

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (8): 1683.doi: 10.7503/cjcu20180081
• Organic Chemistry • Previous Articles Next Articles
WAN Jinlin1, WU Shouqun1, GAN Yiyuan2, MENG Jiao2, WANG Zhenchao2*( ), OUYANG Guiping2*(
), OUYANG Guiping2*( )
)
Received:2018-01-25
Online:2018-08-10
Published:2018-05-27
Supported by:TrendMD:
WAN Jinlin, WU Shouqun, GAN Yiyuan, MENG Jiao, WANG Zhenchao*, OUYANG Guiping*. Synthesis and Antibacterial Activities Evaluation of Chalconesemicarbazone Derivatives Bearing 1,3,4-Thiadiazole Moiety†[J]. Chem. J. Chinese Universities, 2018, 39(8): 1683.
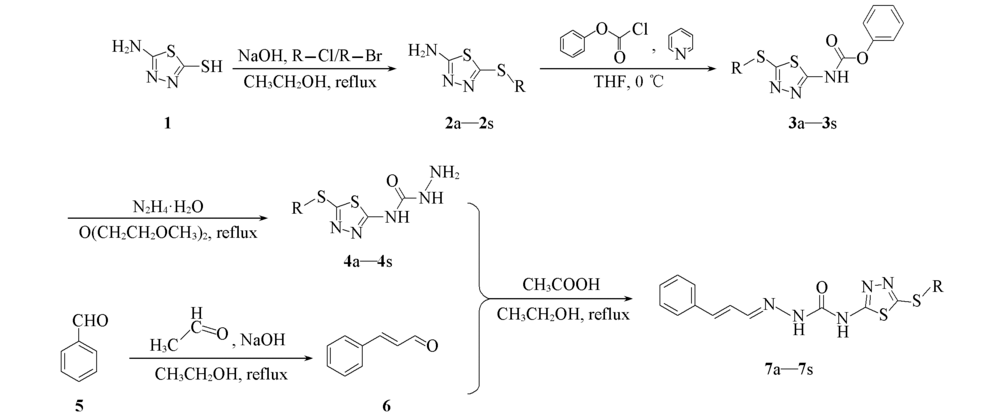
Scheme 1 Scheme 1 Synthetic routes of compounds 7a—7s7a: R=PhCH2; 7b: R=2-ClPhCH2; 7c: R=3-ClPhCH2; 7d: R=4-ClPhCH2; 7e: R=2-OCH3PhCH2; 7f: R=3-OCH3PhCH2; 7g: R=4-OCH3PhCH2; 7h: R=2-CH3PhCH2; 7i: R=3-CH3PhCH2; 7j: R=4-CH3PhCH2; 7k: R=2-FPhCH2; 7l: R=4-FPhCH2; 7m: R=2-CNPhCH2; 7n: R=2,4-diClPhCH2; 7o: R=CH3; 7p: R=C2H5; 7q: R=n-C3H7; 7r: R=n-C4H9; 7s: R=n-C5H11
| Compd. | m. p./℃ | Compd. | m. p./℃ | Compd. | m. p./℃ |
|---|---|---|---|---|---|
| 2a | 157—159(158[ | 2h | 119—121 | 2o | 177—179 |
| 2b | 151—153(151—152[ | 2i | 128—130(128—129[ | 2p | 136—137(136—137[ |
| 2c | 138—140(138—139[ | 2j | 181—183(181—182[ | 2q | 116—118(117—119[ |
| 2d | 167—169(168—169[ | 2k | 143—145(145—146[ | 2r | 103—105 |
| 2e | 100—102 | 2l | 148—150(149—150[ | 2s | 112—113(112—115[ |
| 2f | 148—150 | 2m | 104—106 | ||
| 2g | 151—153 | 2n | 133—135(133—134[ |
Table 1 Melting points of compounds 2a—2s
| Compd. | m. p./℃ | Compd. | m. p./℃ | Compd. | m. p./℃ |
|---|---|---|---|---|---|
| 2a | 157—159(158[ | 2h | 119—121 | 2o | 177—179 |
| 2b | 151—153(151—152[ | 2i | 128—130(128—129[ | 2p | 136—137(136—137[ |
| 2c | 138—140(138—139[ | 2j | 181—183(181—182[ | 2q | 116—118(117—119[ |
| 2d | 167—169(168—169[ | 2k | 143—145(145—146[ | 2r | 103—105 |
| 2e | 100—102 | 2l | 148—150(149—150[ | 2s | 112—113(112—115[ |
| 2f | 148—150 | 2m | 104—106 | ||
| 2g | 151—153 | 2n | 133—135(133—134[ |
| Compd. | m. p./℃ | HRMS(calcd.)*, m/z | 1H NMR(DMSO, 400 MHz), δ |
|---|---|---|---|
| 3a | 170—172 | 344.0517(344.0522) | 12.86(s, 1H), 7.48—7.38(m, 4H), 7.36—7.30(m, 3H), 7.29—7.25(m, 3H), 4.48(s, 2H) |
| 3b | 141—143 | 378.0130(378.0132) | 12.88(s, 1H), 7.52—7.47(m, 2H), 7.44(d, J=8.1 Hz, 2H), 7.36—7.25(m, 5H), 4.55(s, 2H) |
| 3c | 185—187 | 378.0122(378.0132) | 12.89(s, 1H), 7.50(d, J=1.4 Hz, 1H), 7.48—7.43(m, 2H), 7.40—7.30(m, 4H), 7.30—7.26(m, 2H), 4.49(s, 2H) |
| 3d | 172—174 | 378.0129(378.0132) | 12.88(s, 1H), 7.48—7.41(m, 4H), 7.41—7.36(m, 2H), 7.34—7.25(m, 3H), 4.48(s, 2H) |
| Compd. | m. p./℃ | HRMS(calcd.)*, m/z | 1H NMR(DMSO, 400 MHz), δ |
| 3e | 137—139 | 374.0620(374.0628) | 12.88(s, 1H), 7.45(t, J=7.7 Hz, 2H), 7.35—7.24(m, 5H), 7.01(d, J=8.1 Hz, 1H), 6.89(t, J=7.4 Hz, 1H), 4.41(s, 2H), 3.80(s, 3H) |
| 3f | 139—141 | 374.0620(374.0628) | 12.86(s, 1H), 7.45(t, J=7.9 Hz, 2H), 7.34—7.21(m, 4H), 6.98(t, J=5.6 Hz, 2H), 6.85(dd, J=8.2, 2.1 Hz, 1H), 4.45(s, 2H), 3.73(s, 3H) |
| 3g | 199—201 | 374.0619(374.0628) | 12.83(s, 1H), 7.48—7.42(m, 2H), 7.35—7.28(m, 5H), 7.28—7.24(m, 2H), 4.42(s, 2H), 3.73(s, 3H) |
| 3h | 137—139 | 358.0668(358.0678) | 12.84(s, 1H), 7.51—7.40(m, 2H), 7.33—7.25(m, 4H), 7.23—7.17(m, 2H), 7.16—7.10(m, 1H), 4.49(s, 2H), 2.37(s, 3H) |
| 3i | 152—154 | 358.0671(358.0678) | 12.88(s, 1H), 7.50—7.41(m, 2H), 7.33—7.25(m, 3H), 7.21(t, J=9.0, 2.7 Hz, 3H), 7.08(d, J=6.4 Hz, 1H), 4.44(s, 2H, CH2), 2.27(s, 3H, CH3) |
| 3j | 187—189 | 358.0669(358.0678) | 12.85(s, 1H), 7.45(t, J=7.8 Hz, 2H), 7.34—7.24(m, 5H), 7.13(d, J=7.6 Hz, 2H), 4.43(s, 2H), 2.27(s, 3H) |
| 3k | 144—146 | 362.0421(362.0428) | 12.90(s, 1H), 7.49—7.42(m, 3H), 7.38—7.25(m, 4H), 7.19—7.12(m, 2H), 4.48(s, 2H) |
| 3l | 161—163 | 362.0423(362.0428) | 12.86(s, 1H), 7.48—7.42(m, 4H), 7.34—7.25(m, 3H), 7.23—7.12(m, 2H), 4.48(s, 2H) |
| 3m | 120—122 | 369.0467(369.0474) | 12.90(s, 1H), 7.85(dd, J=7.7, 0.9 Hz, 1H), 7.70—7.61(m, 2H), 7.52—7.42(m, 3H), 7.34—7.25(m, 3H), 4.62(s, 2H) |
| 3n | 162—164 | 411.9735(411.9743) | 12.92(s, 1H), 7.63(d, J=12.1 Hz, 1H), 7.53(d, J=8.3 Hz, 1H), 7.46(t, J=7.0 Hz, 2H), 7.39(t, J=7.8 Hz, 1H), 7.30(dd, J=16.2, 8.3 Hz, 3H), 4.53(s, 2H) |
| 3o | 159—161 | 268.0204(268.0209) | 12.85(s, 1H), 7.45(t, J=7.6 Hz, 2H), 7.30(dd, J=16.4, 7.1 Hz, 3H), 2.71(s, 3H) |
| 3p | 206—209 | 282.0361(282.0365) | 8.46(s, 1H), 3.18(q, J=7.3 Hz, 2H), 1.33(t, J=7.3 Hz, 3H) |
| 3q | 190—192 | 296.0518(296.0522) | 8.46(s, 1H), 3.15(t, J=7.1 Hz, 2H), 1.76—1.63(m, 2H), 0.98(t, J=7.3 Hz, 3H) |
| 3r | 181—183 | 310.0669(310.0678) | 8.46(s, 1H), 3.17(t, J=7.3 Hz, 2H), 1.71—1.60(m, 2H), 1.46—1.35(m, 2H), 0.89(t, J=7.4 Hz, 3H) |
| 3s | 108—110 | 324.0826(324.0835) | 12.86(s, 1H), 7.49—7.42(m, 2H), 7.34—7.25(m, 3H), 3.20(t, J=14.6 Hz, 2H), 1.77—1.61(m, 2H), 1.42—1.24(m, 4H), 0.86(t, J=7.2 Hz, 3H) |
| 4a | 196—198 | 282.0469(282.0478) | 8.45(s, 1H), 7.41—7.37(m, 2H), 7.35—7.30(m, 2H), 7.29—7.23(m, 1H), 4.43(s, 2H) |
| 4b | 195—197 | 337.9907(337.9908) | 8.51(s, 1H), 7.51—7.46(m, 2H), 7.37—7.27(m, 2H), 4.51(s, 2H) |
| 4c | 185—187 | 316.0082(316.0088) | 8.45(s, 1H), 7.47(s, 1H), 7.38—7.30(m, 3H), 4.44(s, 2H) |
| 4d | 212—213 | 337.9905(337.9908) | 8.46(s, 1H), 7.43—7.36(m, 4H), 4.43(s, 2H) |
| 4e | 201—203 | 312.0577(312.0583) | 8.49(s, 1H), 7.29(t, J=7.9 Hz, 2H), 7.01(d, J=8.1 Hz, 1H), 6.89(t, J=7.4 Hz, 1H), 4.37(s, 2H), 3.81(s, 3H) |
| 4f | 191—194 | 312.0579(312.0583) | 8.45(s, 1H), 7.24(t, J=8.0 Hz, 1H), 6.95(d, J=7.4 Hz, 2H), 6.84(ddd, J=8.2, 2.5, 1.0 Hz, 1H), 4.40(s, 2H), 3.73(s, 3H) |
| 4g | 211—213 | 312.0577(312.0583) | 8.45(s, 1H), 7.31(d, J=8.7 Hz, 2H), 6.88(d, J=8.7 Hz, 2H), 4.38(s, 2H), 3.73(s, 3H) |
| 4h | 200—202 | 296.0631(296.0634) | 8.48(s, 1H), 7.29(d, J=7.3 Hz, 1H), 7.20(d, J=3.7 Hz, 2H), 7.15(dd, J=11.0, 5.7 Hz, 1H), 4.44(s, 2H), 2.37(s, 3H) |
| 4i | 207—209 | 296.0632(296.0634) | 8.44(s, 1H), 7.23—7.15(m, 3H), 7.08(d, J=7.3 Hz, 1H), 4.39(s, 2H), 2.28(s, 3H) |
| 4j | 189—192 | 318.0450(318.0454) | 8.46(s, 1H), 7.27(d, J=8.0 Hz, 2H), 7.13(d, J=7.8 Hz, 2H), 4.39(s, 2H), 2.27(s, 3H) |
| 4k | 171—173 | 300.0377(300.0384) | 8.48(s, 1H), 7.43(t, J=7.7 Hz, 1H), 7.35(dt, J=13.6, 6.6 Hz, 2H), 7.24—7.13(m, 3H), 4.43(s, 2H) |
| 4l | 197—199 | 300.0377(300.0384) | 8.48(s, 1H), 7.46—7.41(m, 2H), 7.19—7.12(m, 2H), 4.44(s, 2H) |
| 4m | 188—190 | 307.0425(307.0430) | 8.51(s, 1H), 7.85(dd, J=7.7, 1.0 Hz, 1H), 7.69—7.65(m, 1H), 7.64—7.59(m, 1H), 7.49(m, 1H), 4.58(s, 2H) |
| 4n | 183—185 | 371.9517(371.9518) | 7.65(d, J=2.2 Hz, 1H), 7.48(d, J=8.3 Hz, 1H), 7.40(dd, J=8.3, 2.1 Hz, 1H), 4.47(s, 2H) |
| 4o | 209—211 | 228.9980(228.9984) | 8.46(s, 1H), 2.68(s, 3H) |
| Compd. | m. p./℃ | HRMS(calcd.)*, m/z | 1H NMR(DMSO, 400 MHz), δ |
| 4p | 206—209 | 220.0320(220.0321) | 8.46(s, 1H), 3.18(q, J=7.3 Hz, 2H), 1.33(t, J=7.3 Hz, 3H) |
| 4q | 190—192 | 234.0475(234.0478) | 8.46(s, 1H), 3.15(t, J=7.1 Hz, 2H), 1.76—1.63(m, 2H), 0.98(t, J=7.3 Hz, 3H) |
| 4r | 181—183 | 248.0631(248.0634) | 8.46(s, 1H), 3.17(t, J=7.3 Hz, 2H), 1.71—1.60(m, 2H), 1.46—1.40(m, 2H), 0.89(t, J=7.4 Hz, 3H) |
| 4s | 194—196 | 262.0784(262.0791) | 8.45(s, 1H), 3.16(t, J=7.3 Hz, 2H), 1.69—1.64(m, 2H), 1.43—1.24(m, 4H, CH2), 0.86(t, J=7.2 Hz, 3H) |
| 6 | 223—225 | 180.0210(180.0211) | 13.05(s, 1H), 9.99(s, 1H), 8.11—8.01(m, 1H), 7.45—7.38(m, 1H), 7.28—7.20(m, 2H) |
Table 2 Melting points, HRMS and 1H NMR data of compounds 3a—3s, 4a—4s and 6
| Compd. | m. p./℃ | HRMS(calcd.)*, m/z | 1H NMR(DMSO, 400 MHz), δ |
|---|---|---|---|
| 3a | 170—172 | 344.0517(344.0522) | 12.86(s, 1H), 7.48—7.38(m, 4H), 7.36—7.30(m, 3H), 7.29—7.25(m, 3H), 4.48(s, 2H) |
| 3b | 141—143 | 378.0130(378.0132) | 12.88(s, 1H), 7.52—7.47(m, 2H), 7.44(d, J=8.1 Hz, 2H), 7.36—7.25(m, 5H), 4.55(s, 2H) |
| 3c | 185—187 | 378.0122(378.0132) | 12.89(s, 1H), 7.50(d, J=1.4 Hz, 1H), 7.48—7.43(m, 2H), 7.40—7.30(m, 4H), 7.30—7.26(m, 2H), 4.49(s, 2H) |
| 3d | 172—174 | 378.0129(378.0132) | 12.88(s, 1H), 7.48—7.41(m, 4H), 7.41—7.36(m, 2H), 7.34—7.25(m, 3H), 4.48(s, 2H) |
| Compd. | m. p./℃ | HRMS(calcd.)*, m/z | 1H NMR(DMSO, 400 MHz), δ |
| 3e | 137—139 | 374.0620(374.0628) | 12.88(s, 1H), 7.45(t, J=7.7 Hz, 2H), 7.35—7.24(m, 5H), 7.01(d, J=8.1 Hz, 1H), 6.89(t, J=7.4 Hz, 1H), 4.41(s, 2H), 3.80(s, 3H) |
| 3f | 139—141 | 374.0620(374.0628) | 12.86(s, 1H), 7.45(t, J=7.9 Hz, 2H), 7.34—7.21(m, 4H), 6.98(t, J=5.6 Hz, 2H), 6.85(dd, J=8.2, 2.1 Hz, 1H), 4.45(s, 2H), 3.73(s, 3H) |
| 3g | 199—201 | 374.0619(374.0628) | 12.83(s, 1H), 7.48—7.42(m, 2H), 7.35—7.28(m, 5H), 7.28—7.24(m, 2H), 4.42(s, 2H), 3.73(s, 3H) |
| 3h | 137—139 | 358.0668(358.0678) | 12.84(s, 1H), 7.51—7.40(m, 2H), 7.33—7.25(m, 4H), 7.23—7.17(m, 2H), 7.16—7.10(m, 1H), 4.49(s, 2H), 2.37(s, 3H) |
| 3i | 152—154 | 358.0671(358.0678) | 12.88(s, 1H), 7.50—7.41(m, 2H), 7.33—7.25(m, 3H), 7.21(t, J=9.0, 2.7 Hz, 3H), 7.08(d, J=6.4 Hz, 1H), 4.44(s, 2H, CH2), 2.27(s, 3H, CH3) |
| 3j | 187—189 | 358.0669(358.0678) | 12.85(s, 1H), 7.45(t, J=7.8 Hz, 2H), 7.34—7.24(m, 5H), 7.13(d, J=7.6 Hz, 2H), 4.43(s, 2H), 2.27(s, 3H) |
| 3k | 144—146 | 362.0421(362.0428) | 12.90(s, 1H), 7.49—7.42(m, 3H), 7.38—7.25(m, 4H), 7.19—7.12(m, 2H), 4.48(s, 2H) |
| 3l | 161—163 | 362.0423(362.0428) | 12.86(s, 1H), 7.48—7.42(m, 4H), 7.34—7.25(m, 3H), 7.23—7.12(m, 2H), 4.48(s, 2H) |
| 3m | 120—122 | 369.0467(369.0474) | 12.90(s, 1H), 7.85(dd, J=7.7, 0.9 Hz, 1H), 7.70—7.61(m, 2H), 7.52—7.42(m, 3H), 7.34—7.25(m, 3H), 4.62(s, 2H) |
| 3n | 162—164 | 411.9735(411.9743) | 12.92(s, 1H), 7.63(d, J=12.1 Hz, 1H), 7.53(d, J=8.3 Hz, 1H), 7.46(t, J=7.0 Hz, 2H), 7.39(t, J=7.8 Hz, 1H), 7.30(dd, J=16.2, 8.3 Hz, 3H), 4.53(s, 2H) |
| 3o | 159—161 | 268.0204(268.0209) | 12.85(s, 1H), 7.45(t, J=7.6 Hz, 2H), 7.30(dd, J=16.4, 7.1 Hz, 3H), 2.71(s, 3H) |
| 3p | 206—209 | 282.0361(282.0365) | 8.46(s, 1H), 3.18(q, J=7.3 Hz, 2H), 1.33(t, J=7.3 Hz, 3H) |
| 3q | 190—192 | 296.0518(296.0522) | 8.46(s, 1H), 3.15(t, J=7.1 Hz, 2H), 1.76—1.63(m, 2H), 0.98(t, J=7.3 Hz, 3H) |
| 3r | 181—183 | 310.0669(310.0678) | 8.46(s, 1H), 3.17(t, J=7.3 Hz, 2H), 1.71—1.60(m, 2H), 1.46—1.35(m, 2H), 0.89(t, J=7.4 Hz, 3H) |
| 3s | 108—110 | 324.0826(324.0835) | 12.86(s, 1H), 7.49—7.42(m, 2H), 7.34—7.25(m, 3H), 3.20(t, J=14.6 Hz, 2H), 1.77—1.61(m, 2H), 1.42—1.24(m, 4H), 0.86(t, J=7.2 Hz, 3H) |
| 4a | 196—198 | 282.0469(282.0478) | 8.45(s, 1H), 7.41—7.37(m, 2H), 7.35—7.30(m, 2H), 7.29—7.23(m, 1H), 4.43(s, 2H) |
| 4b | 195—197 | 337.9907(337.9908) | 8.51(s, 1H), 7.51—7.46(m, 2H), 7.37—7.27(m, 2H), 4.51(s, 2H) |
| 4c | 185—187 | 316.0082(316.0088) | 8.45(s, 1H), 7.47(s, 1H), 7.38—7.30(m, 3H), 4.44(s, 2H) |
| 4d | 212—213 | 337.9905(337.9908) | 8.46(s, 1H), 7.43—7.36(m, 4H), 4.43(s, 2H) |
| 4e | 201—203 | 312.0577(312.0583) | 8.49(s, 1H), 7.29(t, J=7.9 Hz, 2H), 7.01(d, J=8.1 Hz, 1H), 6.89(t, J=7.4 Hz, 1H), 4.37(s, 2H), 3.81(s, 3H) |
| 4f | 191—194 | 312.0579(312.0583) | 8.45(s, 1H), 7.24(t, J=8.0 Hz, 1H), 6.95(d, J=7.4 Hz, 2H), 6.84(ddd, J=8.2, 2.5, 1.0 Hz, 1H), 4.40(s, 2H), 3.73(s, 3H) |
| 4g | 211—213 | 312.0577(312.0583) | 8.45(s, 1H), 7.31(d, J=8.7 Hz, 2H), 6.88(d, J=8.7 Hz, 2H), 4.38(s, 2H), 3.73(s, 3H) |
| 4h | 200—202 | 296.0631(296.0634) | 8.48(s, 1H), 7.29(d, J=7.3 Hz, 1H), 7.20(d, J=3.7 Hz, 2H), 7.15(dd, J=11.0, 5.7 Hz, 1H), 4.44(s, 2H), 2.37(s, 3H) |
| 4i | 207—209 | 296.0632(296.0634) | 8.44(s, 1H), 7.23—7.15(m, 3H), 7.08(d, J=7.3 Hz, 1H), 4.39(s, 2H), 2.28(s, 3H) |
| 4j | 189—192 | 318.0450(318.0454) | 8.46(s, 1H), 7.27(d, J=8.0 Hz, 2H), 7.13(d, J=7.8 Hz, 2H), 4.39(s, 2H), 2.27(s, 3H) |
| 4k | 171—173 | 300.0377(300.0384) | 8.48(s, 1H), 7.43(t, J=7.7 Hz, 1H), 7.35(dt, J=13.6, 6.6 Hz, 2H), 7.24—7.13(m, 3H), 4.43(s, 2H) |
| 4l | 197—199 | 300.0377(300.0384) | 8.48(s, 1H), 7.46—7.41(m, 2H), 7.19—7.12(m, 2H), 4.44(s, 2H) |
| 4m | 188—190 | 307.0425(307.0430) | 8.51(s, 1H), 7.85(dd, J=7.7, 1.0 Hz, 1H), 7.69—7.65(m, 1H), 7.64—7.59(m, 1H), 7.49(m, 1H), 4.58(s, 2H) |
| 4n | 183—185 | 371.9517(371.9518) | 7.65(d, J=2.2 Hz, 1H), 7.48(d, J=8.3 Hz, 1H), 7.40(dd, J=8.3, 2.1 Hz, 1H), 4.47(s, 2H) |
| 4o | 209—211 | 228.9980(228.9984) | 8.46(s, 1H), 2.68(s, 3H) |
| Compd. | m. p./℃ | HRMS(calcd.)*, m/z | 1H NMR(DMSO, 400 MHz), δ |
| 4p | 206—209 | 220.0320(220.0321) | 8.46(s, 1H), 3.18(q, J=7.3 Hz, 2H), 1.33(t, J=7.3 Hz, 3H) |
| 4q | 190—192 | 234.0475(234.0478) | 8.46(s, 1H), 3.15(t, J=7.1 Hz, 2H), 1.76—1.63(m, 2H), 0.98(t, J=7.3 Hz, 3H) |
| 4r | 181—183 | 248.0631(248.0634) | 8.46(s, 1H), 3.17(t, J=7.3 Hz, 2H), 1.71—1.60(m, 2H), 1.46—1.40(m, 2H), 0.89(t, J=7.4 Hz, 3H) |
| 4s | 194—196 | 262.0784(262.0791) | 8.45(s, 1H), 3.16(t, J=7.3 Hz, 2H), 1.69—1.64(m, 2H), 1.43—1.24(m, 4H, CH2), 0.86(t, J=7.2 Hz, 3H) |
| 6 | 223—225 | 180.0210(180.0211) | 13.05(s, 1H), 9.99(s, 1H), 8.11—8.01(m, 1H), 7.45—7.38(m, 1H), 7.28—7.20(m, 2H) |
| Compd. | Appearance | Yield(%) | m. p. /℃ | HRMS(calcd.), m/z[M+H]+ |
|---|---|---|---|---|
| 7a | White solid | 85 | 189—191 | 396.0940(396.0947) |
| 7b | White solid | 83 | 180—181 | 430.0548(430.0558) |
| 7c | White solid | 78 | 172—173 | 430.0547(430.0558) |
| 7d | White solid | 74 | 200—201 | 430.0549(430.0558) |
| 7e | White solid | 83 | 173—175 | 426.1042(426.1053) |
| 7f | White solid | 81 | 159—161 | 426.1042(426.1053) |
| 7g | White solid | 76 | 177—178 | 426.1043(426.1053) |
| 7h | White solid | 90 | 172—174 | 410.1097(410.1104) |
| 7i | White solid | 89 | 171—173 | 410.1099(410.1104) |
| 7j | White solid | 70 | 173—175 | 410.1101(410.1104) |
| 7k | White solid | 84 | 167—168 | 414.0841(414.0853) |
| 7l | White solid | 79 | 210—211 | 414.0843(414.0853) |
| 7m | Yellow solid | 86 | 165—167 | 421.0889(421.0899) |
| 7n | Yellow solid | 79 | 185—187 | 464.0161(464.0168) |
| 7o | Yellow solid | 77 | 189—191 | 320.0641(320.0634) |
| 7p | Yellow solid | 83 | 197—199 | 334.0784(334.0791) |
| 7q | Yellow solid | 82 | 184—186 | 348.0938(348.0947) |
| 7r | Yellow solid | 81 | 138—140 | 362.1095(362.1104) |
| 7s | Yellow solid | 48 | 139—141 | 376.1250(376.1260) |
Table 3 Appearance, yields, melting points and HRMS data for compounds 7a—7s
| Compd. | Appearance | Yield(%) | m. p. /℃ | HRMS(calcd.), m/z[M+H]+ |
|---|---|---|---|---|
| 7a | White solid | 85 | 189—191 | 396.0940(396.0947) |
| 7b | White solid | 83 | 180—181 | 430.0548(430.0558) |
| 7c | White solid | 78 | 172—173 | 430.0547(430.0558) |
| 7d | White solid | 74 | 200—201 | 430.0549(430.0558) |
| 7e | White solid | 83 | 173—175 | 426.1042(426.1053) |
| 7f | White solid | 81 | 159—161 | 426.1042(426.1053) |
| 7g | White solid | 76 | 177—178 | 426.1043(426.1053) |
| 7h | White solid | 90 | 172—174 | 410.1097(410.1104) |
| 7i | White solid | 89 | 171—173 | 410.1099(410.1104) |
| 7j | White solid | 70 | 173—175 | 410.1101(410.1104) |
| 7k | White solid | 84 | 167—168 | 414.0841(414.0853) |
| 7l | White solid | 79 | 210—211 | 414.0843(414.0853) |
| 7m | Yellow solid | 86 | 165—167 | 421.0889(421.0899) |
| 7n | Yellow solid | 79 | 185—187 | 464.0161(464.0168) |
| 7o | Yellow solid | 77 | 189—191 | 320.0641(320.0634) |
| 7p | Yellow solid | 83 | 197—199 | 334.0784(334.0791) |
| 7q | Yellow solid | 82 | 184—186 | 348.0938(348.0947) |
| 7r | Yellow solid | 81 | 138—140 | 362.1095(362.1104) |
| 7s | Yellow solid | 48 | 139—141 | 376.1250(376.1260) |
| Compd. | 1H NMR(DMSO, 400 MHz), δ | 13C NMR(DMSO, 100 MHz), δ |
|---|---|---|
| 7a | 11.17(d, J=127.5 Hz, 2H), 7.85(d, J=8.0 Hz, 1H), 7.57(d, J=7.3 Hz, 2H), 7.41(dt, J=7.5, 3.9 Hz, 4H), 7.38—7.31(m, 3H), 7.30—7.24(m, 1H), 7.08—6.95(m, 2H), 4.48(s, 2H) | 160.8,158.0, 152.6, 146.2, 138.9,137.2, 136.4, 129.4, 129.0, 128.0, 127.4, 125.6, 38.1 |
| 7b | 11.23(d, J=129.6 Hz, 2H), 7.87(d, J=7.4 Hz, 1H), 7.58(d, J=7.4 Hz, 2H), 7.53—7.48(m, 2H), 7.41(t, J=7.4 Hz, 2H), 7.33(ddd, J=12.3, 6.0, 2.3 Hz, 3H), 7.08—6.97(m, 2H), 4.55(s, 2H) | 161.2, 157.1, 152.5, 146.2, 138.9, 136.3, 134.7, 133.8, 131.9, 130.1, 129.3, 127.8, 127.4, 125.6, 36.3 |
| 7c | 11.22(d, J=118.1 Hz, 2H), 7.87(d, J=7.3 Hz, 1H), 7.58(d, J=7.4 Hz, 2H), 7.50(s, 1H), 7.43—7.33(m, 6H), 7.08—6.95(m, 2H), 4.49(s, 2H) | 161.0, 157.4, 152.5, 146.1, 140.1, 138.9, 136.3, 133.5, 130.8, 129.3, 128.2, 127.9, 127.4, 125.6, 37.2 |
| Compd. | 1H NMR(DMSO, 400 MHz), δ | 13C NMR(DMSO, 100 MHz), δ |
| 7d | 11.20(d, J=130.9 Hz, 2H), 7.86(d, J=7.9 Hz, 1H), 7.57(d, J=7.3 Hz, 2H), 7.42(td, J=8.4, 1.6 Hz, 6H), 7.34(d, J=7.2 Hz, 1H), 7.08—6.95(m, 2H), 4.48(s, 2H) | 160.9, 157.5, 152.5, 146.1, 138.9, 136.6, 136.3, 132.6, 131.3, 129.3, 129.0, 127.4, 125.6, 37.2 |
| 7e | 11.16(d, J=129.2 Hz, 2H), 7.85(d, J=7.5 Hz, 1H), 7.58(d, J=6.9 Hz, 2H), 7.41(t, J=7.4 Hz, 2H), 7.37—7.24(m, 3H), 7.09—6.95(m, 3H), 6.93—6.87(m, 1H), 4.40(s, 2H), 3.81(s, 3H) | 160.9, 158.3, 157.6, 152.6, 146.1, 138.8, 136.4, 130.8, 129.8, 129.3, 127.4, 125.6, 124.8, 120.7, 111.6, 56.0, 33.6 |
| 7f | 11.23(d, J=139.4 Hz, 2H), 7.87(d, J=6.5 Hz, 1H), 7.58(d, J=6.7 Hz, 2H), 7.37(dd, J=21.8, 6.6 Hz, 3H), 7.25(t, J=7.4 Hz, 1H), 7.00(t, J=10.5 Hz, 4H), 6.86(d, J=7.3 Hz, 1H), 4.45(s, 2H), 3.74(s, 3H) | 160.9, 159.7, 157.9, 152.5, 146.2, 138.8, 138.7, 136.4, 130.1, 129.3, 129.3, 127.4, 125.6, 121.6, 115.1, 113.5, 55.5, 38.1 |
| 7g | 11.22(d, J=128.7 Hz, 2H), 7.86(d, J=7.7 Hz, 1H), 7.57(d, J=7.4 Hz, 1H), 7.47—7.37(m, 2H), 7.32(t, J=8.4 Hz, 3H), 7.08—6.96(m, 2H), 6.89(dd, J=7.9, 4.2 Hz, 3H), 4.38(s, 2H), 3.73(s, 3H). | 160.8, 159.1, 156.1, 152.5, 146.2, 138.7, 136.4, 130.8, 129.4, 127.4, 125.6, 114.4, 55.5, 37.8 |
| 7h | 11.21(d, J=136.7 Hz, 2H), 7.86(d, J=6.9 Hz, 1H), 7.56(d, J=7.4 Hz, 2H), 7.39(t, J=7.4 Hz, 2H), 7.31(dd, J=9.8, 7.4 Hz, 2H), 7.22—7.10(m, 3H), 7.06—6.94(m, 2H), 4.47(s, 2H), 2.37(s, 3H) | 160.9, 157.7, 152.6, 146.1, 138.8, 137.2, 136.4, 134.6, 129.4, 129.3, 128.4, 127.4, 126.5, 125.6, 36.7, 19.2 |
| 7i | 11.20(d, J=128.1 Hz, 2H), 7.86(d, J=7.4 Hz, 1H), 7.57(d, J=7.2 Hz, 2H), 7.41(t, J=7.4 Hz, 2H), 7.33(t, J=7.3 Hz, 1H), 7.25—7.18(m, 3H), 7.12—7.05(m, 1H), 7.04—6.95(m, 2H), 4.44(s, 2H), 2.28(s, 3H) | 160.8, 158.0, 152.6, 146.1, 138.8, 138.2, 137.0, 136.3, 130.1, 129.4, 129.3, 128.9, 128.7, 127.4, 126.6, 125.6, 38.1, 21.4 |
| 7j | 11.17(d, J=134.6 Hz, 2H), 7.85(d, J=7.4 Hz, 1H), 7.56(d, J=7.1 Hz, 2H), 7.40(t, J=7.2 Hz, 2H), 7.31(dd, J=20.4, 7.4 Hz, 3H), 7.13(d, J=7.5 Hz, 2H), 7.09—6.93(m, 2H), 4.42(s, 2H), 2.27(s, 3H) | 160.9, 157.7, 152.6, 146.1, 130.9, 130.4, 129.3, 128.4, 127.4, 126.5, 125.6, 36.7, 19.2 |
| 7k | 11.22(d, J=107.8 Hz, 2H), 7.85(d, J=7.3 Hz, 1H), 7.56(d, J=7.3 Hz, 2H), 7.45(td, J=7.7, 1.6 Hz, 1H), 7.39(t, J=7.5 Hz, 2H), 7.34(dt, J=5.2, 2.0 Hz, 2H), 7.24—7.12(m, 2H), 7.06—6.95(m, 2H), 4.47(s, 2H) | 160.9, 161.2, 157.0, 152.6, 146.2, 138.9, 136.3, 131.8, 130.4, 129.3, 127.4, 125.6, 125.0, 124.3, 116.1, 115.9, 32.0 |
| 7l | 11.20(d, J=138.6 Hz, 2H), 7.87(d, J=7.5 Hz, 1H), 7.58(d, J=7.5 Hz, 2H), 7.50—7.44(m, 2H), 7.41(t, J=7.5 Hz, 2H), 7.35(d, J=7.2 Hz, 1H), 7.17(t, J=8.8 Hz, 2H), 7.08—6.95(m, 2H), 4.48(s, 2H) | 163.2, 160.8, 157.6, 152.6, 146.1, 138.8, 136.3, 133.6, 131.5, 129.3, 127.4, 125.6, 115.9, 115.7, 37.3 |
| 7m | 11.22(d, J=108.4 Hz, 2H), 7.86(dd, J=4.5, 3.6 Hz, 2H), 7.70—7.61(m, 2H), 7.57(d, J=7.3 Hz, 2H), 7.50(td, J=7.5, 1.4 Hz, 1H), 7.41(t, J=7.4 Hz, 2H), 7.34(d, J=7.2 Hz, 1H), 7.10—6.95(m, 2H), 4.61(s, 2H) | 161.5, 156.2, 152.5, 146.2, 140.9, 138.9, 136.3, 133.8, 131.0, 129.3, 129.0, 127.4, 125.6, 117.7, 112.3, 36.7 |
| 7n | 11.23(d, J=113.3 Hz, 2H), 7.86(d, J=8.0 Hz, 1H), 7.66(d, J=2.1 Hz, 1H), 7.57(d, J=7.3 Hz, 2H), 7.52(d, J=8.3 Hz, 1H), 7.44—7.37(m, 3H), 7.35(d, J=7.2 Hz, 1H), 7.08—6.95(m, 2H), 4.52(s, 2H) | 161.3, 156.7, 152.6, 146.1, 138.9, 136.3, 134.8, 134.1, 133.7, 133.1, 129.5, 129.3, 128.0, 127.4, 125.6, 35.7 |
| 7o | 11.21(d, J=147.4 Hz, 2H), 7.87(d, J=7.3 Hz, 1H), 7.58(d, J=7.4 Hz, 2H), 7.42(dt, J=14.7, 7.3 Hz, 2H), 7.38—7.23(m, 1H), 7.18—6.93(m, 2H), 2.71(s, 3H) | 160.1, 158.6, 152.6, 146.0, 138.8, 136.4, 129.4, 129.3 127.4, 125.6, 16.4 |
| 7p | 11.18(d, J=144.3 Hz, 2H), 7.86(d, J=7.6 Hz, 1H), 7.58(d, J=7.3 Hz, 2H), 7.41(t, J=7.4 Hz, 2H), 7.34(d, J=7.2 Hz, 1H), 7.09—6.95(m, 2H), 3.21(q, J=7.3 Hz, 2H), 1.35(t, J=7.3 Hz, 3H) | 160.4, 158.4, 152.6, 146.0, 138.8, 136.4, 129.3, 127.4, 125.6, 28.5, 15.3 |
| 7q | 11.20(d, J=148.3 Hz, 2H), 7.87(d, J=7.4 Hz, 1H), 7.58(d, J=7.7 Hz, 2H), 7.41(t, J=7.5 Hz, 2H), 7.34(d, J=7.3 Hz, 1H), 7.08—6.95(m, 2H), 3.18(t, J=7.1 Hz, 2H), 1.78—1.65(m, 2H), 0.99(t, J=7.3 Hz, 3H) | 160.5, 158.6, 152.6, 146.1, 138.8, 136.5, 129.3, 127.4, 125.6, 36.0, 22.9, 13.4 |
| 7r | 11.16(d, J=141.1 Hz, 2H), 7.85(d, J=7.7 Hz, 1H), 7.57(d, J=7.3 Hz, 2H), 7.42—7.39(m, 2H), 7.34(d, J=7.3 Hz, 1H), 7.08—6.96(m, 2H), 3.20(t, J=7.3 Hz, 2H), 1.67(dt, J=14.8, 7.4 Hz, 2H), 1.41(dq, J=14.6, 7.3 Hz, 2H), 0.90(t, J=7.4 Hz, 3H) | 160.4, 158.7, 152.5, 146.1, 138.8, 136.4, 129.4, 127.4, 15.8, 33.8, 31.5, 21.6, 13.9 |
| 7s | 11.20(d, J=145.6 Hz, 2H), 7.86(d, J=7.5 Hz, 1H), 7.58(d, J=7.2 Hz, 2H), 7.44—7.38(m, 3H), 7.08—6.96(m, 2H), 3.19(t, J=7.3 Hz, 2H), 1.74—1.63(m, 2H), 1.41—1.25(m, 4H), 0.87(t, J=7.2 Hz, 3H) | 160.4, 158.7, 152.6, 146.1, 138.8, 129.3, 128.0, 127.4, 125.7, 125.6, 34.0, 30.6, 29.1, 22.1, 14.3 |
Table 4 1H NMR and 13C NMR data of compounds 7a—7s
| Compd. | 1H NMR(DMSO, 400 MHz), δ | 13C NMR(DMSO, 100 MHz), δ |
|---|---|---|
| 7a | 11.17(d, J=127.5 Hz, 2H), 7.85(d, J=8.0 Hz, 1H), 7.57(d, J=7.3 Hz, 2H), 7.41(dt, J=7.5, 3.9 Hz, 4H), 7.38—7.31(m, 3H), 7.30—7.24(m, 1H), 7.08—6.95(m, 2H), 4.48(s, 2H) | 160.8,158.0, 152.6, 146.2, 138.9,137.2, 136.4, 129.4, 129.0, 128.0, 127.4, 125.6, 38.1 |
| 7b | 11.23(d, J=129.6 Hz, 2H), 7.87(d, J=7.4 Hz, 1H), 7.58(d, J=7.4 Hz, 2H), 7.53—7.48(m, 2H), 7.41(t, J=7.4 Hz, 2H), 7.33(ddd, J=12.3, 6.0, 2.3 Hz, 3H), 7.08—6.97(m, 2H), 4.55(s, 2H) | 161.2, 157.1, 152.5, 146.2, 138.9, 136.3, 134.7, 133.8, 131.9, 130.1, 129.3, 127.8, 127.4, 125.6, 36.3 |
| 7c | 11.22(d, J=118.1 Hz, 2H), 7.87(d, J=7.3 Hz, 1H), 7.58(d, J=7.4 Hz, 2H), 7.50(s, 1H), 7.43—7.33(m, 6H), 7.08—6.95(m, 2H), 4.49(s, 2H) | 161.0, 157.4, 152.5, 146.1, 140.1, 138.9, 136.3, 133.5, 130.8, 129.3, 128.2, 127.9, 127.4, 125.6, 37.2 |
| Compd. | 1H NMR(DMSO, 400 MHz), δ | 13C NMR(DMSO, 100 MHz), δ |
| 7d | 11.20(d, J=130.9 Hz, 2H), 7.86(d, J=7.9 Hz, 1H), 7.57(d, J=7.3 Hz, 2H), 7.42(td, J=8.4, 1.6 Hz, 6H), 7.34(d, J=7.2 Hz, 1H), 7.08—6.95(m, 2H), 4.48(s, 2H) | 160.9, 157.5, 152.5, 146.1, 138.9, 136.6, 136.3, 132.6, 131.3, 129.3, 129.0, 127.4, 125.6, 37.2 |
| 7e | 11.16(d, J=129.2 Hz, 2H), 7.85(d, J=7.5 Hz, 1H), 7.58(d, J=6.9 Hz, 2H), 7.41(t, J=7.4 Hz, 2H), 7.37—7.24(m, 3H), 7.09—6.95(m, 3H), 6.93—6.87(m, 1H), 4.40(s, 2H), 3.81(s, 3H) | 160.9, 158.3, 157.6, 152.6, 146.1, 138.8, 136.4, 130.8, 129.8, 129.3, 127.4, 125.6, 124.8, 120.7, 111.6, 56.0, 33.6 |
| 7f | 11.23(d, J=139.4 Hz, 2H), 7.87(d, J=6.5 Hz, 1H), 7.58(d, J=6.7 Hz, 2H), 7.37(dd, J=21.8, 6.6 Hz, 3H), 7.25(t, J=7.4 Hz, 1H), 7.00(t, J=10.5 Hz, 4H), 6.86(d, J=7.3 Hz, 1H), 4.45(s, 2H), 3.74(s, 3H) | 160.9, 159.7, 157.9, 152.5, 146.2, 138.8, 138.7, 136.4, 130.1, 129.3, 129.3, 127.4, 125.6, 121.6, 115.1, 113.5, 55.5, 38.1 |
| 7g | 11.22(d, J=128.7 Hz, 2H), 7.86(d, J=7.7 Hz, 1H), 7.57(d, J=7.4 Hz, 1H), 7.47—7.37(m, 2H), 7.32(t, J=8.4 Hz, 3H), 7.08—6.96(m, 2H), 6.89(dd, J=7.9, 4.2 Hz, 3H), 4.38(s, 2H), 3.73(s, 3H). | 160.8, 159.1, 156.1, 152.5, 146.2, 138.7, 136.4, 130.8, 129.4, 127.4, 125.6, 114.4, 55.5, 37.8 |
| 7h | 11.21(d, J=136.7 Hz, 2H), 7.86(d, J=6.9 Hz, 1H), 7.56(d, J=7.4 Hz, 2H), 7.39(t, J=7.4 Hz, 2H), 7.31(dd, J=9.8, 7.4 Hz, 2H), 7.22—7.10(m, 3H), 7.06—6.94(m, 2H), 4.47(s, 2H), 2.37(s, 3H) | 160.9, 157.7, 152.6, 146.1, 138.8, 137.2, 136.4, 134.6, 129.4, 129.3, 128.4, 127.4, 126.5, 125.6, 36.7, 19.2 |
| 7i | 11.20(d, J=128.1 Hz, 2H), 7.86(d, J=7.4 Hz, 1H), 7.57(d, J=7.2 Hz, 2H), 7.41(t, J=7.4 Hz, 2H), 7.33(t, J=7.3 Hz, 1H), 7.25—7.18(m, 3H), 7.12—7.05(m, 1H), 7.04—6.95(m, 2H), 4.44(s, 2H), 2.28(s, 3H) | 160.8, 158.0, 152.6, 146.1, 138.8, 138.2, 137.0, 136.3, 130.1, 129.4, 129.3, 128.9, 128.7, 127.4, 126.6, 125.6, 38.1, 21.4 |
| 7j | 11.17(d, J=134.6 Hz, 2H), 7.85(d, J=7.4 Hz, 1H), 7.56(d, J=7.1 Hz, 2H), 7.40(t, J=7.2 Hz, 2H), 7.31(dd, J=20.4, 7.4 Hz, 3H), 7.13(d, J=7.5 Hz, 2H), 7.09—6.93(m, 2H), 4.42(s, 2H), 2.27(s, 3H) | 160.9, 157.7, 152.6, 146.1, 130.9, 130.4, 129.3, 128.4, 127.4, 126.5, 125.6, 36.7, 19.2 |
| 7k | 11.22(d, J=107.8 Hz, 2H), 7.85(d, J=7.3 Hz, 1H), 7.56(d, J=7.3 Hz, 2H), 7.45(td, J=7.7, 1.6 Hz, 1H), 7.39(t, J=7.5 Hz, 2H), 7.34(dt, J=5.2, 2.0 Hz, 2H), 7.24—7.12(m, 2H), 7.06—6.95(m, 2H), 4.47(s, 2H) | 160.9, 161.2, 157.0, 152.6, 146.2, 138.9, 136.3, 131.8, 130.4, 129.3, 127.4, 125.6, 125.0, 124.3, 116.1, 115.9, 32.0 |
| 7l | 11.20(d, J=138.6 Hz, 2H), 7.87(d, J=7.5 Hz, 1H), 7.58(d, J=7.5 Hz, 2H), 7.50—7.44(m, 2H), 7.41(t, J=7.5 Hz, 2H), 7.35(d, J=7.2 Hz, 1H), 7.17(t, J=8.8 Hz, 2H), 7.08—6.95(m, 2H), 4.48(s, 2H) | 163.2, 160.8, 157.6, 152.6, 146.1, 138.8, 136.3, 133.6, 131.5, 129.3, 127.4, 125.6, 115.9, 115.7, 37.3 |
| 7m | 11.22(d, J=108.4 Hz, 2H), 7.86(dd, J=4.5, 3.6 Hz, 2H), 7.70—7.61(m, 2H), 7.57(d, J=7.3 Hz, 2H), 7.50(td, J=7.5, 1.4 Hz, 1H), 7.41(t, J=7.4 Hz, 2H), 7.34(d, J=7.2 Hz, 1H), 7.10—6.95(m, 2H), 4.61(s, 2H) | 161.5, 156.2, 152.5, 146.2, 140.9, 138.9, 136.3, 133.8, 131.0, 129.3, 129.0, 127.4, 125.6, 117.7, 112.3, 36.7 |
| 7n | 11.23(d, J=113.3 Hz, 2H), 7.86(d, J=8.0 Hz, 1H), 7.66(d, J=2.1 Hz, 1H), 7.57(d, J=7.3 Hz, 2H), 7.52(d, J=8.3 Hz, 1H), 7.44—7.37(m, 3H), 7.35(d, J=7.2 Hz, 1H), 7.08—6.95(m, 2H), 4.52(s, 2H) | 161.3, 156.7, 152.6, 146.1, 138.9, 136.3, 134.8, 134.1, 133.7, 133.1, 129.5, 129.3, 128.0, 127.4, 125.6, 35.7 |
| 7o | 11.21(d, J=147.4 Hz, 2H), 7.87(d, J=7.3 Hz, 1H), 7.58(d, J=7.4 Hz, 2H), 7.42(dt, J=14.7, 7.3 Hz, 2H), 7.38—7.23(m, 1H), 7.18—6.93(m, 2H), 2.71(s, 3H) | 160.1, 158.6, 152.6, 146.0, 138.8, 136.4, 129.4, 129.3 127.4, 125.6, 16.4 |
| 7p | 11.18(d, J=144.3 Hz, 2H), 7.86(d, J=7.6 Hz, 1H), 7.58(d, J=7.3 Hz, 2H), 7.41(t, J=7.4 Hz, 2H), 7.34(d, J=7.2 Hz, 1H), 7.09—6.95(m, 2H), 3.21(q, J=7.3 Hz, 2H), 1.35(t, J=7.3 Hz, 3H) | 160.4, 158.4, 152.6, 146.0, 138.8, 136.4, 129.3, 127.4, 125.6, 28.5, 15.3 |
| 7q | 11.20(d, J=148.3 Hz, 2H), 7.87(d, J=7.4 Hz, 1H), 7.58(d, J=7.7 Hz, 2H), 7.41(t, J=7.5 Hz, 2H), 7.34(d, J=7.3 Hz, 1H), 7.08—6.95(m, 2H), 3.18(t, J=7.1 Hz, 2H), 1.78—1.65(m, 2H), 0.99(t, J=7.3 Hz, 3H) | 160.5, 158.6, 152.6, 146.1, 138.8, 136.5, 129.3, 127.4, 125.6, 36.0, 22.9, 13.4 |
| 7r | 11.16(d, J=141.1 Hz, 2H), 7.85(d, J=7.7 Hz, 1H), 7.57(d, J=7.3 Hz, 2H), 7.42—7.39(m, 2H), 7.34(d, J=7.3 Hz, 1H), 7.08—6.96(m, 2H), 3.20(t, J=7.3 Hz, 2H), 1.67(dt, J=14.8, 7.4 Hz, 2H), 1.41(dq, J=14.6, 7.3 Hz, 2H), 0.90(t, J=7.4 Hz, 3H) | 160.4, 158.7, 152.5, 146.1, 138.8, 136.4, 129.4, 127.4, 15.8, 33.8, 31.5, 21.6, 13.9 |
| 7s | 11.20(d, J=145.6 Hz, 2H), 7.86(d, J=7.5 Hz, 1H), 7.58(d, J=7.2 Hz, 2H), 7.44—7.38(m, 3H), 7.08—6.96(m, 2H), 3.19(t, J=7.3 Hz, 2H), 1.74—1.63(m, 2H), 1.41—1.25(m, 4H), 0.87(t, J=7.2 Hz, 3H) | 160.4, 158.7, 152.6, 146.1, 138.8, 129.3, 128.0, 127.4, 125.7, 125.6, 34.0, 30.6, 29.1, 22.1, 14.3 |
| Compd. | Inhibition rate(%) | |||||
|---|---|---|---|---|---|---|
| X. oryzae | R. solanacearum | X. citri | ||||
| 200 μg/mL | 100 μg/mL | 200 μg/mL | 100 μg/mL | 200 μg/mL | 100 μg/mL | |
| 7a | 81.02±1.15 | 72.38±0.35 | 90.78±0.47 | 61.56±0.38 | 61.72±2.45 | 36.35±0.32 |
| 7b | 88.34±0.65 | 67.62±1.36 | 71.35±4.43 | 57.32±0.58 | 46.26±1.25 | 28.11±2.23 |
| 7c | 72.13±1.25 | 47.04±1.21 | 58.63±1.19 | 31.25±1.28 | 58.47±0.57 | 39.04±0.92 |
| 7d | 81.56±0.11 | 59.92±0.45 | 66.27±0.66 | 51.33±0.58 | 41.35±6.34 | 19.06±0.35 |
| 7e | 90.00±0.23 | 82.11±1.32 | 91.92±1.03 | 78.83±1.24 | 89.62±2.15 | 72.37±7.14 |
| 7f | 74.56±0.12 | 43.22±0.72 | 69.33±1.11 | 56.46±0.26 | 66.25±1.02 | 34.32±0.15 |
| 7g | 96.00±0.33 | 73.11±0.26 | 93.22±0.66 | 88.17±0.15 | 75.11±0.24 | 41.31±0.44 |
| 7h | 92.12±0.15 | 80.05±0.65 | 88.13±1.15 | 62.05±0.45 | 79.63±0.65 | 58.59±6.42 |
| 7i | 100.03±0.24 | 100.17±1.44 | 91.03±8.24 | 79.82±4.06 | 87.25±6.44 | 66.93±1.43 |
| 7j | 100.82±0.75 | 100.08±1.02 | 100.18±5.45 | 100.25±8.15 | 100.06±8.25 | 100.32±4.36 |
| 7k | 100.02±1.16 | 100.15±1.21 | 100.34±2.83 | 100.01±6.54 | 100.25±1.93 | 100.17±8.04 |
| 7l | 54.13±0.61 | 39.57±2.35 | 81.03±8.20 | 57.24±0.26 | 100.02±2.92 | 100.28±3.15 |
| 7m | 62.78±0.72 | 39.52±1.22 | 82.93±4.21 | 64.82±5.42 | 66.46±9.01 | 47.82±4.33 |
| 7n | 100.33±0.43 | 100.01±1.15 | 100.32±0.14 | 100.35±5.00 | 62.39±6.34 | 35.52±0.25 |
| 7o | 71.22±0.15 | 42.38±0.55 | 60.88±0.37 | 31.66±1.38 | 62.52±1.45 | 31.35±0.22 |
| 7p | 56.04±1.25 | 39.88±0.75 | 100.09±4.34 | 100.62±4.13 | 59.77±2.36 | 38.36±9.23 |
| 7q | 67.26±0.55 | 59.65±2.42 | 100.26±5.82 | 100.35±6.74 | 100.05±3.11 | 100.14±2.17 |
| 7r | 100.06±1.66 | 100.27±1.03 | 100.02±0.32 | 100.52±2.11 | 63.22±0.23 | 39.37±0.11 |
| 7s | 61.11±0.34 | 42.66±2.16 | 100.61±1.23 | 100.86±8.07 | 67.42±0.14 | 48.73±8.14 |
| Bismerthiazolb | 74.25±0.45 | 53.11±0.65 | 100.26±1.17 | 100.02±4.53 | 70.22±6.23 | 48.85±7.42 |
| Thiodiazole-copperb | 70.17±1.35 | 41.56 ±1.01 | 55.63±1.20 | 41.32±5.39 | 63.51±8.15 | 44.27±3.46 |
Table 5 Inhibition rates of the title compounds 7a—7pa
| Compd. | Inhibition rate(%) | |||||
|---|---|---|---|---|---|---|
| X. oryzae | R. solanacearum | X. citri | ||||
| 200 μg/mL | 100 μg/mL | 200 μg/mL | 100 μg/mL | 200 μg/mL | 100 μg/mL | |
| 7a | 81.02±1.15 | 72.38±0.35 | 90.78±0.47 | 61.56±0.38 | 61.72±2.45 | 36.35±0.32 |
| 7b | 88.34±0.65 | 67.62±1.36 | 71.35±4.43 | 57.32±0.58 | 46.26±1.25 | 28.11±2.23 |
| 7c | 72.13±1.25 | 47.04±1.21 | 58.63±1.19 | 31.25±1.28 | 58.47±0.57 | 39.04±0.92 |
| 7d | 81.56±0.11 | 59.92±0.45 | 66.27±0.66 | 51.33±0.58 | 41.35±6.34 | 19.06±0.35 |
| 7e | 90.00±0.23 | 82.11±1.32 | 91.92±1.03 | 78.83±1.24 | 89.62±2.15 | 72.37±7.14 |
| 7f | 74.56±0.12 | 43.22±0.72 | 69.33±1.11 | 56.46±0.26 | 66.25±1.02 | 34.32±0.15 |
| 7g | 96.00±0.33 | 73.11±0.26 | 93.22±0.66 | 88.17±0.15 | 75.11±0.24 | 41.31±0.44 |
| 7h | 92.12±0.15 | 80.05±0.65 | 88.13±1.15 | 62.05±0.45 | 79.63±0.65 | 58.59±6.42 |
| 7i | 100.03±0.24 | 100.17±1.44 | 91.03±8.24 | 79.82±4.06 | 87.25±6.44 | 66.93±1.43 |
| 7j | 100.82±0.75 | 100.08±1.02 | 100.18±5.45 | 100.25±8.15 | 100.06±8.25 | 100.32±4.36 |
| 7k | 100.02±1.16 | 100.15±1.21 | 100.34±2.83 | 100.01±6.54 | 100.25±1.93 | 100.17±8.04 |
| 7l | 54.13±0.61 | 39.57±2.35 | 81.03±8.20 | 57.24±0.26 | 100.02±2.92 | 100.28±3.15 |
| 7m | 62.78±0.72 | 39.52±1.22 | 82.93±4.21 | 64.82±5.42 | 66.46±9.01 | 47.82±4.33 |
| 7n | 100.33±0.43 | 100.01±1.15 | 100.32±0.14 | 100.35±5.00 | 62.39±6.34 | 35.52±0.25 |
| 7o | 71.22±0.15 | 42.38±0.55 | 60.88±0.37 | 31.66±1.38 | 62.52±1.45 | 31.35±0.22 |
| 7p | 56.04±1.25 | 39.88±0.75 | 100.09±4.34 | 100.62±4.13 | 59.77±2.36 | 38.36±9.23 |
| 7q | 67.26±0.55 | 59.65±2.42 | 100.26±5.82 | 100.35±6.74 | 100.05±3.11 | 100.14±2.17 |
| 7r | 100.06±1.66 | 100.27±1.03 | 100.02±0.32 | 100.52±2.11 | 63.22±0.23 | 39.37±0.11 |
| 7s | 61.11±0.34 | 42.66±2.16 | 100.61±1.23 | 100.86±8.07 | 67.42±0.14 | 48.73±8.14 |
| Bismerthiazolb | 74.25±0.45 | 53.11±0.65 | 100.26±1.17 | 100.02±4.53 | 70.22±6.23 | 48.85±7.42 |
| Thiodiazole-copperb | 70.17±1.35 | 41.56 ±1.01 | 55.63±1.20 | 41.32±5.39 | 63.51±8.15 | 44.27±3.46 |
| Compd. | X. oryzae | R. solanacearu | X. citri | ||||||
|---|---|---|---|---|---|---|---|---|---|
| EC50/ (μg·mL-1) | Regression equation | r | EC50/ (μg·mL-1) | Regression equation | r | EC50/ (μg·mL-1) | Regression equation | r | |
| 7e | 38.02±2.15 | y=1.7584x+2.2116 | 0.97 | 47.86±0.64 | y=2.1183x+1.4312 | 0.97 | 67.20±2.24 | y=1.7527x+1.8076 | 0.98 |
| 7g | 36.88±0.29 | y=2.0126x+1.8664 | 0.95 | 63.09±0.45 | y=1.7527x+1.8076 | 0.96 | — | — | — |
| 7h | 41.30±2.51 | y=1.8947x+1.9382 | 0.98 | — | — | — | — | — | — |
| 7i | 63.09±1.71 | y=2.3573x+0.7284 | 0.96 | — | — | — | — | — | — |
| 7j | 15.13±0.64 | y=2.9303x+1.5332 | 0.96 | 30.90±1.03 | y=1.9523x+2.0932 | 0.96 | 24.49±0.61 | y=1.5289x+2.8762 | 0.99 |
| 7k | 21.33±1.44 | y=1.1424x+3.4811 | 0.97 | 24.54±0.53 | y=1.5243x+2.8742 | 0.97 | 14.79±1.82 | y=3.4379x+0.9771 | 0.98 |
| 7l | — | — | — | — | — | — | 51.28±3.21 | y=1.4098x+2.5822 | 0.99 |
| 7n | 75.36±1.73 | y=2.1535x+0.9522 | 0.96 | 86.09±2.13 | y=1.4033x+2.2843 | 0.98 | — | — | — |
| 7p | — | — | — | 37.15±2.35 | y=1.2443x+3.0454 | 0.97 | — | — | — |
| 7q | — | — | — | 23.98±1.41 | y=2.8346x+1.0746 | 0.98 | 48.86±2.21 | y=1.9589x+1.6904 | 0.97 |
| 7r | 36.89±2.12 | y=2.5243x+1.0433 | 0.97 | 39.81±2.82 | y=1.3243x+2.8753 | 0.96 | — | — | — |
| 7s | — | — | — | 56.75±0.61 | y=1.8564x+1.7434 | 0.98 | — | — | — |
| Bismert- | 92.23±0.7 | y=1.5038x+2.0433 | 0.98 | 58.88±1.01 | y=1.2144x+2.8443 | 0.99 | 123.02±1.11 | y=1.5143x+1.8278 | 0.99 |
| hiazol | |||||||||
Table 6 Antibacterial activities of some title compounds
| Compd. | X. oryzae | R. solanacearu | X. citri | ||||||
|---|---|---|---|---|---|---|---|---|---|
| EC50/ (μg·mL-1) | Regression equation | r | EC50/ (μg·mL-1) | Regression equation | r | EC50/ (μg·mL-1) | Regression equation | r | |
| 7e | 38.02±2.15 | y=1.7584x+2.2116 | 0.97 | 47.86±0.64 | y=2.1183x+1.4312 | 0.97 | 67.20±2.24 | y=1.7527x+1.8076 | 0.98 |
| 7g | 36.88±0.29 | y=2.0126x+1.8664 | 0.95 | 63.09±0.45 | y=1.7527x+1.8076 | 0.96 | — | — | — |
| 7h | 41.30±2.51 | y=1.8947x+1.9382 | 0.98 | — | — | — | — | — | — |
| 7i | 63.09±1.71 | y=2.3573x+0.7284 | 0.96 | — | — | — | — | — | — |
| 7j | 15.13±0.64 | y=2.9303x+1.5332 | 0.96 | 30.90±1.03 | y=1.9523x+2.0932 | 0.96 | 24.49±0.61 | y=1.5289x+2.8762 | 0.99 |
| 7k | 21.33±1.44 | y=1.1424x+3.4811 | 0.97 | 24.54±0.53 | y=1.5243x+2.8742 | 0.97 | 14.79±1.82 | y=3.4379x+0.9771 | 0.98 |
| 7l | — | — | — | — | — | — | 51.28±3.21 | y=1.4098x+2.5822 | 0.99 |
| 7n | 75.36±1.73 | y=2.1535x+0.9522 | 0.96 | 86.09±2.13 | y=1.4033x+2.2843 | 0.98 | — | — | — |
| 7p | — | — | — | 37.15±2.35 | y=1.2443x+3.0454 | 0.97 | — | — | — |
| 7q | — | — | — | 23.98±1.41 | y=2.8346x+1.0746 | 0.98 | 48.86±2.21 | y=1.9589x+1.6904 | 0.97 |
| 7r | 36.89±2.12 | y=2.5243x+1.0433 | 0.97 | 39.81±2.82 | y=1.3243x+2.8753 | 0.96 | — | — | — |
| 7s | — | — | — | 56.75±0.61 | y=1.8564x+1.7434 | 0.98 | — | — | — |
| Bismert- | 92.23±0.7 | y=1.5038x+2.0433 | 0.98 | 58.88±1.01 | y=1.2144x+2.8443 | 0.99 | 123.02±1.11 | y=1.5143x+1.8278 | 0.99 |
| hiazol | |||||||||
| [1] | Niu C., Li G., Mai D.N., Chem. J. Chinese Universities, 2014, 35(6), 1204—1211 |
| (牛超, 李根, 买迪娜. 高等学校化学学报, 2014, 35(6), 1204—1211) | |
| [2] | Beaudoin D., Maris T., Wuest J.D., J. Chem. Pharm. Res., 2013, 5(11), 830—834 |
| [3] | Singh H.P., Pandeya S. N., Chandra C. S., Sharma C. S, Med. Chem. Res., 2011, 20(1), 74—80 |
| [4] | Singh H.P., Chanuhan C. S., Pandeya S. N., Sharma C. S., Srivastava B., Singhal M, Der. Pharm. Lett., 2010, 2(2), 460—462 |
| [5] | Singhal M., Paul A.,Singh P.H., Dubey S. K., Songara R. K., Int. J. Pharmaceut. Sci. Drug Res., 2011, 3(2), 150—154 |
| [6] | Jafri L., Ansari F.L., Jamil M., Kalsoom S., Qureishi S., Mirza B, Chem. Biol. Drug Des., 2012, 79(6), 950—959 |
| [7] | Singhal M., Paul A., Internal. J. Chem. Pharm. Res., 2011, 10(2), 2602—2604 |
| [8] | Demirbas N., Karaoglu S.A., Demirbas A., Sanca K., Eur. J. Med. Chem., 2004, 39(9), 793—804 |
| [9] | Wang M.J., Lu J. R., Xin C. W., Liu J. B., Mu J. B., Zhang H., Zhang R. B., Yang X. Y., Wang H. W., Chem. J. Chinese Universities, 2015, 36(3), 469—476 |
| (王美君, 卢俊瑞, 辛春伟, 刘金彪, 穆江蓓, 张贺, 张瑞波, 杨旭云, 王宏韫. 高等学校化学学报, 2015, 36(3), 469—476) | |
| [10] | Azam M.A., Kumar B. R. P., Shalinis S., Surech B., Reddy T. K., Reddy C. D., Indian. J. Pharm. Sci., 2008, 70(5), 672—677 |
| [11] | Foroumadi A., Emami S., Hassanzadeh A., Rajaee M., Sokhanvar K., Moshafi M.H., Bioorg. Med. Chem. Lett., 2005, 15(20), 4488—4492 |
| [12] | Chou J.Y., Lai S. Y., Pan S. L., Jow G. M., Chern J. W., Guh J. H, Biochem. Pharmacol., 2003, 66(1), 115—124 |
| [13] | Martinez A., Alonso D., Castro A., Aran V.J., Cardelus I., Banos E, Arch. Pharm., 2010, 332(6), 191—194 |
| [14] | Jin G.Y., Hou Z., Zhao G. F., Cao C. Y., Li Y. C., Chem. J. Chinese Universities, 1997, 18(4), 409—412 |
| (金桂玉, 侯震, 赵国峰, 曹春阳, 李煜昶. 高等学校化学学报, 1997, 18(4), 409—412) | |
| [15] | Raj M.M., Patel H. V., Raj L. M., Patel N. K., Int. J. Pharm, Chem. Biol. Sci., 2013, 3(3), 814—819 |
| [16] | Zhao J., Chen B.Q., Shi Y. P., Shi Y. P., Liu Y. M., Zhao H. C., Cheng J., Chinese Chem. Lett., 2012, 23(7), 817—819 |
| [17] | Sheng C.Q., Che X. Y., Wang W. Y., Wang S. Z., Cao Y. B., Miao Z. Y., Yao J. Z., Zhang W. N., Eur. J. Med. Chem., 2011, 46(11), 5276—5282 |
| [18] | Ma J.J., Bao G. L., Wang L. M., Li W. T., Xu B. X., Du B. Q., Lv J., Zhai X., Gong P., Eur. J. Med. Chem., 2015, 96, 173—186 |
| [19] | Mortimer D., Whiting M., Harrity J.P. A., Jones S., Coldham L, Tetrahedron Lett., 2014, 55(6), 1255—1257 |
| [20] | Cami G.E., González M. L., Ruiz F. S., Pedregosa J. C., J. Phys. Chem. Solids, 2005, 66(6), 936—945 |
| [21] | Liu H.L., Wu R. M., Sun Y. Y., Ye Y., Chen J., Luo X. M., Shen X., Liu H, Bioorg. Med. Chem., 2014, 22(22), 6344—6352 |
| [22] | Shen L.H., Li H. Y., Shang H. X., Tian S. T., Lai Y. S., Liu L. J., Chinese Chem. Lett., 2013, 24(4), 299—302 |
| [23] | Zhao J., Xuan L.N., Zhao H. C., Cheng J., Fu X. Y., Li S., Jing F., Liu Y. M., Chen B. Q., Chem. Res. Chinese Universities, 2014, 30(5), 764—769 |
| [24] | Foroumadi A., Firoozpour L., Emami S., Mansouri S., Ebrahimabadi A.H., Asadipour A., Amini M., Saeid-Adeli N., Shafiee A, Arch. Pharm. Res., 2007, 30(2), 138—145 |
| [25] | Zhao J., Chen B.Q., Shi Y. P., Liu Y. M., Zhao H. C., Cheng J., Chinese Chem. Lett., 2012, 23(7), 817—819 |
| [26] | Su S.H., Zhou X., Liao G. P., Qi P. Y., Jin L. H., Molecules, 2016, 22(1), 64—81 |
| [1] | HU Haocheng, LI Wenli, ZHANG Jianing, LIU Yubo. Extraction, Structure Characterization and Biological Activities of Oligosaccharides from Auricularia heimuer [J]. Chem. J. Chinese Universities, 2021, 42(8): 2465. |
| [2] | MAO Long, LIU Yuejun, FAN Shuhong. Preparation and Properties of Polypyrrole Modified Layered Clay/poly(ε-caprolactone) Antibacterial Nanocomposites [J]. Chem. J. Chinese Universities, 2019, 40(8): 1726. |
| [3] | LI Pu,CHEN Ying,XIA Rongjiao,GUO Tao,ZHANG Min,JIANG Shichun,ANG Xu,HE Ming,XUE Wei. Synthesis and Biological Activities of Myricetin Derivatives Containing Quinoxaline† [J]. Chem. J. Chinese Universities, 2019, 40(5): 909. |
| [4] | LI Bing,WANG Xuemin,BAI Fengying,LIU Shuqing. Synthesises, Structures and Antibacterial Activities of a Series of Rare Earth Nitrogen Heterocyclic Complexes† [J]. Chem. J. Chinese Universities, 2019, 40(4): 632. |
| [5] | JIA Yunjing, SHI Wensi, HU Feiliu, ZHU Huajie, LIU Li, MA Zhengyue. Cytotoxic Activity of Trichothecene Compounds and Derivatives from Myrothecium sp.† [J]. Chem. J. Chinese Universities, 2018, 39(8): 1668. |
| [6] | ZHANG Bingyang, MA Yangyang, GUO Hua, ZHU Huajie, LI Wan. Absolute Configuration Determination of Two Drimane Sesquiterpenoids from the Endophytic Fungi Talaromyces Purpureogenus of Panax notoginseng† [J]. Chem. J. Chinese Universities, 2017, 38(6): 1046. |
| [7] | DONG Xiaoming, YUAN Baoming, CHEN Bingpeng, HE Chaoliang, WANG Jincheng, CHEN Xuesi. In vitro Antibacterial Activity of PLGA-PEG-PLGA Thermosensitive Hydrogels Loaded with Vancomycin [J]. Chem. J. Chinese Universities, 2017, 38(5): 866. |
| [8] | FU Ranran, JI Xiujie, LIU Chao, REN Yanfei, WANG Gang, CHENG Bowen. Fabrication of Cellulose/Nano Lamellar ZnO Composite Antibacterial Fibers Using Ionic Liquid† [J]. Chem. J. Chinese Universities, 2017, 38(12): 2344. |
| [9] | MA Yangyang, CAI Hui, DU Min, CAO Fei, ZHU Huajie. Fractionation of Azaphilones from Secondary Metabolites of Marine-Derived Fungus Penicillim pinophilum and Their Antibacterial Activity† [J]. Chem. J. Chinese Universities, 2017, 38(11): 1963. |
| [10] | XIAO Wei, RUAN Xianghui, LI Qin, ZHANG Juping, ZHONG Xinmin, XIE Yan, WANG Xiaobin, HUANG Minguo, XUE Wei. Synthesis and Antibacterial Activities of Myricetin Derivatives ontaining Acidamide Moiety† [J]. Chem. J. Chinese Universities, 2017, 38(1): 35. |
| [11] | GAO Tong, CAI Siyuan, XU Lanlan, CAO Fei, ZHU Huajie. Citrinin Derivatives from Marine-derived Fungus Penicillim Grisefulvum and Antibacterial Activity† [J]. Chem. J. Chinese Universities, 2016, 37(7): 1282. |
| [12] | WANG Hongyun, LIU Jinbiao, LU Junrui, YING Ming, YANG Xuyun, YANG Shuxun, MA Yao. Synthesis and Antibacterial Activities of 5-Methyl-1,2,4-triazole-3-thione Glucosides Compounds† [J]. Chem. J. Chinese Universities, 2016, 37(2): 246. |
| [13] | XU Lanlan, ZHAO Qiqi, YU He, WANG Jingchen, WANG Huijun, YANG Qin, ZHU Huajie, LI Yan. Absolute Configuration Determination of One New Compound Trichoderol A from Trichoderma sp. Fungus† [J]. Chem. J. Chinese Universities, 2016, 37(11): 1972. |
| [14] | ZHANG Ruibo, LU Junrui, LIU Jinbiao, MU Jiangbei, YANG Xuyun, WANG Hongyun, WANG Meijun, ZHANG He, ZHANG Mei. Synthesis and Antibacterial Activities of 3-S-(β-D-Glucosides)-1,2,4-triazole† [J]. Chem. J. Chinese Universities, 2015, 36(8): 1521. |
| [15] | WU Yao, LIANG Wenjing, LI Cheng, SHANG Xu, CONG Lina. Effects of Temperature on the Antibacterial Activity and Structural Change of the C-terminal Polypeptide of the Sea Cucumber Lysozyme† [J]. Chem. J. Chinese Universities, 2014, 35(5): 1044. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||