

Chem. J. Chinese Universities ›› 2017, Vol. 38 ›› Issue (5): 743.doi: 10.7503/cjcu20160956
• Analytical Chemistry • Previous Articles Next Articles
HUANG Yandong, WU Ruofei, CHU Yanqiu*( ), DING Chuanfan*(
), DING Chuanfan*( )
)
Received:2016-12-29
Online:2017-05-10
Published:2017-04-14
Contact:
CHU Yanqiu,DING Chuanfan
E-mail:chuyq@fudan.edu.cn;cfding@fudan.edu.cn
CLC Number:
TrendMD:
HUANG Yandong, WU Ruofei, CHU Yanqiu, DING Chuanfan. Effect of Side Chain of α-Amino Acids and Esters on the Stability Constants for β-Cyclodextrin Complexes†[J]. Chem. J. Chinese Universities, 2017, 38(5): 743.
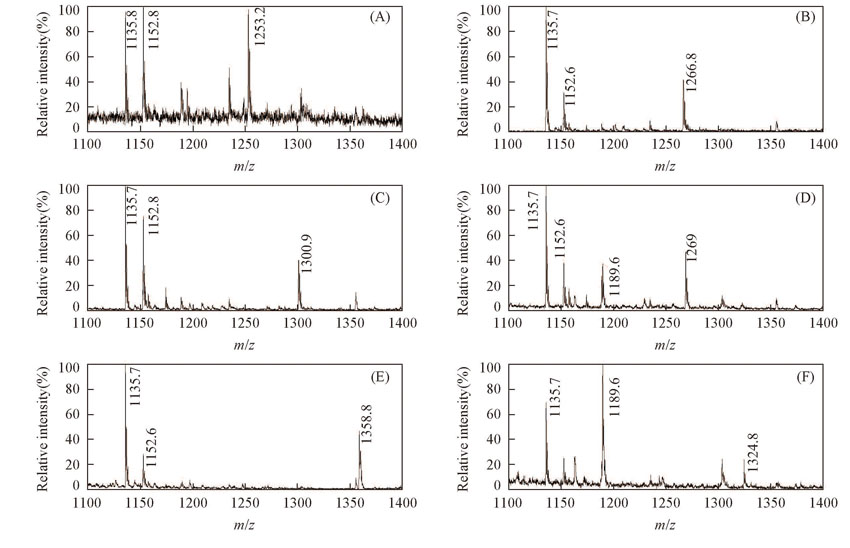
Fig.2 Mass spectra for β-CD with amino acid and esters(A) n-Val; (B) Leu; (C) Phe; (D) Asp; (E) Asp-4-benzyl ester; (F) Asp-4-t-butyl ester.[β-CD]=[Amino acids or esters]=2.0×10-4 mol/L.
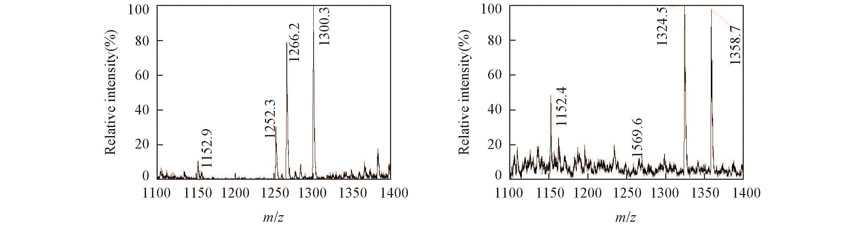
Fig.4 Competitive reaction mass spectra for β-CD with amino acid and esters in groups(A) β-CD with n-Val, Leu, Phe;(B) β-CD with Asp, Asp-4-benzyl ester, Asp-4-t-butyl ester.[β-CD]=[Amino acids or esters]=2.0×10-4 mol/L.
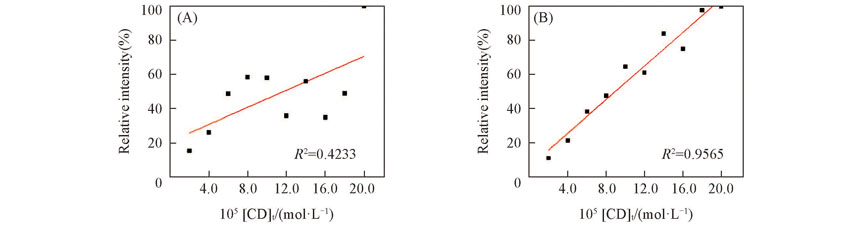
Fig.6 Linear fitting results of concentration and relative intensity of β-CD(A) Without internal standard; (B) with internal standard K5. [K5]=2.0×10-4 mol/L.
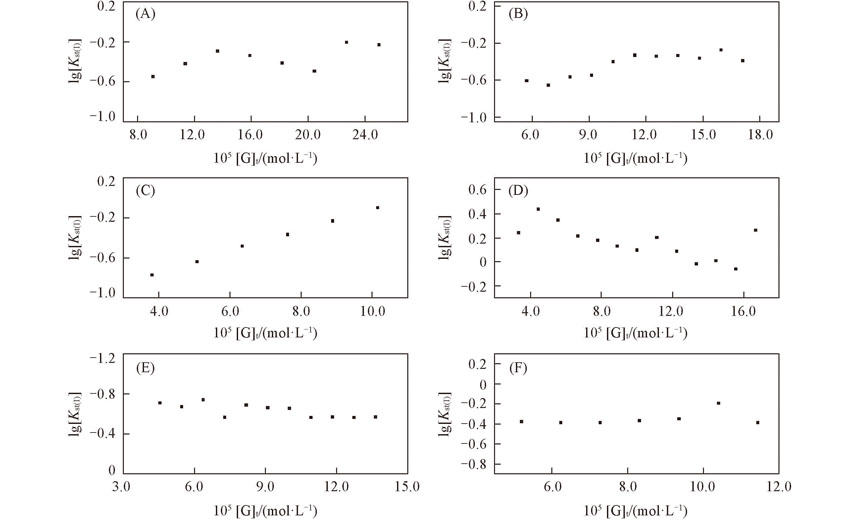
Fig.7 lg[Kst(I)] with variation in concentration of guest compounds(A) n-Val; (B)Leu; (C)Phe; (D)Asp; (E)Asp-4-benzyl ester; (F)Asp-4-t-butyl ester.[β-CD]=1.0×10-4 mol/L, [Internal standard K5]=2.0×10-4 mol/L.
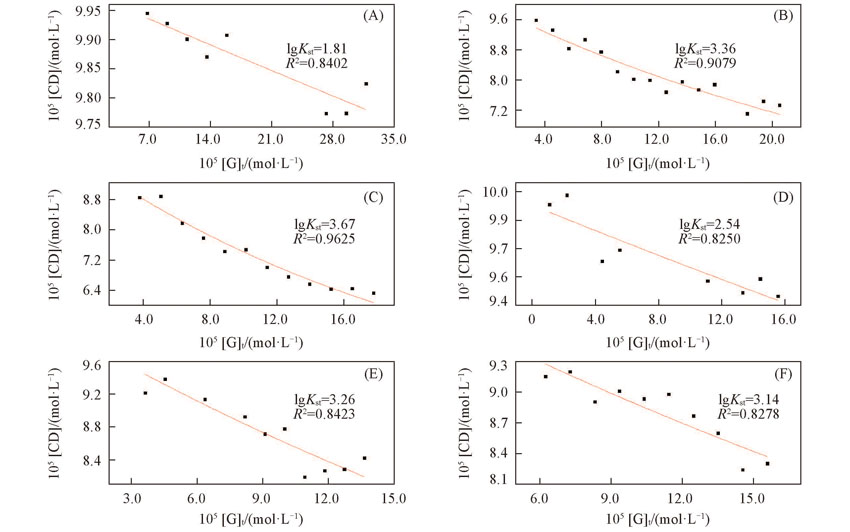
Fig.8 Nonlinear fitting curves of [CD] and [G]t(A) n-Val; (B) Leu; (C) Phe; (D) Asp; (E) Asp-4-benzyl ester; (F) Asp-4-t-butyl ester.[β-CD]= 1.0×10-4 mol/L, [Internal standard K5]=2.0×10-4 mol/L.
| [1] | Szejtli J., Cyclodextrin Technology, Springer, Berlin, 1988, 1—185 |
| [2] | Chen Y., Liu Y., Chin. J. Org. Chem., 2012, 32(5), 805—814 |
| (陈湧, 刘育.有机化学, 2012,32(5), 805—814) | |
| [3] | Yu Z., Cui M., Yan C. Y., Song F. R., Liu Z. Q., Liu S. Y., Zhang H. X., J. Mass Spectrom., 2010, 45(4), 444—450 |
| [4] | Zhao Y. Y., Wang L. H., Guo Z. M., Chi X. F., Ma X. C., Qi Y., Fang S. N., Li X. L., Liang X. M., Chem. Res. Chinese Universities, 2015, 31(1), 44—52 |
| [5] | He X. D., Xu C. S., Chu Y. Q., Ding C. F., Acta Chim. Sinica, 2013, 71, 397—404 |
| (何小丹, 许崇晟, 储艳秋, 丁传凡. 化学学报, 2013, 71, 397—404) | |
| [6] | Pandey S., Kumar B., Swamy S. M. V., Gupta A., Int. J. Pharm. Technol., 2010, 2, 281—319 |
| [7] | Chen B., Chen J., Yang L. M., Zhao G. C., Ding G. W., Chem. Res. Chinese Universities, 2016, 32(2), 278—283 |
| [8] | Lu H. J., Yu C. T., Guo Y. L., Acta Chim. Sinica, 2002, 60(5), 882—885 |
| (陆豪杰, 余翀天, 郭寅龙.化学学报, 2002,60(5), 882—885) | |
| [9] | Caso J. V., Russo L., Palmieri M., Malgieri G., Galdiero S., Falanga A., Isernia C., Iacovino R., Amino Acids, 2015, 47(10), 2215—2227 |
| [10] | Kucerova G., Prochazkova H., Kalikova K., Tesarova E., J. Chromatog. A, 2016, 1467, 356—362 |
| [11] | Borisov Y. A., Kieslev S. S., Russian J. Phys. Chemistry A, 2016, 90(9), 1822—1827 |
| [12] | Cho E., Kim H., Choi Y., Paik S. R., Jung S., Scientific Reports, 2016, 6, 31115—31125 |
| [13] | Roy M. N., Roy A., Saha S., Carbohydrate Polymers, 2016, 151, 458—466 |
| [14] | Caso J. V., Russo L., Palmieri M., Malgieri G., Galdiero S., Falanga A., Isernia C., Iacovino R., Amino Acids, 2015, 47, 2215—2227 |
| [15] | Tang K. Q., Page J. S., Smith R. D., J. Am. Soc. Mass Spectrom., 2004, 15(10), 1416—1423 |
| [16] | Wortmann A., Rossi F., Lelais G., Zenobi R., J. Mass Spectrom., 2005, 40(6), 777—784 |
| [17] | Wang W., Kitova E. N., Klassen J. S., J. Am. Chem. Soc., 2003, 125(45), 13630—13631 |
| [18] | Nyadong L., Green M. D., De Jesus V. R., Newton P. N., Fernandez F. M., Anal. Chem., 2007, 79(5), 2150—2157 |
| [19] | Schmidt A. C., Neustadt M., Otto M., J. Mass Spectrom., 2007, 42(6), 771—780 |
| [20] | Frycak P., Schug K. A., Anal. Chem., 2008, 80(5), 1385—1393 |
| [21] | Young B. L., Cooks R. G., Int. J. Mass Spectrom., 2007, 267(1—3), 199—204 |
| [22] | Zhang H. R., Chen G., Wang L., Ding L., Tian Y., Jin W., Zhang H., Int. J. Mass Spectrom., 2006, 252(1), 1—10 |
| [23] | Konig S., Hasche A., Pallast S., Krieglstein J., Klumpp S., J. Am. Soc. Mass Spectrom., 2008, 19(1), 91—95 |
| [24] | Bligh S. W., Haley T., Lowe P. N., J. Mol. Recognit., 2003, 16(3), 139—148 |
| [25] | Barylyuk K., Balabin R. M., Grunstein D., Kikkeri R., Frankevich V., Seeberger P. H., Zenobi R., J. Am. Soc. Mass Spectrom., 2011, 22, 1167—1171 |
| [26] | Ramirez J., Ahn S., Grigorean G., Lebrilla C. B., J. Am. Chem. Soc., 2000, 122(29), 6884—6890 |
| [27] | Shen W., Shao X. G., Cai W. S., Chem. J. Chinese Universities, 2016, 37(10), 1809—1816 |
| (沈文, 邵学广, 蔡文生.高等学校化学学报, 2016,37(10), 1809—1816) | |
| [28] | Han B. H., Liu Y., Chen R. T., Chinese J. Anal. Chem., 2000, 11(28), 1355—1358 |
| (韩宝航, 刘育, 陈荣悌.分析化学, 2000,11(28), 1355—1358) | |
| [29] | Pan T. T., Chu Y. Q., Zhou M. F., Ding C. F., Lü N., Weng Z. H., Li J. Q., Acta Chim. Sinica, 2008, 66(22), 2462—2468 |
| (潘婷婷, 储艳秋, 周鸣飞, 丁传凡, 吕娜, 翁志洁, 李建其.化学学报, 2008,66(22), 2462—2468) | |
| [30] | Chu Y. Q., Dai X. H., Jiang D., Jiang G. Y., Fang X., Ding C. F., Rapid Commun. Mass Spectrom., 2010, 24(15), 2255—2261 |
| [31] | He X. D., Jiang D., Chen C., Chu Y. Q., Ding C. F., Weng Z. F., Li J. Q., Acta Phys. Chim. Sin., 2010, 26(10), 2604—2612 |
| (何小丹, 姜丹, 陈琛, 储艳秋, 丁传凡, 翁志洁, 李建基.物理化学学报, 2010,26(10), 2604—2612) | |
| [32] | Wei W. H., Chu Y. Q., Wang R., He X. D., Ding C. F., Rapid Commun. Mass Spectrom., 2015, 29(10), 927—936 |
| [33] | Claudia K., Ulrike H., Chirality, 2004, 16, 509—515 |
| [34] | Wei W. H., Chu Y. Q., Ding C. F., Anal. Lett., 2014, 47(13), 2221—2237 |
| [1] | DENG Jiewei, YANG Yunyun, LIN Li, LUAN Tiangang. Rapid Classification of Daphniamagna and Daphnia pulex by Surface-coated Probe Nanoelectrospray Ionization Mass Spectrometry Lipidomics [J]. Chem. J. Chinese Universities, 2020, 41(9): 2011. |
| [2] | LI Xiaoqian, ZHANG Hua, LU Haijian, LIU Chang, LIU Qinglong, MA Xiayu, FANG Yuanping, LIANG Dapeng. Mechanism of Photocatalytic Degradation of Rhodamine B by TiO2 Nanowire Array with Internal Extraction Electrospray Ionization Mass Spectrometry [J]. Chem. J. Chinese Universities, 2020, 41(9): 2003. |
| [3] | WANG Xiaoqun, HUANG Guangming. Investigation of Lipid Distributions by Trifluoroacetic Acid-enhanced Desorption Electrospray Ionization Mass Spectrometry Imaging [J]. Chem. J. Chinese Universities, 2020, 41(12): 2673. |
| [4] | LI Cheng,WANG Chengjian,JIN Wanjun,HAN Jianli,YANG Meifang,GAO Xi,HUANG Linjuan,WANG Zhongfu. Mass Spectrometric Analysis of N-Glycans of Glycoprotein Separated by SDS-PAGE Gel from Ginkgo Seed† [J]. Chem. J. Chinese Universities, 2019, 40(1): 69. |
| [5] | WU Fangling,CHU Yanqiu,CHEN Xin,WEI Wanghui,DING Chuanfan. Critical Factors Affecting Noncovalent Interaction Between Pentapeptides Explored by Electrospray Ionization Mass Spectrometry† [J]. Chem. J. Chinese Universities, 2018, 39(9): 1927. |
| [6] | KE Mufang, HAN Jing, ZHU Tenggao, LIU Wenjie, ZHANG Hua, KOU Wei, LIANG Dapeng. Direct and Rapid Analysis of Avocado Using Internal Extractive Electrospray Ionization Mass Spectrometry† [J]. Chem. J. Chinese Universities, 2017, 38(5): 738. |
| [7] | PENG Yifang, WANG Chengjian, WANG Jingjing, LI Lingmei, JIN Wanjun, QIANG Shan, SHI Hongdan, ZHANG Ying, HUANG Linjuan, WANG Zhongfu. Analysis of Antigenic Determinant Glycans of Peanut Allergy Glycoprotein Ara h1 by Mass Spectrometry† [J]. Chem. J. Chinese Universities, 2016, 37(9): 1622. |
| [8] | LI Penghui, DENG Lingli, LUO Jiao, LI Wei, NING Jing, DING Jianhua, WU Xiaoping. EESI-MS Detection and Statistical Analysis of Multi-batch of Exhaled Breath Metabolomics Data of Liver Failure Patients [J]. Chem. J. Chinese Universities, 2016, 37(4): 626. |
| [9] | WU Ruofei, CHU Yanqiu, XU Chongsheng, LIU Zhipan, DING Chuanfan. Fragmentation Reaction of Complexes of Alkali Metal Cations with Tripeptides in Gas Phase† [J]. Chem. J. Chinese Universities, 2016, 37(12): 2150. |
| [10] | CHEN Huanhuan, ZHAO Xia, LUAN Xiaohong, YU Guangli. Application of Electrospray Tandem Mass Spectrometry in Sequence Analysis of Oligosaccharides† [J]. Chem. J. Chinese Universities, 2015, 36(1): 1. |
| [11] | DAI Yulin, YU Shanshan, ZHANG Ying, HAO Ying, ZHONG Wei, YUE Hao, LIU Shuying. Studies on the Isoflavone in Extract of the Flower of Pueraria Lobata by RRLC-Q-TOF MS/MS† [J]. Chem. J. Chinese Universities, 2014, 35(7): 1396. |
| [12] | FANG Feifei, DU Shangguang, DAI Ximo, GUO Xiali, CHEN Huanwen, LUO Liping. Rapid Analysis Alkaloids in Lotus Seeds by Extractive Electrospray Ionization Mass Spectrometry† [J]. Chem. J. Chinese Universities, 2014, 35(4): 730. |
| [13] | PAN Liying, WANG Chengjian, YUAN Jiangbei, ZHANG Ying, HUANG Linjuan, WANG Zhongfu. Qualitation and Quantitation for Comparative Analysis of N-glycans from Human Hepatocellular Carcinoma HepG2 and Normal Liver Cells L02 by Electrospray Ionization Mass Spectrometry† [J]. Chem. J. Chinese Universities, 2014, 35(2): 237. |
| [14] | HUANG Jia-Hua, GONG Zhen-Bin, LIN Ji-Jun, DUAN Hua-Ling. Measurement of Complexation Stability Constant and Coordinating Number of Poly(ethylenimine) with Metals [J]. Chem. J. Chinese Universities, 2012, 33(12): 2633. |
| [15] | XU Niu-Sheng, YANG Hong-Mei, CUI Meng, SONG Feng-Rui, LIU Zhi-Qiang, LIU Shu-Ying. Investigation of Interaction of Fangchinoline with G-quadruplex DNA by Electrospray Ionization Mass Spectrometry [J]. Chem. J. Chinese Universities, 2012, 33(11): 2430. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||