |
| Manidipine | 10.97 | 11.75 | 1.52 | 37072 | 35721 | 25 | 15 |
| Benidipine | 6.23 | 6.64 | 1.54 | 54853 | 52014 | 10 | 20 |
| Cilnidipine | 7.37 | 8.16 | 1.50 | 13795 | 12876 | 25 | 25 |
| Nitredipine | 11.88 | 12.56 | 1.15 | 25603 | 20230 | 10 | 15 |
| Felodipine | 15.90 | 16.76 | 1.14 | 12065 | 10274 | 18 | 25 |
| Lercanidipine | 5.70 | 6.13 | 0.63 | 11254 | 10235 | 12 | 25 |
| Lacidipine | 29.12 | 29.83 | 0.32 | 10242 | 10112 | 12 | 20 |
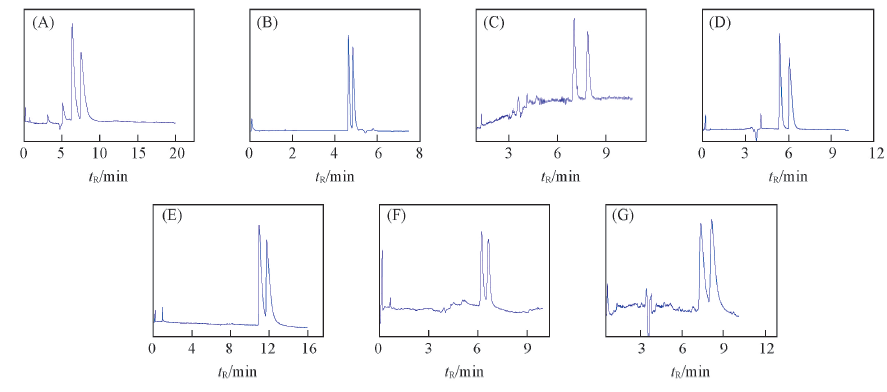
Fig.7 Electrochromatograms of some dihydropyridine enantiomers on SECDP (A) Amlodipine besytle; (B) amlodipine mesylate; (C) nimodipine; (D) nicardipine; (E) manidipine; (F) benidipine; (G) cilnidipine.
| [1] | Ribeiro A. R., Afonso C. M., Castro P. M. L., Tiritan M. E., Ecotoxicol. Environ. Saf., 2013, 87, 108—114 |
| [2] | Barnes B. J., Howard P. A., Lai S. M., Grauer D. W., Muehlebach G. F., Hosp. Pharm., 2012, 47(8), 617—625 |
| [3] | Xiao M., Xu B. Y., Chen Y. B., Li S. Y., Contemp. Med., 2013, 19(15), 132—133 |
| (肖敏, 徐八一, 陈益彪, 李淑云. 当代医学, 2013, 19(15), 132—133) | |
| [4] | Lu J., China Pharm., 2013, 22(4),86—87(卢军. 中国药业, 2013, 22(4), 86—87) |
| [5] | Yoon Y. J., Kim K. B., Kim H., Seo K. A., Kim H. S., Cha I. J., Kim E. Y., Liu K. H. Shin J. G., Drug Metab. Dispos., 2007, 35(9), 1518—1524 |
| [6] | Inotsume N., Nakano M., J Biochem. Biophys. Methods, 2002, 54, 255—274 |
| [7] | Uno T., Ohkubo T., Sugawara K., J. Chromatogr. B, 1997, 698(1/2), 181—186 |
| [8] | Fu Q., He L. C., Chin. J. Anal. Chem., 2005, 33(12), 1779—1782 |
| (傅强, 贺浪冲. 分析化学, 2005, 33(12), 1779—1782) | |
| [9] | Xu L. F., Lu Y., Li Y., Xu X., Chin. J. Chromatogr., 2010, 28(4), 426—429 |
| (徐丽芳, 鲁耀, 李奕, 许旭. 色谱, 2010, 28(4), 426—429) | |
| [10] | Tang X. D., Wang S. Y., Cong J. X., Wang Z. C., Chin. J. Spectr. Lab., 2011, 28(5), 2573—2577 |
| (唐晓丹, 王绍艳, 丛景香, 王智超. 光谱实验室, 2011, 28(5), 2573—2577) | |
| [11] | Li C. P., Zeng H. C., Lu L., Gao W. L., Shan W. G., Chin. J. Pharm. Anal., 2010, 30(4), 661—663 |
| (李成平, 曾怀超, 鲁琳, 高伟梁, 单伟光. 药物分析杂志, 2010, 30(4), 661—663) | |
| [12] | Lammerhofer M., Gyllenhaal O., Lindner W., J. Pharm. Biomed. Anal., 2004, 35(2), 259—266 |
| [13] | Chen J., Yao X. N., Lin L. N., Guo X. J., J. Shenyang Pharm. Univ., 2010, 27(2), 132—134 |
| (陈静, 姚鑫宁, 林丽娜, 郭兴杰. 沈阳药科大学学报, 2010, 27(2), 132—134) | |
| [14] | Cucinotta V., Contino A., Giuffrida A., Maccarrone G., Messina M., J. Chromatogr. A, 2010, 1217(7), 953—967 |
| [15] | Juvancz Z., Kendrovics R. B., Lvanyi R., Szente L., Electrophoresis, 2008, 29(8), 1701—1712 |
| [16] | Wang R., Jia Z. P., Fan J. J., Chen L. R., Xie H., Ma J., Ge X., Zhang Q., Ao Y., Wang J., Chromatographia, 2007, 65(9/10), 575—579 |
| [17] | Li B. H., Yang G. L., Wang D. X., Zhang Z. F., Chen Y., Chin. J. Chromatogr., 2002, 20(4), 338—340 |
| (李保会, 杨更亮, 王德先, 张哲峰, 陈义. 色谱, 2002, 20(4), 338—340) | |
| [18] | Hu Y. L., Zhao M., Guo W. W., Zhang L. J., Xu S. Y., Guo X. J., J. Shenyang Pharm. Univ., 2013, 30(2), 114—119 |
| (胡艳丽, 赵旻, 郭雯雯, 张丽娟, 徐淑英, 郭兴杰. 沈阳药科大学学报, 2013, 30(2), 114—119) | |
| [19] | Fakhari A. R., Nojavan S., Haghgoo S., Mohammadi A., Electrophoresis, 2008, 29(22), 4583—4592 |
| [20] | Xu S. J., Wu M. J., Chin. J. Anal. Chem., 2004, 32(1), 46—49 |
| (许菽娟, 吴明嘉. 分析化学, 2004, 32(1), 46—49) | |
| [21] | Cai C., Wang Y. C., Deng F., J. Hangzhou Normal Univ., 2012, 11(2), 122—125 |
| (蔡诚, 王园朝, 邓飞. 杭州师范大学学报, 2012, 11(2), 122—125) | |
| [22] | Li Y., Zhu P. Y., Song F. Y., Chin. J. Pharm.Anal., 2011, 31(2), 306—310 |
| (李艺, 朱培仪, 宋粉云. 药物分析杂志, 2011, 31(2), 306—310) | |
| [23] | Van E. A., Detaevernier M. R., Michotte Y., J. Pharm. Biomed. Anal., 2004, 36(4), 799—805 |
| [24] | Zhang W.B., The Theoretical Basis for Capillary Electrochromatography, Science Press, Beijing, 2006, 23—35 |
| (张维冰. 毛细管电色谱理论基础, 北京: 科学出版社, 2006, 23—35) | |
| [25] | Wren A. C., Rowe C. R., J. Chromatogr. A, 1992, 603(1/2), 235—241 |
| [26] | Wang S. Y., Dai Y., Wu J. H., Zhou J., Tang J., Tang W. H., J. Chromatogr. A, 2013, 1227, 84—92 |
| [27] | Li Y. J., Song C. H., Zhang L. Y., Zhang W. B., Fu H. G., Talanta, 2010, 80(3), 1378—1384 |
| [28] | Xia D. H., Ren X. D., Jiao L., Li H., Chem. Res. Chinese Universities, 2012, 28(2), 282—286 |
| [29] | Yuan R., Wang Y., Ding G., Anal. Sci., 2010, 26(9), 943—947 |
| (Ed.: N, K) |
| [1] | PENG Yuyu,WANG Yu,YU Xinyao,ZENG Julan,XIAO Zhongliang,CAO Zhong. Rapid and Sensitive Detection of L-Cysteine Based on Mono(6-mercapto-6-deoxy)-β-cyclodextrin Modified Gold Electrode † [J]. Chem. J. Chinese Universities, 2020, 41(2): 268. |
| [2] | TIAN Yao,ZHANG Chunquan,WANG Wenzhe,ZHOU Yingfang,LU Yitong,ZHANG Peng,JIA Zhenfu,ZHOU Chengyu,CHEN Shilan. Preparation of Polyrotaxane Cross-linking Agent with “Pulley” Effect and Its Potential Application in Swelling Grain Used as Profile Control and Water Plugging Agent† [J]. Chem. J. Chinese Universities, 2018, 39(9): 2098. |
| [3] | FENG Wenya, QIAO Juan, QI Li, LI Zhiwei. Sepatation of Antipyretic Analgesics by CapillaryElectrochromatography with Block Copolymer Coating† [J]. Chem. J. Chinese Universities, 2018, 39(8): 1640. |
| [4] | ZHOU Min, XU Xiaoying, LONG Yuande. Enantioseparation of Seventeen Kinds of β-Lactams on Carboxymethyl-β-cyclodextrin Chiral Stationary Phase and Research on Enantioseparation Mechanism [J]. Chem. J. Chinese Universities, 2018, 39(6): 1164. |
| [5] | YANG Qinghua, WANG Longgang, LIU Jie, LU Yong, CHEN Tianyun. Preparation and Characterization of Star-shaped β-Cyclodextrin Based Polymer† [J]. Chem. J. Chinese Universities, 2018, 39(4): 793. |
| [6] | LIN Musong, PENG Lei, FU Qiang, QIAN Yihua, CHEN Tiansheng, ZHANG Sheng, MA Xiaoqian. Research on Self-healing Insulating Material Based on Host-guest Cooperation† [J]. Chem. J. Chinese Universities, 2018, 39(11): 2572. |
| [7] | CHEN Tao,HUANG Chan. β-Cyclodextrin Based Star-like Polymer for Loading Chlorambucil† [J]. Chem. J. Chinese Universities, 2018, 39(10): 2350. |
| [8] | JIAN Yuhang, YAN Shifeng, LI Xing, HUANG Yanan, YIN Jingbo. Synthesis and Characterization of Injectable Hydrogels Based on Star β-CD-g-poly(L-glutamic acid) [J]. Chem. J. Chinese Universities, 2017, 38(8): 1489. |
| [9] | ZHOU Xinhui, WANG Haishui. Co-effect of L-Cysteine Self-assembled Monolayers and Mixed Solvents on Chiral Separation of DL-glutamic Acid† [J]. Chem. J. Chinese Universities, 2017, 38(6): 1076. |
| [10] | HUANG Yandong, WU Ruofei, CHU Yanqiu, DING Chuanfan. Effect of Side Chain of α-Amino Acids and Esters on the Stability Constants for β-Cyclodextrin Complexes† [J]. Chem. J. Chinese Universities, 2017, 38(5): 743. |
| [11] | HE Meiying, LUO Jianhui, YANG Bowen, XIA Bibo, LI Yuanyang, ZHANG Shuming, JIANG Bo. Sol-gel Preparation of Hydrophobic Silica Coating-materials with Ultralow Refractive Index† [J]. Chem. J. Chinese Universities, 2017, 38(11): 2077. |
| [12] | ZHANG Jing, CHEN Linfeng, ZHU Yaxian, ZHANG Yong. Effects of Hydroxypropyl-β-cyclodextrin(HPCD) on the Interaction of 1-Hydroxypyrene with Bovine Serum Albumin† [J]. Chem. J. Chinese Universities, 2017, 38(1): 28. |
| [13] | WANG Huichun, WANG Fachun, LI Baolin. Preparation of Cyclodextrin-based Mesoporous Carbon and Its Catalytic Performance† [J]. Chem. J. Chinese Universities, 2016, 37(11): 2076. |
| [14] | CHENG Biaoping, LI Laisheng, ZHOU Rendan, LI Liang, ZHANG Hongfu. Enantioseparations of Triazole Chiral Pesticides on Two β-Cyclodextrin-bonded Stationary Phases with Different Linkages by HPLC† [J]. Chem. J. Chinese Universities, 2015, 36(5): 872. |
| [15] | ZHANG Huanhuan, CHEN Jiyun, ZHOU Sunying. Preparation of Open Tubular Capillary Electrochromatography Column with Nano-chitosan Coating and Its Application for Basic Proteins Analyzing† [J]. Chem. J. Chinese Universities, 2015, 36(4): 631. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||