

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (1): 12.doi: 10.7503/cjcu20130671
• Articles: Inorganic Chemistry • Previous Articles Next Articles
Received:2013-07-17
Online:2014-01-10
Published:2013-12-13
Contact:
KOU Huizhong
E-mail:kouhz@mail.tsinghua.edu.cn
Supported by:CLC Number:
TrendMD:
SHI Wenbo, KOU Huizhong. Synthesis, Crystal Structure and Magnetic Properties of Tetranuclear MnⅡ2MnⅢ2 Complexes Based on 2-(Hydroxymethyl)-N-methylimidazole†[J]. Chem. J. Chinese Universities, 2014, 35(1): 12.
| Compound | 1 | 2 | 3·6CH3CN |
|---|---|---|---|
| Empirical formula | C36H56 | C30 | |
| Formula weight | 1315.67 | 1205.51 | 1274.64 |
| Crystal system | Monoclinic | Monoclinic | Triclinic |
| Space group | C2/c | P21/c | P-1 |
| a/nm | 2.8773(4) | 1.2276(2) | 1.0440(3) |
| b/nm | 1.8427(2) | 1.0738(2) | 1.1281(4) |
| c/nm | 1.0991(2) | 1.8502(2) | 1.3646(3) |
| α/(°) | 90 | 90 | 80.57(1) |
| β/(°) | 109.77(1) | 105.66(1) | 69.69(1) |
| γ/(°) | 90 | 90 | 79.59(1) |
| Volume/nm3 | 5.484(1) | 2.3482(5) | 0.7101(2) |
| Z | 4 | 4 | 1 |
| ρcalcd/(g·cm-3) | 1.594 | 1.705 | 1.437 |
| Absorption coefficient/mm-1 | 1.079 | 1.251 | 1.076 |
| Obsd. data[I>2σ(I)] | 1894 | 1601 | 6118 |
| GOF | 0.960 | 0.964 | 1.055 |
| R1[I>2σ(I)] | 0.0916 | 0.0862 | 0.0477 |
| wR2(All data) | 0.1663 | 0.1627 | 0.1356 |
Table 1 Crystallographic data for complexes 1—3
| Compound | 1 | 2 | 3·6CH3CN |
|---|---|---|---|
| Empirical formula | C36H56 | C30 | |
| Formula weight | 1315.67 | 1205.51 | 1274.64 |
| Crystal system | Monoclinic | Monoclinic | Triclinic |
| Space group | C2/c | P21/c | P-1 |
| a/nm | 2.8773(4) | 1.2276(2) | 1.0440(3) |
| b/nm | 1.8427(2) | 1.0738(2) | 1.1281(4) |
| c/nm | 1.0991(2) | 1.8502(2) | 1.3646(3) |
| α/(°) | 90 | 90 | 80.57(1) |
| β/(°) | 109.77(1) | 105.66(1) | 69.69(1) |
| γ/(°) | 90 | 90 | 79.59(1) |
| Volume/nm3 | 5.484(1) | 2.3482(5) | 0.7101(2) |
| Z | 4 | 4 | 1 |
| ρcalcd/(g·cm-3) | 1.594 | 1.705 | 1.437 |
| Absorption coefficient/mm-1 | 1.079 | 1.251 | 1.076 |
| Obsd. data[I>2σ(I)] | 1894 | 1601 | 6118 |
| GOF | 0.960 | 0.964 | 1.055 |
| R1[I>2σ(I)] | 0.0916 | 0.0862 | 0.0477 |
| wR2(All data) | 0.1663 | 0.1627 | 0.1356 |
| Mn1—O1A | 0.1903(6) | Mn1—O2 | 0.1971(6) |
|---|---|---|---|
| Mn1—O3 | 0.1883(6) | Mn1—O4 | 0.2179(7) |
| Mn1—O2A | 0.2230(6) | Mn1—N8 | 0.1986(7) |
| Mn2—O1 | 0.2182(6) | Mn2—O2 | 0.2406(6) |
| Mn2—O3 | 0.2164(6) | Mn2—N1 | 0.2199(9) |
| Mn2—N3 | 0.2131(9) | Mn2—N5 | 0.2168(8) |
| Mn1—O2—Mn2 | 98.9(2) | Mn1—O3—Mn2 | 110.9(3) |
| Mn1A—O1—Mn2 | 110.1(3) | Mn1A—O2—Mn2 | 92.5(2) |
| Mn1—O2—Mn1A | 103.4(3) |
Table 2 Selected bond distances(nm) and bond angles(°) for complexe 1
| Mn1—O1A | 0.1903(6) | Mn1—O2 | 0.1971(6) |
|---|---|---|---|
| Mn1—O3 | 0.1883(6) | Mn1—O4 | 0.2179(7) |
| Mn1—O2A | 0.2230(6) | Mn1—N8 | 0.1986(7) |
| Mn2—O1 | 0.2182(6) | Mn2—O2 | 0.2406(6) |
| Mn2—O3 | 0.2164(6) | Mn2—N1 | 0.2199(9) |
| Mn2—N3 | 0.2131(9) | Mn2—N5 | 0.2168(8) |
| Mn1—O2—Mn2 | 98.9(2) | Mn1—O3—Mn2 | 110.9(3) |
| Mn1A—O1—Mn2 | 110.1(3) | Mn1A—O2—Mn2 | 92.5(2) |
| Mn1—O2—Mn1A | 103.4(3) |
| Mn1—O1 | 0.1907(5) | Mn1—O2 | 0.1939(5) |
|---|---|---|---|
| Mn1—O2A | 0.2326(5) | Mn1—O3A | 0.1874(5) |
| Mn1—N1 | 0.1981(9) | Mn1—N7 | 0.2174(8) |
| Mn2—O1 | 0.2131(6) | Mn2—O2 | 0.2344(5) |
| Mn2—O3 | 0.2215(6) | Mn2—O1W | 0.2260(6) |
| Mn2—N3 | 0.2168(9) | Mn2—N5 | 0.2160(8) |
| Mn1—O1—Mn2 | 108.1(3) | Mn1—O2—Mn2 | 99.2(2) |
| Mn1A—O2—Mn2 | 94.5(2) | Mn1A—O3—Mn2 | 113.7(3) |
| Mn1—O2—Mn1A | 101.3(2) |
Table 3 Selected bond distances(nm) and bond angles(°) for complexe 2
| Mn1—O1 | 0.1907(5) | Mn1—O2 | 0.1939(5) |
|---|---|---|---|
| Mn1—O2A | 0.2326(5) | Mn1—O3A | 0.1874(5) |
| Mn1—N1 | 0.1981(9) | Mn1—N7 | 0.2174(8) |
| Mn2—O1 | 0.2131(6) | Mn2—O2 | 0.2344(5) |
| Mn2—O3 | 0.2215(6) | Mn2—O1W | 0.2260(6) |
| Mn2—N3 | 0.2168(9) | Mn2—N5 | 0.2160(8) |
| Mn1—O1—Mn2 | 108.1(3) | Mn1—O2—Mn2 | 99.2(2) |
| Mn1A—O2—Mn2 | 94.5(2) | Mn1A—O3—Mn2 | 113.7(3) |
| Mn1—O2—Mn1A | 101.3(2) |
| Mn1—O1 | 0.1871(2) | Mn1—O2 | 0.1906(2) |
|---|---|---|---|
| Mn1—O3 | 0.2353(2) | Mn1—O3A | 0.1930(2) |
| Mn1—N3 | 0.2021(2) | Mn1—Cl1 | 0.2509(1) |
| Mn2—O1 | 0.2296(2) | Mn2—O2A | 0.2139(2) |
| Mn2—O3 | 0.2380(2) | Mn2—N1 | 0.2160(2) |
| Mn2—N5 | 0.2177(2) | Mn2—Cl2 | 0.2466(1) |
| Mn1—O1—Mn2 | 113.23(9) | Mn1—O3—Mn2 | 94.95(6) |
| Mn1—O2—Mn2A | 108.66(8) | Mn1A—O3—Mn2 | 98.99(8) |
| Mn1—O3—Mn1A | 101.56(7) |
Table 4 Selected bond distances(nm) and bond angles(°) for complexe 3·6CH3CN
| Mn1—O1 | 0.1871(2) | Mn1—O2 | 0.1906(2) |
|---|---|---|---|
| Mn1—O3 | 0.2353(2) | Mn1—O3A | 0.1930(2) |
| Mn1—N3 | 0.2021(2) | Mn1—Cl1 | 0.2509(1) |
| Mn2—O1 | 0.2296(2) | Mn2—O2A | 0.2139(2) |
| Mn2—O3 | 0.2380(2) | Mn2—N1 | 0.2160(2) |
| Mn2—N5 | 0.2177(2) | Mn2—Cl2 | 0.2466(1) |
| Mn1—O1—Mn2 | 113.23(9) | Mn1—O3—Mn2 | 94.95(6) |
| Mn1—O2—Mn2A | 108.66(8) | Mn1A—O3—Mn2 | 98.99(8) |
| Mn1—O3—Mn1A | 101.56(7) |
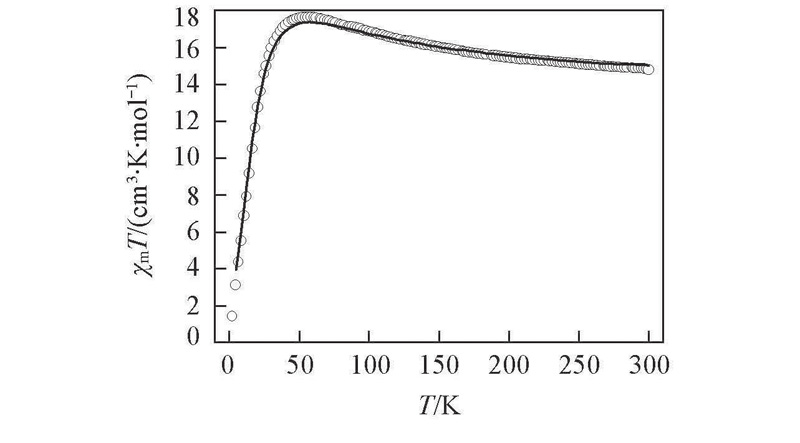
Fig.6 Temperature dependence of χmT for complex 3·6H2OThe solid line represents the best fit results(5—300 K) using the parameters discussed in the text.
| Compound | (Jbb/kB)/ K | MnⅢ—O—MnⅢ/ (°) | (Jwb/kB)/ K | MnⅢ—O—MnⅡ/ (°) | (°) | Ref. |
|---|---|---|---|---|---|---|
| [Mn4(hmmi)6Cl4](3) | 14.4 | 101.56 | -1.35 | 94.95, 98.99, 108.66, 113.23 | 104.0 | This work |
| [Mn4(hmp)6(Hhmp)2](ClO4)4 | 0.25 | 102.90 | -0.92 | 94.35, 97.24, 105.36, 110.59 | 101.9 | [ |
| [Mn4(hmp)4(acac)2(MeO)2](ClO4)2 | 2.7 | 99.38 | 0.39 | 96.84, 107.19 | 102.0 | [ |
| [Mn4(hmp)6(NO3)2(MeCN)2](ClO4)2 | 5.0 | 99.55 | 0.5 | 95.33, 99.77, 107.76, 109.34 | 103.1 | [ |
| [Mn4(hmp)6(H2O)4](ClO4)4 | 5.0 | 98.9 | 0.55 | 93.8, 100.0, 106.6, 111.8 | 103.1 | [ |
| [Mn4(hmmi)6(DMF)2(N3)2](ClO4)2(1) | 4.5 | 103.4 | 0.63 | 92.5, 98.9, 110.1, 110.9 | 103.1 | This work |
| [Mn4(hmp)6(CH3CN)2(H2O)4](ClO4)4 | 8.56 | 100.49 | 0.66 | 94.41, 99.84, 107.64, 113.28 | 103.8 | [ |
| [Mn4(hmp)6(H2O)2(NO3)2](NO3)2 | 7.1 | 98.81 | 0.80 | 95.00, 100.43, 107.53, 111.68 | 103.7 | [ |
| {[Mn4(hmp)6(NO3)2][FeNO(CN)5]}n | 9.78 | 99.01 | 0.91 | 94.09, 99.83, 107.73, 110.36 | 103.0 | [ |
| [Mn4(hmp)6(dca)2](ClO4)2 | 9.12 | 98.19, 98.61 | 1.02 | 95.42, 99.73, 106.25, 109.12 | 102.6 | [ |
| [Mn4(hmp)4(Hpdm)2(dca)2](ClO4)2 | 12.66 | 100.60 | 1.15 | 94.67, 100.78, 109.06, 111.05 | 102.4 | [ |
| [Mn4(hmp)6(H2O)2(NO3)2](ClO4)2 | 13.3 | 99.44 | 1.24 | 95.31, 100.25, 107.63, 110.77 | 103.5 | [ |
| [Mn4(hmp)6(dae-c)2(H2O)2](ClO4)2 | 12.26 | 100.51 | 1.25 | 94.07, 99.91, 107.75, 109.19 | 102.7 | [ |
| [Mn4(hmp)6(dae-o)2(ClO4)2] | 9.18 | 99.44 | 1.26 | 96.70, 109.70 | 103.2 | [ |
| [Mn4(hmp)4Br2(OMe)2(dca)2] | 10.89 | 97.71 | 1.30 | 97.23, 98.40, 109.10, 109.69 | 103.6 | [ |
| [Mn4(hmp)6(NO3)2(dca)2] | 9.80 | 98.91 | 1.61 | 95.49, 101.14, 108.06, 109.84 | 103.6 | [ |
| [Mn4(hmp)6(NO3)4] | 3.2 | 99.03 | 2.1 | 96.89, 99.47, 107.42, 110.67 | 103.6 | [ |
| [Mn4(hmp)6(N3)4] | 3.96 | 100.48 | 2.78 | 94.70, 111.73, 97.81, 108.23 | 103.1 | [ |
| [Mn4(hmmi)6(H2O)2(N3)2](ClO4)2(2) | 13.7 | 101.3 | 2.37 | 94.5, 99.2, 108.1, 113.7 | 103.9 | This work |
Table 5 Comparison of the magnetic property for butterfly Mn4 complexes
| Compound | (Jbb/kB)/ K | MnⅢ—O—MnⅢ/ (°) | (Jwb/kB)/ K | MnⅢ—O—MnⅡ/ (°) | (°) | Ref. |
|---|---|---|---|---|---|---|
| [Mn4(hmmi)6Cl4](3) | 14.4 | 101.56 | -1.35 | 94.95, 98.99, 108.66, 113.23 | 104.0 | This work |
| [Mn4(hmp)6(Hhmp)2](ClO4)4 | 0.25 | 102.90 | -0.92 | 94.35, 97.24, 105.36, 110.59 | 101.9 | [ |
| [Mn4(hmp)4(acac)2(MeO)2](ClO4)2 | 2.7 | 99.38 | 0.39 | 96.84, 107.19 | 102.0 | [ |
| [Mn4(hmp)6(NO3)2(MeCN)2](ClO4)2 | 5.0 | 99.55 | 0.5 | 95.33, 99.77, 107.76, 109.34 | 103.1 | [ |
| [Mn4(hmp)6(H2O)4](ClO4)4 | 5.0 | 98.9 | 0.55 | 93.8, 100.0, 106.6, 111.8 | 103.1 | [ |
| [Mn4(hmmi)6(DMF)2(N3)2](ClO4)2(1) | 4.5 | 103.4 | 0.63 | 92.5, 98.9, 110.1, 110.9 | 103.1 | This work |
| [Mn4(hmp)6(CH3CN)2(H2O)4](ClO4)4 | 8.56 | 100.49 | 0.66 | 94.41, 99.84, 107.64, 113.28 | 103.8 | [ |
| [Mn4(hmp)6(H2O)2(NO3)2](NO3)2 | 7.1 | 98.81 | 0.80 | 95.00, 100.43, 107.53, 111.68 | 103.7 | [ |
| {[Mn4(hmp)6(NO3)2][FeNO(CN)5]}n | 9.78 | 99.01 | 0.91 | 94.09, 99.83, 107.73, 110.36 | 103.0 | [ |
| [Mn4(hmp)6(dca)2](ClO4)2 | 9.12 | 98.19, 98.61 | 1.02 | 95.42, 99.73, 106.25, 109.12 | 102.6 | [ |
| [Mn4(hmp)4(Hpdm)2(dca)2](ClO4)2 | 12.66 | 100.60 | 1.15 | 94.67, 100.78, 109.06, 111.05 | 102.4 | [ |
| [Mn4(hmp)6(H2O)2(NO3)2](ClO4)2 | 13.3 | 99.44 | 1.24 | 95.31, 100.25, 107.63, 110.77 | 103.5 | [ |
| [Mn4(hmp)6(dae-c)2(H2O)2](ClO4)2 | 12.26 | 100.51 | 1.25 | 94.07, 99.91, 107.75, 109.19 | 102.7 | [ |
| [Mn4(hmp)6(dae-o)2(ClO4)2] | 9.18 | 99.44 | 1.26 | 96.70, 109.70 | 103.2 | [ |
| [Mn4(hmp)4Br2(OMe)2(dca)2] | 10.89 | 97.71 | 1.30 | 97.23, 98.40, 109.10, 109.69 | 103.6 | [ |
| [Mn4(hmp)6(NO3)2(dca)2] | 9.80 | 98.91 | 1.61 | 95.49, 101.14, 108.06, 109.84 | 103.6 | [ |
| [Mn4(hmp)6(NO3)4] | 3.2 | 99.03 | 2.1 | 96.89, 99.47, 107.42, 110.67 | 103.6 | [ |
| [Mn4(hmp)6(N3)4] | 3.96 | 100.48 | 2.78 | 94.70, 111.73, 97.81, 108.23 | 103.1 | [ |
| [Mn4(hmmi)6(H2O)2(N3)2](ClO4)2(2) | 13.7 | 101.3 | 2.37 | 94.5, 99.2, 108.1, 113.7 | 103.9 | This work |
| [1] | Sessoli R., Gatteschi D., Caneschi A., Novak M. A., Nature, 1993, 365, 141—143 |
| [2] | Affronte M., J. Mater. Chem., 2009, 19, 1731—1737 |
| [3] | Sanudo E. C., Brechin E. K., Boskovic C., Wernsdorfer W., Yoo J., Yamaguchi A., Concolino T. R., Abboud K. A., Rheingold A. L., Ishimoto H., Hendrickson D. N., Christou G., Polyhedron, 2003, 22, 2267—2271 |
| [4] | Dey S.K., Honecker A., Mitra P., Mandal S. K., Mukherjee A., Eur. J. Inorg. Chem., 2012, 5814—5824 |
| [5] | Harden N. C., Bolcar M. A., Wernsdorfer W., Abboud K. A., Streib W. E., Christou G., Inorg. Chem., 2003, 42, 7067—7076 |
| [6] | Stamatatos T.C., Abboud K. A., Wernsdorfer W., Christou G., Polyhedron, 2007, 26,2042—2046 |
| [7] | Taguchi T., Wernsdorfer W., Abboud K. A., Christou G., Inorg. Chem., 2010, 49, 199—208 |
| [8] | Mondal K. C., Drew M. G. B., Mukherjee P. S., Inorg. Chem., 2007, 46, 5625—5629 |
| [9] | Zhao J. P., Zhao R., Yang Q., Hu B. W., Liu F. C., Bu X. H., Dalton Trans., 2013, 42, 14509—14515 |
| [10] | Wang Q. L., Li C. H., Cheng P., Yan S. P., Liao D. Z., Jiang Z. H., Chem. J. Chinese Universties, 2001, 22(10), 1632—1633 |
| (王庆伦, 李春晖, 程鹏, 阎世平, 廖代正, 姜宗慧.高等学校化学学报, 2001,22(10), 1632—1633) | |
| [11] | Yu Y., Liu D., Hu W. W., Li J., Peng Y., Zhou Q., Yang F., Li G. H., Shi Z., Feng S. H., Chem. Res. Chinese Universties, 2012, 28, 186—190 |
| [12] | Vallina A. T., Lecren L., Li Y. G., Roubeau O., Clerac R., Z. Anorg. Allg. Chem., 2007, 633, 2400—2407 |
| [13] | Feng P. L., Beedle C. C., Wernsdorfer W., Koo C., Nakano M., Hill S., Hendrickson D. N., Inorg. Chem., 2007, 46, 8126—8128 |
| [14] | Papatriantafyllopoulou C., Abboud K. A., Christou G., Inorg. Chem., 2011, 50, 8959—8966 |
| [15] | Saha A., Thompson M., Abboud K. A., Wernsdorfer W., Christou. G., Inorg. Chem., 2011, 50, 10476—10485 |
| [16] | Lecren L., Wernsdorfer W., Li Y. G., Roubeau O., Miyasaka H., Clerac R., J. Am. Chem. Soc., 2005, 127, 11311—11317 |
| [17] | Lecren L., Roubeau O., Coulon C., Li Y. G., le Goff X. F., Wernsdorfer W., Miyasaka H., Clerac R., J. Am. Chem. Soc., 2005, 127, 17353—17363 |
| [18] | Miyasaka H., Nakata K., Lecren L., Coulon C., Nakazawa Y., Fujisaki T., Sugiura K., Yamashita M., Clerac R., J. Am. Chem. Soc., 2006, 128, 3770—3783 |
| [19] | Morimoto M., Miyasaka H., Yamashita M., Irie M., J. Am. Chem. Soc., 2009, 131, 9823—9835 |
| [20] | Lecren L., Li Y. G., Wernsdorfer W., Roubeau O., Miyasaka H., Clerac R., Inorg. Chem. Comm., 2005, 8, 626—630 |
| [21] | Yang E. C., Harden N., Wernsdorfer W., Zakharov L., Brechin E. K., Rheingold A. L., Christou G., Hendrickson D. N., Polyhedron, 2003, 49, 1857—1863 |
| [22] | Yoo J., Yamaguchi A., Nakano M., Krystek J., Streib W. E., Brunel L. C., Ishimoto H., Christou G., Hendrickson D. N., Inorg. Chem., 2001, 40, 4604—4616 |
| [23] | Hendrickson D. N., Christou G., Ishimoto H., Yoo J., Brechin E. K., Yamaguchi A., Rumberger E. M., Aubin S. M. J., Sun Z., Aromi G., Polyhedron, 2001, 20, 1479—1488 |
| [24] | Saha A., Abboud K. A., Christou G., Inorg. Chem., 2011, 50, 12774—12784 |
| [25] | Kushch L. A., Sasnovskaya V. D., Yagubskii E. B., Khasanov S. S., Simonov S. V., Shibaeva R. P., Korolev A. V., Starichenko D. V., Anokhin A. O., Irkhin V. Y., Shvachko Y. N., Inorg. Chim. Acta, 2011, 378, 169—173 |
| [26] | Sivanesan D., Son K., Lee H. J., Park K. T., Jang Z., Suh B. J., Yoon S., Polyhedron, 2013, 50, 339—344 |
| [27] | Na L. Y., Ning G. L., Zhang F. J., Wang B., Chem. J. Chinese Universties, 2007, 28(3), 406—409 |
| (那立艳, 宁桂玲, 张风杰, 王冰.高等学校化学学报, 2007,28(3), 406—409) | |
| [28] | Lu Z., Fan C., Inorg. Chem. Commun., 2011, 14, 1329—1332 |
| [29] | He F., Tong M. L., Chen X. M., Inorg. Chem., 2005, 44, 8285—8292 |
| [30] | Wei N., Murthy N. N., Tyeklar Z., Karlin K. D., Inorg. Chem., 1994, 33, 1177—1183 |
| [31] | Kou H. Z., Liao D. Z., Univ. Chem., 2000, 15, 26—28 |
| (寇会忠, 廖代正.大学化学, 2000, 15, 26—28) | |
| [32] | Brese N. E., O’ Keeffe M., Acta Cryst., 1991, B47, 192—197 |
| [33] | Ge C. H., Ni Z. H., Liu C. M., Cui A. L., Zhang D. Q., Kou H. Z., Inorg. Chem. Commun., 2008, 11, 675—677 |
| [1] | GUO Biao, ZHAO Chencan, LIU Xinxin, YU Zhou, ZHOU Lijing, YUAN Hongming, ZHAO Zhen. Effects of Surface Hydrothermal Carbon Layer on the Photocatalytic Activity of Magnetic NiFe2O4 Octahedron [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220472. |
| [2] | XU Fei, LI Gangmei, HAN Songde, WANG Guoming. Photochromism and Photomagnetism in Two Dinuclear Lanthanide Complexes [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210337. |
| [3] | HONG Yangyu, XING Hongzhu, BING Qiming, GAO Xuwen, QI Bin, CHEN Yakun, SU Tan, ZOU Bo. Synthesis and Fluorescence Properties of Novel Ce3+-doped Manganese Phosphite Open-framework Materials [J]. Chem. J. Chinese Universities, 2021, 42(9): 2725. |
| [4] | RUAN Zeyu, DU Shannan, HUANG Guozhang, TONG Mingliang, LIU Junliang. Atropisomerism and Magnetic Properties of an in situ Synthesized Chiral Nickel Complex [J]. Chem. J. Chinese Universities, 2021, 42(3): 709. |
| [5] | ZHANG Lin, ZHANG Wei, YUE Xin, LI Pengjie, YANG Zuoyin, PU Min, LEI Ming. Theoretical Study on Mechanism of CO2 Hydrogenation to Formic Acid Catalyzed by Manganese Complex † [J]. Chem. J. Chinese Universities, 2019, 40(9): 1911. |
| [6] | WANG Xiaohui, WANG Kexin, LIU Junping, HONG Xia. Mesoporous-structure Enhanced Photothermal Effect of Fe3O4 Superparticles@mesoporous Silica [J]. Chem. J. Chinese Universities, 2019, 40(8): 1586. |
| [7] | WANG Zhixiu, MU Ying, WANG Yilin, SUN Xiaoyuan, SU Tan, LIU Jingyao. Proton Conduction Property of a Manganese Phosphite Open Framework Compound† [J]. Chem. J. Chinese Universities, 2018, 39(6): 1138. |
| [8] | WANG Fengmei, XU Guangwei, JIN Chengchang. Synthesis and Electrochemical Performance for Supercapacitors of Bi-doped α-MnO2 Nanorods [J]. Chem. J. Chinese Universities, 2018, 39(3): 530. |
| [9] | FENG Dongyang,GUO Di,LIU Xiaoxia. Functionalization of Carbon Electrode and Subsequent Electrochemical Deposition of Nanostructured Manganese Oxide† [J]. Chem. J. Chinese Universities, 2018, 39(10): 2280. |
| [10] | QIU Jiaxin,JIANG Qi,GAO Yike,PENG Junqi,DUAN Zhihong,LU Xiaoying. Electrochemical Studies on the Working Mechanism of Lithium-rich Manganese Based Material Coated by MnO2 † [J]. Chem. J. Chinese Universities, 2018, 39(10): 2238. |
| [11] | YU Ze, GAO He, ZHENG Kai, LIANG Daxin, LIU Zhiming, PANG Guangsheng, FANG Zhenxing. Preparation and Properties of Surface Functionalized Wood by Nanoscale Manganese Oxide† [J]. Chem. J. Chinese Universities, 2017, 38(9): 1518. |
| [12] | GAO Lijuan, WANG Li, WANG Shengyan, JING Shubo. Influence of Solvent on Structure of Ni(Ⅱ) Metal-organic Frameworks† [J]. Chem. J. Chinese Universities, 2016, 37(9): 1589. |
| [13] | SONG Min, ZHANG Linping, ZHONG Yi, XU Hong, MAO Zhiping. Catalytic Properties of Manganese Complex of Cyclic Polyamine Encapsulated in Ethyl Cellulose Microcapsules† [J]. Chem. J. Chinese Universities, 2014, 35(9): 1941. |
| [14] | HE Yongke, YAN Yan, YANG Fen, WU Junbiao, SONG Xiaowei. Ionothermal Syntheses and Characterization of Open-framework Manganese Phosphites (NH4)2Mn3(HPO3)4 and Mn(HPO3)† [J]. Chem. J. Chinese Universities, 2014, 35(9): 1859. |
| [15] | LI Xiaoli, LI Zhiguo, LI Bin, REN Yuting. Preparation and Application of Self-assembly of Amphiphilic Cyclotriphosphazene† [J]. Chem. J. Chinese Universities, 2014, 35(8): 1675. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
