

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (5): 909.doi: 10.7503/cjcu20180828
• Organic Chemistry • Previous Articles Next Articles
LI Pu, CHEN Ying, XIA Rongjiao, GUO Tao, ZHANG Min, JIANG Shichun, ANG Xu, HE Ming, XUE Wei*( )
)
Received:2018-12-10
Online:2019-04-12
Published:2019-04-12
Contact:
XUE Wei
E-mail:wxue@gzu.edu.cn
Supported by:CLC Number:
TrendMD:
LI Pu,CHEN Ying,XIA Rongjiao,GUO Tao,ZHANG Min,JIANG Shichun,ANG Xu,HE Ming,XUE Wei. Synthesis and Biological Activities of Myricetin Derivatives Containing Quinoxaline†[J]. Chem. J. Chinese Universities, 2019, 40(5): 909.
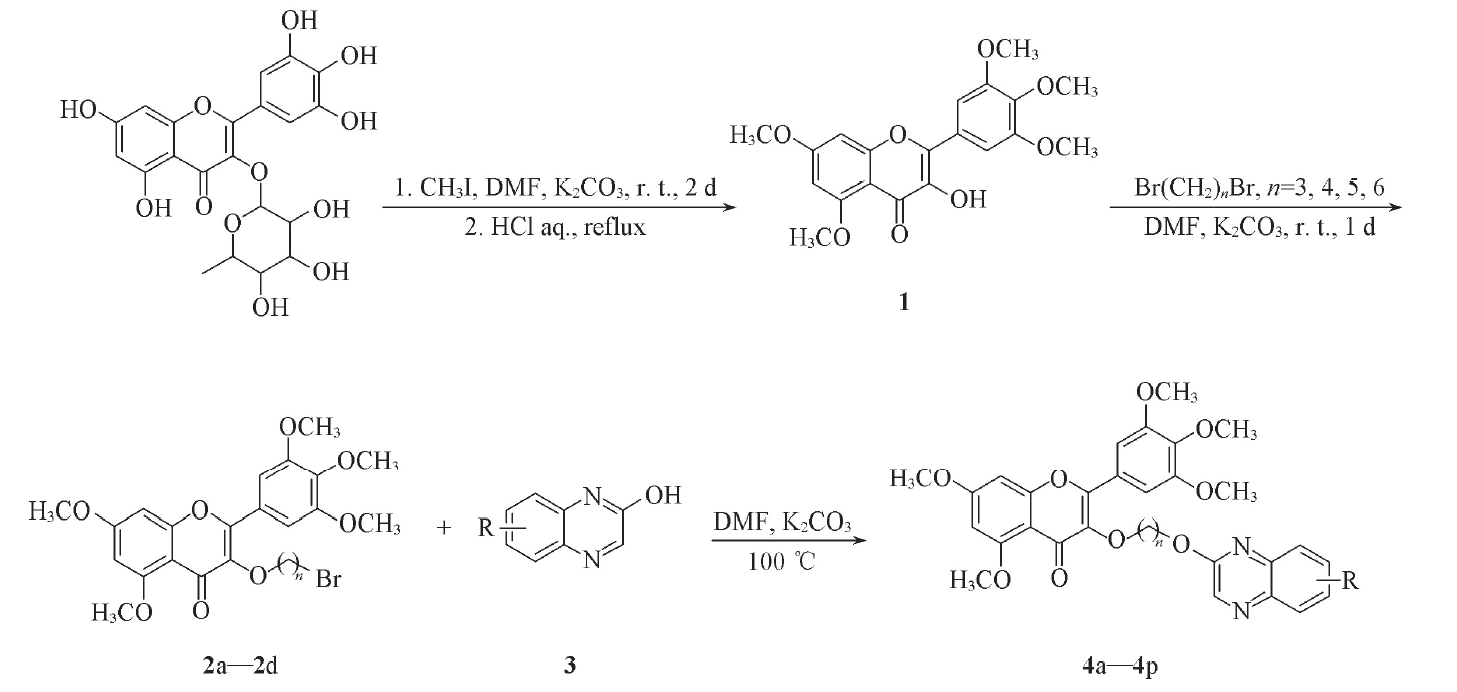
Scheme 1 Synthetic routes of target compounds 4a─4p2a: n=3; 2b: n=4; 2c: n=5; 2d: n=6; 4a: n=3, R=H; 4b: n=3, R=3-CH3; 4c: n=3, R=6-Cl; 4d: n=3, R=3-OH; 4e: n=4, R=H; 4f: n=4, R=3-CH3; 4g: n=4, R=6-Cl; 4h: n=4, R=3-OH; 4i: n=5, R=H; 4j: n=5, R=3-CH3; 4k: n=5, R=6-Cl; 4l: n=5, R=3-OH; 4m: n=6, R=H; 4n: n=6, R=3-CH3; 4o: n=6, R=6-Cl; 4p: n=6, R=3-OH
| Compd. | Appearance | Yield(%) | m. p./℃ | HRMS(calcd. ), m/z[M+H]+ |
|---|---|---|---|---|
| 1 | Yellow solid | 48.8 | 228—230(227—229[ | |
| 2a | White solid | 83.4 | 138—140(141—142[ | |
| 2b | White solid | 71.2 | 152—153(154—156[ | |
| 2c | White solid | 65.5 | 149—151 | |
| 2d | White solid | 58.1 | 150—152 | |
| 4a | White solid | 45.3 | 79—81 | 575.20241(575.20215) |
| 4b | Yellow solid | 63.2 | 98—100 | 589.21806(589.21686) |
| 4c | Yellow solid | 48.7 | 164—166 | 609.16343(609.16229) |
| 4d | Yellow solid | 52.2 | 113—115 | 613.17927(613.17822) |
| 4e | Yellow solid | 47.6 | 58—60 | 589.21806(589.21747) |
| 4f | Yellow solid | 49.4 | 103—105 | 603.23371(603.23242) |
| 4g | Yellow solid | 57.3 | 124—126 | 623.17908(623.17780) |
| 4h | Yellow solid | 54.5 | 84—86 | 605.21297(605.21216) |
| 4i | Yellow solid | 41.7 | 152—154 | 603.23371(603.23236) |
| 4j | Yellow solid | 51.6 | 89—91 | 617.24936(617.24792) |
| 4k | Yellow solid | 60.3 | 131—133 | 659.17668(659.17493) |
| 4l | Yellow solid | 56.0 | 88—90 | 605.21297(605.21216) |
| 4m | White solid | 44.1 | 96—98 | 655.20524(655.20654) |
| 4n | White solid | 53.2 | 127—129 | 631.26501(631.26349) |
| 4o | Yellow solid | 57.4 | 83—85 | 651.21038(651.21038) |
| 4p | White solid | 48.6 | 76—78 | 633.24427(633.24274) |
Table 1 Appearance, yields, melting points and HRMS data of compounds 1, 2a—2d and 4a—4p
| Compd. | Appearance | Yield(%) | m. p./℃ | HRMS(calcd. ), m/z[M+H]+ |
|---|---|---|---|---|
| 1 | Yellow solid | 48.8 | 228—230(227—229[ | |
| 2a | White solid | 83.4 | 138—140(141—142[ | |
| 2b | White solid | 71.2 | 152—153(154—156[ | |
| 2c | White solid | 65.5 | 149—151 | |
| 2d | White solid | 58.1 | 150—152 | |
| 4a | White solid | 45.3 | 79—81 | 575.20241(575.20215) |
| 4b | Yellow solid | 63.2 | 98—100 | 589.21806(589.21686) |
| 4c | Yellow solid | 48.7 | 164—166 | 609.16343(609.16229) |
| 4d | Yellow solid | 52.2 | 113—115 | 613.17927(613.17822) |
| 4e | Yellow solid | 47.6 | 58—60 | 589.21806(589.21747) |
| 4f | Yellow solid | 49.4 | 103—105 | 603.23371(603.23242) |
| 4g | Yellow solid | 57.3 | 124—126 | 623.17908(623.17780) |
| 4h | Yellow solid | 54.5 | 84—86 | 605.21297(605.21216) |
| 4i | Yellow solid | 41.7 | 152—154 | 603.23371(603.23236) |
| 4j | Yellow solid | 51.6 | 89—91 | 617.24936(617.24792) |
| 4k | Yellow solid | 60.3 | 131—133 | 659.17668(659.17493) |
| 4l | Yellow solid | 56.0 | 88—90 | 605.21297(605.21216) |
| 4m | White solid | 44.1 | 96—98 | 655.20524(655.20654) |
| 4n | White solid | 53.2 | 127—129 | 631.26501(631.26349) |
| 4o | Yellow solid | 57.4 | 83—85 | 651.21038(651.21038) |
| 4p | White solid | 48.6 | 76—78 | 633.24427(633.24274) |
| Compd. | 1H NMR(400 MHz), δ | 13C NMR(101 MHz), δ |
|---|---|---|
| 2c | 7.37(s, 2H), 6.81(s, 1H), 6.48(s, 1H), 3.94(t, J=5.6 Hz, 2H), 3.90(s, 3H), 3.88(s, 6H), 3.85(s, 3H), 3.77(s, 3H), 3.48(t, J=6.7 Hz, 2H), 1.89—1.72(m, 2H), 1.71—1.62(m, 2H), 1.51—1.40(m, 2H) | |
| 2d | 7.37(s, 2H), 6.84(s, 1H), 6.49(s, 1H), 3.95(t, J=6.0 Hz, 2H), 3.90(s, 3H), 3.87(s, 6H), 3.85(s, 3H), 3.76(s, 3H), 3.58(t, J=6.6 Hz, 2H), 1.67—1.64(m, 2H), 1.58—1.47(m, 2H), 1.43—1.35(m, 2H), 1.29—1.23(m, 2H) | |
| 4a | 8.53(s, 1H), 8.00(d, J=9.2 Hz, 1H), 7.82(d, J=8.6 Hz, 1H), 7.76(t, J=7.6 Hz, 1H), 7.64(t, J=6.8 Hz, 1H), 7.39(s, 1H), 7.38(s, 1H), 6.83(s, 1H), 6.49(s, 1H), 4.54(t, J=6.6 Hz, 2H), 4.16(t, J=6.1 Hz, 2H), 3.90(s, 3H), 3.85(s, 6H), 3.84(s, 3H), 3.70(s, 3H), 2.28—2.07(m, 2H) | 172.6, 164.2, 160.7, 158.6, 157.4, 153.2, 152.2, 140.3, 140.2, 140.1, 139.9, 138.8, 130.9, 129.1, 127.3, 127.2, 126.0, 108.9, 106.2, 96.4, 93.6, 69.0, 63.9, 60.6, 56.6, 56.5, 29.6 |
| 4b | 7.88(d, J=8.2 Hz, 1H), 7.74(t, J=7.7 Hz, 1H), 7.65(d, J=6.8 Hz, 1H), 7.57(t, J=6.8 Hz, 1H), 7.39(s, 1H), 7.36(s, 1H), 6.83(s, 1H), 6.46(s, 1H), 4.53(t, J=6.4 Hz, 2H), 4.09(t, J=6.2 Hz, 2H), 3.91(s, 3H), 3.85(s, 6H), 3.84(s, 3H), 3.70(s, 3H), 2.43(s, 3H), 2.27—2.06(m, 2H) | 172.6, 164.2, 160.7, 158.6, 156.2, 154.6, 153.1, 152.1, 148.3, 140.4, 139.6, 138.4, 129.5, 128.2, 126.9, 126.0, 123.7, 108.9, 106.2, 96.3, 93.4, 69.9, 68.9, 63.7, 60.5, 56.4, 29.6, 20.4 |
| Compd. | 1H NMR(400 MHz), δ | 13C NMR(101 MHz), δ |
| 4c | 8.57(s, 1H), 8.07(s, 1H), 7.81(d, J=12.0 Hz, 1H), 7.67(d, J=11.3 Hz, 1H), 7.38(s, 1H), 7.36(s, 1H), 6.81(s, 1H), 6.48(s, 1H), 4.53(t, J=6.5 Hz, 2H), 4.07(t, J=6.2 Hz, 2H), 3.89(s, 3H), 3.85(s, 6H), 3.84(s, 3H), 3.70(s, 3H), 2.24—2.00(m, 2H) | 172.6, 164.2, 160.8, 160.7, 158.6, 157.7, 154.4, 153.2, 153.1, 152.1, 141.5, 140.3, 140.2, 139.8, 139.1, 138.9, 134.1, 131.2, 129.1, 128.0, 126.0, 108.9, 106.2, 96.4, 93.5, 68.9, 64.2, 60.6, 60.6, 56.5, 29.5 |
| 4d | 7.69(d, J=10.0 Hz, 1H), 7.43(d, J=9.8 Hz, 1H), 7.40(s, 1H), 7.39(s, 1H), 7.25(t, J=6.6 Hz, 1H), 7.06(t, J=7.1 Hz, 1H), 6.86(s, 1H), 6.50(s, 1H), 4.27(t, J=6.8 Hz, 2H), 4.09(t, J=5.9 Hz, 2H), 3.91(s, 3H), 3.85(s, 3H), 3.83(s, 6H), 3.73(s, 3H), 2.07—2.00(m, 2H) | 172.6, 166.8, 164.3, 160.7, 158.7, 153.2, 152.1, 140.4, 140.2, 139.8, 133.5, 132.1, 129.6, 129.2, 126.3, 126.0, 108.9, 106.1, 96.4, 93.9, 93.5, 73.8, 65.4, 60.6, 56.5, 56.3, 29.5 |
| 4e | 8.54(s, 1H), 7.99(d, J=7.0 Hz, 1H), 7.81(d, J=8.3 Hz, 1H), 7.74(t, J=6.9 Hz, 1H), 7.62(t, J=6.8 Hz, 1H), 7.37(s, 2H), 6.81(s, 1H), 6.47(s, 1H), 4.45(t, J=6.3 Hz, 2H), 4.01(t, J=6.1 Hz, 2H), 3.90(s, 3H), 3.86(s, 6H), 3.84(s, 3H), 3.73(s, 3H), 1.95—1.89(m, 2H), 1.88—1.82(m, 2H) | 172.7, 164.2, 160.8, 158.6, 157.5, 153.2, 152.1, 140.4, 140.3, 140.1, 139.9, 138.8, 130.8, 129.1, 127.3, 127.2, 126.1, 108.9, 106.3, 96.4, 93.6, 71.7, 66.3, 60.6, 56.6, 56.5, 26.7, 25.4 |
| 4f | 7.87(d, J=8.1 Hz, 1H), 7.70(t, J=8.9 Hz, 1H), 7.60(d, J=7.0 Hz, 1H), 7.44(s, 1H), 7.41(s, 1H), 7.31(t, J=7.6 Hz, 1H), 6.66(s, 1H), 6.40(s, 1H), 4.52(t, J=6.2 Hz, 2H), 4.13(t, J=6.2 Hz, 2H), 3.91(s, 3H), 3.89(s, 6H), 3.86(s, 3H), 3.79(s, 3H), 2.45(s, 3H), 2.08—2.01(m, 2H), 1.96—1.91(m, 2H) | 173.7, 165.3, 159.9, 157.5, 154.5, 153.1, 153.1, 149.4, 141.4, 139.8, 134.0, 133.9, 130.8, 129.9, 129.3, 127.9, 124.2, 110.3, 107.2, 96.8, 94.0, 72.3, 67.3, 61.1, 56.8, 56.8, 28.1, 26.6, 20.9 |
| 4g | 8.43(s, 1H), 7.98(s, 1H), 7.79(d, J=10.3 Hz, 1H), 7.70(d, J=9.5 Hz, 1H), 7.37(s, 1H), 7.34(s, 1H), 6.82(s, 1H), 6.49(s, 1H), 4.44(t, J=6.4 Hz, 2H), 4.01(t, J=6.4 Hz, 2H), 3.90(s, 3H), 3.86(s, 6H), 3.82(s, 3H), 3.71(s, 3H), 2.05—1.86(m, 2H), 1.85—1.75(m, 2H) | 172.7, 164.2, 160.7, 158.6, 154.4, 153.1, 152.1, 141.6, 139.8, 134.0, 131.7, 131.2, 127.9, 127.7, 117.3, 108.9, 106.2, 106.1, 96.4, 93.6, 71.5, 66.6, 60.6, 56.5, 56.5, 27.4, 26.7 |
| 4h | 7.61(d, J=9.5 Hz, 1H), 7.47(d, J=10.1 Hz, 1H), 7.38(s, 1H), 7.36(s, 1H), 7.30(t, J=7.2 Hz, 1H), 7.21(t, J=6.8 Hz, 1H), 6.81(s, 1H), 6.46(s, 1H), 4.36(t, J=6.4 Hz, 2H), 4.00(t, J=6.9 Hz, 2H), 3.89(s, 3H), 3.83(s, 6H), 3.81(s, 3H), 3.72(s, 3H), 2.01—1.83(m, 2H), 1.79—1.64(m, 2H) | 172.7, 164.2, 160.8, 158.6, 153.9, 153.3, 153.2, 153.1, 152.1, 140.3, 139.9, 126.8, 126.1, 126.0, 124.3, 115.9, 108.9, 106.2, 96.4, 93.6, 71.6, 60.6, 60.5, 56.5, 56.4, 27.5, 26.7 |
| 4i | 8.20(s, 1H), 8.00(d, J=8.1 Hz, 1H), 7.82(d, J=7.3 Hz, 1H), 7.74(t, J=6.9 Hz, 1H), 7.63(t, J=6.5 Hz, 1H), 7.40(s, 1H), 7.37(s, 1H), 6.83(s, 1H), 6.50(s, 1H), 4.40(t, J=6.5 Hz, 2H), 3.97(t, J=6.4 Hz, 2H), 3.90(s, 3H), 3.86(s, 6H), 3.85(s, 3H), 3.73(s, 3H), 1.86—1.74(m, 2H), 1.72—1.58(m, 2H), 1.57—1.43(m, 2H) | 172.7, 164.2, 160.8, 158.6, 154.6, 153.2, 150.6, 140.3, 140.0, 131.6, 130.8, 130.3, 129.1, 127.3, 126.1, 123.8, 115.2, 109.0, 106.3, 96.4, 93.6, 72.0, 66.6, 60.7, 56.6, 56.5, 29.8, 28.3, 23.3 |
| 4j | 7.89(d, J=7.3 Hz, 1H), 7.77(t, J=7.5 Hz, 1H), 7.63(d, J=8.3 Hz, 1H), 7.54(t, J=7.7 Hz, 1H), 7.39(s, 1H), 7.37(s, 1H), 6.80(s, 1H), 6.46(s, 1H), 4.39(t, J=6.3 Hz, 2H), 3.96(t, J=6.4 Hz, 2H), 3.90(s, 3H), 3.88(s, 6H), 3.85(s, 3H), 3.75(s, 3H), 2.43(s, 3H), 1.91—1.75(m, 2H), 1.74—1.64(m, 2H), 1.57—1.46(m, 2H) | 172.6, 164.1, 160.7, 158.6, 156.3, 154.5, 153.1, 151.8, 148.3, 140.5, 139.8, 139.7, 138.4, 130.1, 129.4, 128.2, 126.9, 123.6, 108.9, 106.2, 106.1, 96.3, 93.5, 71.9, 66.6, 60.6, 56.5, 56.5, 29.8, 28.31, 23.3, 20.4 |
| 4k | 8.26(s, 1H), 8.06(s, 1H), 7.80(d, J=9.6 Hz, 1H), 7.70(d, J=9.7 Hz, 1H), 7.38(s, 1H), 7.37(s, 1H), 6.83(s, 1H), 6.49(s, 1H), 4.40(t, J=6.4 Hz, 2H), 3.97(t, J=6.4 Hz, 2H), 3.90(s, 3H), 3.86(s, 6H), 3.84(s, 3H), 3.73(s, 3H), 1.94—1.73(m, 2H), 1.72—1.62(m, 2H), 1.59—1.44(m, 2H) | 172.7, 164.2, 160.7, 158.6, 157.8, 153.1, 152.1, 141.6, 140.4, 139.8, 139.0, 138.9, 132.0, 131.2, 129.1, 128.0, 126.1, 108.9, 106.2, 96.4, 93.6, 71.9, 66.9, 65.5, 60.6, 56.5, 29.8, 28.2, 22.6 |
| 4l | 7.70(d, J=10.7 Hz, 1H), 7.51(d, J=9.6 Hz, 1H), 7.39(s, 1H), 7.37(s, 1H), 7.22(t, J=10.4 Hz, 1H), 7.05(t, J=9.2 Hz, 1H), 6.85(s, 1H), 6.50(s, 1H), 4.23(t, J=9.5 Hz, 2H), 3.97(t, J=9.1 Hz, 2H), 3.90(s, 3H), 3.85(s, 6H), 3.82(s, 3H), 3.72(s, 3H), 1.91—1.74(m, 2H), 1.72—1.65(m, 2H), 1.54—1.46(m, 2H) | 172.7, 164.2, 160.8, 158.6, 154.0, 153.2, 153.2, 153.1, 152.1, 140.3, 139.9, 126.8, 126.1, 126.0, 124.3, 115.9, 108.9, 106.2, 96.4, 93.6, 71.6, 60.6, 60.5, 56.5, 56.4, 27.5, 26.7 |
| Compd. | 1H NMR(400 MHz), δ | 13C NMR(101 MHz), δ |
| 4m | 7.66(s, 1H), 7.49(d, J=8.7 Hz, 1H), 7.44(d, J=8.0 Hz, 1H), 7.42(s, 1H), 7.42(s, 1H), 7.30(t, J=10.7 Hz, 1H), 7.21(t, J=11.4 Hz, 1H), 6.67(s, 1H), 6.41(s, 1H), 4.22(t, J=5.4 Hz, 2H), 4.01(t, J=5.1 Hz, 2H), 3.91(s, 3H), 3.90(s, 6H), 3.87(s, 3H), 3.79(s, 3H), 1.81—1.78(m, 2H), 1.72—1.68(m, 2H), 1.52—1.49(m, 2H), 1.46—1.42(m, 2H) | 173.9, 165.3, 162.2, 159.9, 155.1, 154.4, 153.1, 151.4, 141.8, 141.4, 132.5, 132.2, 128.6, 128.1, 127.5, 127.4, 125.1, 107.3, 107.3, 96.8, 94.0, 73.0, 73.0, 61.1, 57.1, 56.7, 27.9, 27.6, 26.9 |
| 4n | 7.74(d, J=9.2 Hz, 1H), 7.63(d, J=8.2 Hz, 1H), 7.49(t, J=6.9 Hz, 1H), 7.41(t, J=8.3 Hz, 1H), 7.32(s, 1H), 7.32(s, 1H), 6.58(s, 1H), 6.32(s, 1H), 4.35(t, J=6.5 Hz, 2H), 3.94(t, J=6.5 Hz, 2H), 3.78(s, 3H), 3.78(s, 6H), 3.77(s, 3H), 3.67(s, 3H), 2.44(s, 3H), 1.97—1.92(m, 2H), 1.92—1.87(m, 2H), 1.49—1.42(m, 2H), 1.41—1.33(m, 2H) | 173.7, 165.3, 162.3, 160.0, 157.6, 154.5, 153.0, 149.4, 141.9, 141.4, 141.1, 139.8, 130.0, 129.3, 127.9, 127.5, 127.4, 110.4, 107.4, 96.8, 94.0, 73.0, 67.5, 61.1, 57.0, 56.8, 27.0, 27.0, 26.9, 20.9 |
| 4o | 8.61(s, 1H), 8.05(s, 1H), 7.83(d, J=8.9 Hz, 1H), 7.74(d, J=7.4 Hz, 1H), 7.38(s, 1H), 7.37(s, 1H), 6.83(s, 1H), 6.49(s, 1H), 4.40(t, J=6.6 Hz, 2H), 3.95(t, J=6.7 Hz, 2H), 3.90(s, 3H), 3.86(s, 3H), 3.85(s, 6H), 3.74(s, 3H), 1.80—1.67(m, 2H), 1.66—1.59(m, 2H), 1.49—1.41(m, 2H), 1.40—1.31(m, 2H) | 172.7, 164.2, 160.8, 158.6, 157.8, 153.1, 152.0, 141.7, 140.4, 139.9, 139.0, 138.9, 131.2, 131.1, 129.1, 127.9, 126.1, 108.9, 106.3, 96.4, 93.6, 72.0, 66.8, 60.6, 56.5, 56.5, 30.0, 28.5, 25.7 |
| 4p | 7.84(d, J=7.9 Hz, 1H), 7.66(d, J=7.1 Hz, 1H), 7.60(t, J=6.4 Hz, 1H), 7.39(t, J=7.0 Hz, 1H), 7.40(s, 2H), 7.36(s, 1H), 6.70(s, 1H), 6.44(s, 1H), 4.32(t, J=6.6 Hz, 2H), 4.04(t, J=6.3 Hz, 2H), 3.94(s, 3H), 3.93(s, 3H), 3.92(s, 6H), 3.82(s, 3H), 1.97—1.82(m, 2H), 1.73—1.63(m, 2H), 1.49—1.37(m, 2H), 1.31—1.11(m, 2H) | 173.8, 165.3, 162.2, 159.9, 154.5, 153.0, 151.4, 141.8, 141.4, 134.7, 132.3, 131.5, 130.1, 128.4, 127.5, 124.4, 110.4, 107.3, 96.8, 94.0, 72.9, 67.5, 61.1, 57.1, 56.7, 28.3, 27.6, 26.8 |
Table 2 1H NMR and 13C NMR data of compounds 2c, 2d and 4a—4p
| Compd. | 1H NMR(400 MHz), δ | 13C NMR(101 MHz), δ |
|---|---|---|
| 2c | 7.37(s, 2H), 6.81(s, 1H), 6.48(s, 1H), 3.94(t, J=5.6 Hz, 2H), 3.90(s, 3H), 3.88(s, 6H), 3.85(s, 3H), 3.77(s, 3H), 3.48(t, J=6.7 Hz, 2H), 1.89—1.72(m, 2H), 1.71—1.62(m, 2H), 1.51—1.40(m, 2H) | |
| 2d | 7.37(s, 2H), 6.84(s, 1H), 6.49(s, 1H), 3.95(t, J=6.0 Hz, 2H), 3.90(s, 3H), 3.87(s, 6H), 3.85(s, 3H), 3.76(s, 3H), 3.58(t, J=6.6 Hz, 2H), 1.67—1.64(m, 2H), 1.58—1.47(m, 2H), 1.43—1.35(m, 2H), 1.29—1.23(m, 2H) | |
| 4a | 8.53(s, 1H), 8.00(d, J=9.2 Hz, 1H), 7.82(d, J=8.6 Hz, 1H), 7.76(t, J=7.6 Hz, 1H), 7.64(t, J=6.8 Hz, 1H), 7.39(s, 1H), 7.38(s, 1H), 6.83(s, 1H), 6.49(s, 1H), 4.54(t, J=6.6 Hz, 2H), 4.16(t, J=6.1 Hz, 2H), 3.90(s, 3H), 3.85(s, 6H), 3.84(s, 3H), 3.70(s, 3H), 2.28—2.07(m, 2H) | 172.6, 164.2, 160.7, 158.6, 157.4, 153.2, 152.2, 140.3, 140.2, 140.1, 139.9, 138.8, 130.9, 129.1, 127.3, 127.2, 126.0, 108.9, 106.2, 96.4, 93.6, 69.0, 63.9, 60.6, 56.6, 56.5, 29.6 |
| 4b | 7.88(d, J=8.2 Hz, 1H), 7.74(t, J=7.7 Hz, 1H), 7.65(d, J=6.8 Hz, 1H), 7.57(t, J=6.8 Hz, 1H), 7.39(s, 1H), 7.36(s, 1H), 6.83(s, 1H), 6.46(s, 1H), 4.53(t, J=6.4 Hz, 2H), 4.09(t, J=6.2 Hz, 2H), 3.91(s, 3H), 3.85(s, 6H), 3.84(s, 3H), 3.70(s, 3H), 2.43(s, 3H), 2.27—2.06(m, 2H) | 172.6, 164.2, 160.7, 158.6, 156.2, 154.6, 153.1, 152.1, 148.3, 140.4, 139.6, 138.4, 129.5, 128.2, 126.9, 126.0, 123.7, 108.9, 106.2, 96.3, 93.4, 69.9, 68.9, 63.7, 60.5, 56.4, 29.6, 20.4 |
| Compd. | 1H NMR(400 MHz), δ | 13C NMR(101 MHz), δ |
| 4c | 8.57(s, 1H), 8.07(s, 1H), 7.81(d, J=12.0 Hz, 1H), 7.67(d, J=11.3 Hz, 1H), 7.38(s, 1H), 7.36(s, 1H), 6.81(s, 1H), 6.48(s, 1H), 4.53(t, J=6.5 Hz, 2H), 4.07(t, J=6.2 Hz, 2H), 3.89(s, 3H), 3.85(s, 6H), 3.84(s, 3H), 3.70(s, 3H), 2.24—2.00(m, 2H) | 172.6, 164.2, 160.8, 160.7, 158.6, 157.7, 154.4, 153.2, 153.1, 152.1, 141.5, 140.3, 140.2, 139.8, 139.1, 138.9, 134.1, 131.2, 129.1, 128.0, 126.0, 108.9, 106.2, 96.4, 93.5, 68.9, 64.2, 60.6, 60.6, 56.5, 29.5 |
| 4d | 7.69(d, J=10.0 Hz, 1H), 7.43(d, J=9.8 Hz, 1H), 7.40(s, 1H), 7.39(s, 1H), 7.25(t, J=6.6 Hz, 1H), 7.06(t, J=7.1 Hz, 1H), 6.86(s, 1H), 6.50(s, 1H), 4.27(t, J=6.8 Hz, 2H), 4.09(t, J=5.9 Hz, 2H), 3.91(s, 3H), 3.85(s, 3H), 3.83(s, 6H), 3.73(s, 3H), 2.07—2.00(m, 2H) | 172.6, 166.8, 164.3, 160.7, 158.7, 153.2, 152.1, 140.4, 140.2, 139.8, 133.5, 132.1, 129.6, 129.2, 126.3, 126.0, 108.9, 106.1, 96.4, 93.9, 93.5, 73.8, 65.4, 60.6, 56.5, 56.3, 29.5 |
| 4e | 8.54(s, 1H), 7.99(d, J=7.0 Hz, 1H), 7.81(d, J=8.3 Hz, 1H), 7.74(t, J=6.9 Hz, 1H), 7.62(t, J=6.8 Hz, 1H), 7.37(s, 2H), 6.81(s, 1H), 6.47(s, 1H), 4.45(t, J=6.3 Hz, 2H), 4.01(t, J=6.1 Hz, 2H), 3.90(s, 3H), 3.86(s, 6H), 3.84(s, 3H), 3.73(s, 3H), 1.95—1.89(m, 2H), 1.88—1.82(m, 2H) | 172.7, 164.2, 160.8, 158.6, 157.5, 153.2, 152.1, 140.4, 140.3, 140.1, 139.9, 138.8, 130.8, 129.1, 127.3, 127.2, 126.1, 108.9, 106.3, 96.4, 93.6, 71.7, 66.3, 60.6, 56.6, 56.5, 26.7, 25.4 |
| 4f | 7.87(d, J=8.1 Hz, 1H), 7.70(t, J=8.9 Hz, 1H), 7.60(d, J=7.0 Hz, 1H), 7.44(s, 1H), 7.41(s, 1H), 7.31(t, J=7.6 Hz, 1H), 6.66(s, 1H), 6.40(s, 1H), 4.52(t, J=6.2 Hz, 2H), 4.13(t, J=6.2 Hz, 2H), 3.91(s, 3H), 3.89(s, 6H), 3.86(s, 3H), 3.79(s, 3H), 2.45(s, 3H), 2.08—2.01(m, 2H), 1.96—1.91(m, 2H) | 173.7, 165.3, 159.9, 157.5, 154.5, 153.1, 153.1, 149.4, 141.4, 139.8, 134.0, 133.9, 130.8, 129.9, 129.3, 127.9, 124.2, 110.3, 107.2, 96.8, 94.0, 72.3, 67.3, 61.1, 56.8, 56.8, 28.1, 26.6, 20.9 |
| 4g | 8.43(s, 1H), 7.98(s, 1H), 7.79(d, J=10.3 Hz, 1H), 7.70(d, J=9.5 Hz, 1H), 7.37(s, 1H), 7.34(s, 1H), 6.82(s, 1H), 6.49(s, 1H), 4.44(t, J=6.4 Hz, 2H), 4.01(t, J=6.4 Hz, 2H), 3.90(s, 3H), 3.86(s, 6H), 3.82(s, 3H), 3.71(s, 3H), 2.05—1.86(m, 2H), 1.85—1.75(m, 2H) | 172.7, 164.2, 160.7, 158.6, 154.4, 153.1, 152.1, 141.6, 139.8, 134.0, 131.7, 131.2, 127.9, 127.7, 117.3, 108.9, 106.2, 106.1, 96.4, 93.6, 71.5, 66.6, 60.6, 56.5, 56.5, 27.4, 26.7 |
| 4h | 7.61(d, J=9.5 Hz, 1H), 7.47(d, J=10.1 Hz, 1H), 7.38(s, 1H), 7.36(s, 1H), 7.30(t, J=7.2 Hz, 1H), 7.21(t, J=6.8 Hz, 1H), 6.81(s, 1H), 6.46(s, 1H), 4.36(t, J=6.4 Hz, 2H), 4.00(t, J=6.9 Hz, 2H), 3.89(s, 3H), 3.83(s, 6H), 3.81(s, 3H), 3.72(s, 3H), 2.01—1.83(m, 2H), 1.79—1.64(m, 2H) | 172.7, 164.2, 160.8, 158.6, 153.9, 153.3, 153.2, 153.1, 152.1, 140.3, 139.9, 126.8, 126.1, 126.0, 124.3, 115.9, 108.9, 106.2, 96.4, 93.6, 71.6, 60.6, 60.5, 56.5, 56.4, 27.5, 26.7 |
| 4i | 8.20(s, 1H), 8.00(d, J=8.1 Hz, 1H), 7.82(d, J=7.3 Hz, 1H), 7.74(t, J=6.9 Hz, 1H), 7.63(t, J=6.5 Hz, 1H), 7.40(s, 1H), 7.37(s, 1H), 6.83(s, 1H), 6.50(s, 1H), 4.40(t, J=6.5 Hz, 2H), 3.97(t, J=6.4 Hz, 2H), 3.90(s, 3H), 3.86(s, 6H), 3.85(s, 3H), 3.73(s, 3H), 1.86—1.74(m, 2H), 1.72—1.58(m, 2H), 1.57—1.43(m, 2H) | 172.7, 164.2, 160.8, 158.6, 154.6, 153.2, 150.6, 140.3, 140.0, 131.6, 130.8, 130.3, 129.1, 127.3, 126.1, 123.8, 115.2, 109.0, 106.3, 96.4, 93.6, 72.0, 66.6, 60.7, 56.6, 56.5, 29.8, 28.3, 23.3 |
| 4j | 7.89(d, J=7.3 Hz, 1H), 7.77(t, J=7.5 Hz, 1H), 7.63(d, J=8.3 Hz, 1H), 7.54(t, J=7.7 Hz, 1H), 7.39(s, 1H), 7.37(s, 1H), 6.80(s, 1H), 6.46(s, 1H), 4.39(t, J=6.3 Hz, 2H), 3.96(t, J=6.4 Hz, 2H), 3.90(s, 3H), 3.88(s, 6H), 3.85(s, 3H), 3.75(s, 3H), 2.43(s, 3H), 1.91—1.75(m, 2H), 1.74—1.64(m, 2H), 1.57—1.46(m, 2H) | 172.6, 164.1, 160.7, 158.6, 156.3, 154.5, 153.1, 151.8, 148.3, 140.5, 139.8, 139.7, 138.4, 130.1, 129.4, 128.2, 126.9, 123.6, 108.9, 106.2, 106.1, 96.3, 93.5, 71.9, 66.6, 60.6, 56.5, 56.5, 29.8, 28.31, 23.3, 20.4 |
| 4k | 8.26(s, 1H), 8.06(s, 1H), 7.80(d, J=9.6 Hz, 1H), 7.70(d, J=9.7 Hz, 1H), 7.38(s, 1H), 7.37(s, 1H), 6.83(s, 1H), 6.49(s, 1H), 4.40(t, J=6.4 Hz, 2H), 3.97(t, J=6.4 Hz, 2H), 3.90(s, 3H), 3.86(s, 6H), 3.84(s, 3H), 3.73(s, 3H), 1.94—1.73(m, 2H), 1.72—1.62(m, 2H), 1.59—1.44(m, 2H) | 172.7, 164.2, 160.7, 158.6, 157.8, 153.1, 152.1, 141.6, 140.4, 139.8, 139.0, 138.9, 132.0, 131.2, 129.1, 128.0, 126.1, 108.9, 106.2, 96.4, 93.6, 71.9, 66.9, 65.5, 60.6, 56.5, 29.8, 28.2, 22.6 |
| 4l | 7.70(d, J=10.7 Hz, 1H), 7.51(d, J=9.6 Hz, 1H), 7.39(s, 1H), 7.37(s, 1H), 7.22(t, J=10.4 Hz, 1H), 7.05(t, J=9.2 Hz, 1H), 6.85(s, 1H), 6.50(s, 1H), 4.23(t, J=9.5 Hz, 2H), 3.97(t, J=9.1 Hz, 2H), 3.90(s, 3H), 3.85(s, 6H), 3.82(s, 3H), 3.72(s, 3H), 1.91—1.74(m, 2H), 1.72—1.65(m, 2H), 1.54—1.46(m, 2H) | 172.7, 164.2, 160.8, 158.6, 154.0, 153.2, 153.2, 153.1, 152.1, 140.3, 139.9, 126.8, 126.1, 126.0, 124.3, 115.9, 108.9, 106.2, 96.4, 93.6, 71.6, 60.6, 60.5, 56.5, 56.4, 27.5, 26.7 |
| Compd. | 1H NMR(400 MHz), δ | 13C NMR(101 MHz), δ |
| 4m | 7.66(s, 1H), 7.49(d, J=8.7 Hz, 1H), 7.44(d, J=8.0 Hz, 1H), 7.42(s, 1H), 7.42(s, 1H), 7.30(t, J=10.7 Hz, 1H), 7.21(t, J=11.4 Hz, 1H), 6.67(s, 1H), 6.41(s, 1H), 4.22(t, J=5.4 Hz, 2H), 4.01(t, J=5.1 Hz, 2H), 3.91(s, 3H), 3.90(s, 6H), 3.87(s, 3H), 3.79(s, 3H), 1.81—1.78(m, 2H), 1.72—1.68(m, 2H), 1.52—1.49(m, 2H), 1.46—1.42(m, 2H) | 173.9, 165.3, 162.2, 159.9, 155.1, 154.4, 153.1, 151.4, 141.8, 141.4, 132.5, 132.2, 128.6, 128.1, 127.5, 127.4, 125.1, 107.3, 107.3, 96.8, 94.0, 73.0, 73.0, 61.1, 57.1, 56.7, 27.9, 27.6, 26.9 |
| 4n | 7.74(d, J=9.2 Hz, 1H), 7.63(d, J=8.2 Hz, 1H), 7.49(t, J=6.9 Hz, 1H), 7.41(t, J=8.3 Hz, 1H), 7.32(s, 1H), 7.32(s, 1H), 6.58(s, 1H), 6.32(s, 1H), 4.35(t, J=6.5 Hz, 2H), 3.94(t, J=6.5 Hz, 2H), 3.78(s, 3H), 3.78(s, 6H), 3.77(s, 3H), 3.67(s, 3H), 2.44(s, 3H), 1.97—1.92(m, 2H), 1.92—1.87(m, 2H), 1.49—1.42(m, 2H), 1.41—1.33(m, 2H) | 173.7, 165.3, 162.3, 160.0, 157.6, 154.5, 153.0, 149.4, 141.9, 141.4, 141.1, 139.8, 130.0, 129.3, 127.9, 127.5, 127.4, 110.4, 107.4, 96.8, 94.0, 73.0, 67.5, 61.1, 57.0, 56.8, 27.0, 27.0, 26.9, 20.9 |
| 4o | 8.61(s, 1H), 8.05(s, 1H), 7.83(d, J=8.9 Hz, 1H), 7.74(d, J=7.4 Hz, 1H), 7.38(s, 1H), 7.37(s, 1H), 6.83(s, 1H), 6.49(s, 1H), 4.40(t, J=6.6 Hz, 2H), 3.95(t, J=6.7 Hz, 2H), 3.90(s, 3H), 3.86(s, 3H), 3.85(s, 6H), 3.74(s, 3H), 1.80—1.67(m, 2H), 1.66—1.59(m, 2H), 1.49—1.41(m, 2H), 1.40—1.31(m, 2H) | 172.7, 164.2, 160.8, 158.6, 157.8, 153.1, 152.0, 141.7, 140.4, 139.9, 139.0, 138.9, 131.2, 131.1, 129.1, 127.9, 126.1, 108.9, 106.3, 96.4, 93.6, 72.0, 66.8, 60.6, 56.5, 56.5, 30.0, 28.5, 25.7 |
| 4p | 7.84(d, J=7.9 Hz, 1H), 7.66(d, J=7.1 Hz, 1H), 7.60(t, J=6.4 Hz, 1H), 7.39(t, J=7.0 Hz, 1H), 7.40(s, 2H), 7.36(s, 1H), 6.70(s, 1H), 6.44(s, 1H), 4.32(t, J=6.6 Hz, 2H), 4.04(t, J=6.3 Hz, 2H), 3.94(s, 3H), 3.93(s, 3H), 3.92(s, 6H), 3.82(s, 3H), 1.97—1.82(m, 2H), 1.73—1.63(m, 2H), 1.49—1.37(m, 2H), 1.31—1.11(m, 2H) | 173.8, 165.3, 162.2, 159.9, 154.5, 153.0, 151.4, 141.8, 141.4, 134.7, 132.3, 131.5, 130.1, 128.4, 127.5, 124.4, 110.4, 107.3, 96.8, 94.0, 72.9, 67.5, 61.1, 57.1, 56.7, 28.3, 27.6, 26.8 |
| Compd. | n | R | Inhibition ratea (%) | |||
|---|---|---|---|---|---|---|
| X. citri | X. oryzae | |||||
| 100 μg/mL | 50 μg/mL | 100 μg/mL | 50 μg/mL | |||
| 4a | 3 | H | 73.69±5.97 | 67.82±2.64 | 40.78±3.60 | 33.63±5.14 |
| 4b | 3 | 3-CH3 | 83.52±2.19 | 68.13±1.20 | 49.33±2.85 | 44.71±4.65 |
| 4c | 3 | 6-Cl | 73.17±7.31 | 63.18±6.12 | 50.23±4.81 | 43.66±8.00 |
| 4d | 3 | 3-OH | 77.50±2.97 | 67.71±7.99 | 52.78±5.55 | 43.77±5.57 |
| 4e | 4 | H | 75.23±9.52 | 62.10±2.25 | 55.17±5.65 | 42.52±5.98 |
| 4f | 4 | 3-CH3 | 74.61±2.36 | 65.50±5.74 | 61.30±4.24 | 53.27±8.16 |
| 4g | 4 | 6-Cl | 74.20±9.83 | 61.79±7.18 | 47.19±5.92 | 37.91±6.71 |
| 4h | 4 | 3-OH | 77.96±1.47 | 68.07±6.74 | 53.22±6.21 | 42.31±2.88 |
| 4i | 5 | H | 80.38±6.90 | 69.88±4.33 | 47.57±5.99 | 37.48±2.00 |
| 4j | 5 | 3-CH3 | 76.67±2.63 | 66.63±6.14 | 50.23±5.71 | 44.80±1.41 |
| 4k | 5 | 6-Cl | 81.00±8.11 | 69.41±4.79 | 36.18±0.32 | 31.99±6.24 |
| 4l | 5 | 3-OH | 73.07±4.23 | 67.35±7.10 | 49.61±3.08 | 38.20±8.51 |
| 4m | 6 | H | 76.00±7.20 | 65.55±4.94 | 54.74±8.50 | 46.65±7.34 |
| 4n | 6 | 3-CH3 | 77.45±1.75 | 67.35±6.36 | 47.95±7.65 | 39.92±9.61 |
| 4o | 6 | 6-Cl | 76.21±5.41 | 68.13±7.63 | 29.61±2.46 | 18.79±4.44 |
| 4p | 6 | 3-OH | 77.45±3.28 | 66.37±3.42 | 33.72±1.93 | 21.23±6.28 |
| Myricetin | 49.38±4.06 | 40.37±0.41 | 41.38±5.94 | 32.32±2.85 | ||
| Bismerthiazolb | 59.47±7.65 | 49.49±5.74 | 47.03±3.06 | 39.59±6.08 | ||
| Thiodiazole-copperb | 56.90±1.47 | 47.94±8.47 | 43.23±3.01 | 38.18±6.32 | ||
Table 3 Antibacterial activities of target compounds 4a—4p
| Compd. | n | R | Inhibition ratea (%) | |||
|---|---|---|---|---|---|---|
| X. citri | X. oryzae | |||||
| 100 μg/mL | 50 μg/mL | 100 μg/mL | 50 μg/mL | |||
| 4a | 3 | H | 73.69±5.97 | 67.82±2.64 | 40.78±3.60 | 33.63±5.14 |
| 4b | 3 | 3-CH3 | 83.52±2.19 | 68.13±1.20 | 49.33±2.85 | 44.71±4.65 |
| 4c | 3 | 6-Cl | 73.17±7.31 | 63.18±6.12 | 50.23±4.81 | 43.66±8.00 |
| 4d | 3 | 3-OH | 77.50±2.97 | 67.71±7.99 | 52.78±5.55 | 43.77±5.57 |
| 4e | 4 | H | 75.23±9.52 | 62.10±2.25 | 55.17±5.65 | 42.52±5.98 |
| 4f | 4 | 3-CH3 | 74.61±2.36 | 65.50±5.74 | 61.30±4.24 | 53.27±8.16 |
| 4g | 4 | 6-Cl | 74.20±9.83 | 61.79±7.18 | 47.19±5.92 | 37.91±6.71 |
| 4h | 4 | 3-OH | 77.96±1.47 | 68.07±6.74 | 53.22±6.21 | 42.31±2.88 |
| 4i | 5 | H | 80.38±6.90 | 69.88±4.33 | 47.57±5.99 | 37.48±2.00 |
| 4j | 5 | 3-CH3 | 76.67±2.63 | 66.63±6.14 | 50.23±5.71 | 44.80±1.41 |
| 4k | 5 | 6-Cl | 81.00±8.11 | 69.41±4.79 | 36.18±0.32 | 31.99±6.24 |
| 4l | 5 | 3-OH | 73.07±4.23 | 67.35±7.10 | 49.61±3.08 | 38.20±8.51 |
| 4m | 6 | H | 76.00±7.20 | 65.55±4.94 | 54.74±8.50 | 46.65±7.34 |
| 4n | 6 | 3-CH3 | 77.45±1.75 | 67.35±6.36 | 47.95±7.65 | 39.92±9.61 |
| 4o | 6 | 6-Cl | 76.21±5.41 | 68.13±7.63 | 29.61±2.46 | 18.79±4.44 |
| 4p | 6 | 3-OH | 77.45±3.28 | 66.37±3.42 | 33.72±1.93 | 21.23±6.28 |
| Myricetin | 49.38±4.06 | 40.37±0.41 | 41.38±5.94 | 32.32±2.85 | ||
| Bismerthiazolb | 59.47±7.65 | 49.49±5.74 | 47.03±3.06 | 39.59±6.08 | ||
| Thiodiazole-copperb | 56.90±1.47 | 47.94±8.47 | 43.23±3.01 | 38.18±6.32 | ||
| Compd. | n | R | Toxic regression equation | r | EC50/(μg·mL-1) |
|---|---|---|---|---|---|
| 4a | 3 | H | y=0.8555x+3.9292 | 0.9368 | 17.85±2.27 |
| 4b | 3 | 3-CH3 | y=0.9079x+4.0182 | 0.9033 | 12.06±2.84 |
| 4c | 3 | 6-Cl | y=0.7629x+4.0511 | 0.9859 | 17.53±2.94 |
| 4d | 3 | 3-OH | y=0.8701x+3.9962 | 0.9876 | 14.24±2.13 |
| 4e | 4 | H | y=0.7769x+4.0440 | 0.9713 | 17.00±3.11 |
| 4f | 4 | 3-CH3 | y=0.7701x+4.1052 | 0.9911 | 14.52±1.80 |
| 4g | 4 | 6-Cl | y=0.9324x+3.7319 | 0.9869 | 22.91±4.26 |
| 4h | 4 | 3-OH | y=0.9139x+3.9345 | 0.9988 | 14.65±3.15 |
| 4i | 5 | H | y=0.8168x+4.0913 | 0.8317 | 12.38±1.74 |
| 4j | 5 | 3-CH3 | y=0.7878x+4.1041 | 0.9667 | 13.72±1.31 |
| 4k | 5 | 6-Cl | y=0.9325x+3.9408 | 0.9719 | 13.67±2.35 |
| 4l | 5 | 3-OH | y=0.6705x+4.2733 | 0.9944 | 12.13±1.91 |
| 4m | 6 | H | y=0.7829x+4.0987 | 0.9867 | 14.17±1.37 |
| 4n | 6 | 3-CH3 | y=0.8137x+4.0627 | 0.9771 | 14.19±1.66 |
| 4o | 6 | 6-Cl | y=0.6998x+4.2666 | 0.9710 | 11.17±2.20 |
| 4p | 6 | 3-OH | y=0.7367x+4.2104 | 0.9845 | 11.80±0.72 |
| Myricetin | y=0.6520x+3.6763 | 0.9838 | 107.20±1.63 | ||
| Bismerthiazola | y=0.8357x+3.5466 | 0.9876 | 54.85±1.37 | ||
| Thiediazole-coppera | y=0.8874x+3.4149 | 0.9984 | 61.13±3.37 |
Table 4 EC50 values of some compounds against X. citri
| Compd. | n | R | Toxic regression equation | r | EC50/(μg·mL-1) |
|---|---|---|---|---|---|
| 4a | 3 | H | y=0.8555x+3.9292 | 0.9368 | 17.85±2.27 |
| 4b | 3 | 3-CH3 | y=0.9079x+4.0182 | 0.9033 | 12.06±2.84 |
| 4c | 3 | 6-Cl | y=0.7629x+4.0511 | 0.9859 | 17.53±2.94 |
| 4d | 3 | 3-OH | y=0.8701x+3.9962 | 0.9876 | 14.24±2.13 |
| 4e | 4 | H | y=0.7769x+4.0440 | 0.9713 | 17.00±3.11 |
| 4f | 4 | 3-CH3 | y=0.7701x+4.1052 | 0.9911 | 14.52±1.80 |
| 4g | 4 | 6-Cl | y=0.9324x+3.7319 | 0.9869 | 22.91±4.26 |
| 4h | 4 | 3-OH | y=0.9139x+3.9345 | 0.9988 | 14.65±3.15 |
| 4i | 5 | H | y=0.8168x+4.0913 | 0.8317 | 12.38±1.74 |
| 4j | 5 | 3-CH3 | y=0.7878x+4.1041 | 0.9667 | 13.72±1.31 |
| 4k | 5 | 6-Cl | y=0.9325x+3.9408 | 0.9719 | 13.67±2.35 |
| 4l | 5 | 3-OH | y=0.6705x+4.2733 | 0.9944 | 12.13±1.91 |
| 4m | 6 | H | y=0.7829x+4.0987 | 0.9867 | 14.17±1.37 |
| 4n | 6 | 3-CH3 | y=0.8137x+4.0627 | 0.9771 | 14.19±1.66 |
| 4o | 6 | 6-Cl | y=0.6998x+4.2666 | 0.9710 | 11.17±2.20 |
| 4p | 6 | 3-OH | y=0.7367x+4.2104 | 0.9845 | 11.80±0.72 |
| Myricetin | y=0.6520x+3.6763 | 0.9838 | 107.20±1.63 | ||
| Bismerthiazola | y=0.8357x+3.5466 | 0.9876 | 54.85±1.37 | ||
| Thiediazole-coppera | y=0.8874x+3.4149 | 0.9984 | 61.13±3.37 |
| Compd. | n | R | Toxic regression equation | r | EC50/(μg·mL-1) |
|---|---|---|---|---|---|
| 4c | 3 | 6-Cl | y=0.7670x+3.5327 | 0.9862 | 81.85±3.03 |
| 4d | 3 | 3-OH | y=0.6330x+3.7945 | 0.9972 | 80.25±2.31 |
| 4e | 4 | H | y=0.8403x+3.4619 | 0.9880 | 67.67±1.81 |
| 4f | 4 | 3-CH3 | y=0.7279x+3.8807 | 0.9811 | 34.49±2.14 |
| 4g | 4 | 6-Cl | y=0.7356x+3.4632 | 0.9966 | 122.79±0.94 |
| 4h | 4 | 3-OH | y=0.8820x+3.3230 | 0.9929 | 79.68±2.18 |
| 4i | 5 | H | y=0.9043x+3.1931 | 0.9840 | 99.57±2.57 |
| 4j | 5 | 3-CH3 | y=0.6945x+3.6513 | 0.9897 | 87.49±2.42 |
| 4m | 6 | H | y=0.9009x+3.3445 | 0.9956 | 68.80±2.52 |
| 4n | 6 | 3-CH3 | y=0.7117x+3.5476 | 0.9975 | 109.84±1.09 |
| Myricetin | y=0.6512x+3.4715 | 0.9862 | 222.44±2.37 | ||
| Bismerthiazol* | y=0.5525x+3.8806 | 0.9947 | 148.20±2.44 | ||
| Thiediazole-copper* | y=0.5876x+3.6813 | 0.9950 | 175.47±2.09 |
Table 5 EC50 values of some compounds against X. oryzae
| Compd. | n | R | Toxic regression equation | r | EC50/(μg·mL-1) |
|---|---|---|---|---|---|
| 4c | 3 | 6-Cl | y=0.7670x+3.5327 | 0.9862 | 81.85±3.03 |
| 4d | 3 | 3-OH | y=0.6330x+3.7945 | 0.9972 | 80.25±2.31 |
| 4e | 4 | H | y=0.8403x+3.4619 | 0.9880 | 67.67±1.81 |
| 4f | 4 | 3-CH3 | y=0.7279x+3.8807 | 0.9811 | 34.49±2.14 |
| 4g | 4 | 6-Cl | y=0.7356x+3.4632 | 0.9966 | 122.79±0.94 |
| 4h | 4 | 3-OH | y=0.8820x+3.3230 | 0.9929 | 79.68±2.18 |
| 4i | 5 | H | y=0.9043x+3.1931 | 0.9840 | 99.57±2.57 |
| 4j | 5 | 3-CH3 | y=0.6945x+3.6513 | 0.9897 | 87.49±2.42 |
| 4m | 6 | H | y=0.9009x+3.3445 | 0.9956 | 68.80±2.52 |
| 4n | 6 | 3-CH3 | y=0.7117x+3.5476 | 0.9975 | 109.84±1.09 |
| Myricetin | y=0.6512x+3.4715 | 0.9862 | 222.44±2.37 | ||
| Bismerthiazol* | y=0.5525x+3.8806 | 0.9947 | 148.20±2.44 | ||
| Thiediazole-copper* | y=0.5876x+3.6813 | 0.9950 | 175.47±2.09 |
| Compd. | n | R | Inhibition rate(%) | ||
|---|---|---|---|---|---|
| Curative | Protection | Inactivation | |||
| 4a | 3 | H | 39.2±4.5 | 40.1±3.9 | 63.2±6.6 |
| 4b | 3 | 3-CH3 | 38.6±4.7 | 40.2±4.9 | 60.0±5.5 |
| 4c | 3 | 6-Cl | 43.8±1.0 | 47.3±4.6 | 69.2±8.4 |
| 4d | 3 | 3-OH | 35.4±7.5 | 46.3±2.5 | 44.5±5.7 |
| 4e | 4 | H | 41.5±5.0 | 50.5±5.6 | 75.1±5.1 |
| 4f | 4 | 3-CH3 | 50.3±3.3 | 52.6±5.2 | 67.5±8.1 |
| 4g | 4 | 6-Cl | 51.6±6.6 | 59.8±5.1 | 77.7±6.4 |
| 4h | 4 | 3-OH | 33.7±3.5 | 43.3±4.4 | 65.8±4.2 |
| 4i | 5 | H | 47.9±0.7 | 49.1±8.5 | 66.4±7.3 |
| 4j | 5 | 3-CH3 | 44.4±5.1 | 40.7±4.5 | 62.8±3.6 |
| 4k | 5 | 6-Cl | 49.0±7.2 | 53.0±5.6 | 73.6±2.5 |
| 4l | 5 | 3-OH | 40.5±5.7 | 44.0±2.6 | 54.0±4.1 |
| 4m | 6 | H | 51.4±2.6 | 62.3±4.9 | 78.3±1.5 |
| 4n | 6 | 3-CH3 | 48.7±6.6 | 47.3±9.2 | 51.6±2.7 |
| 4o | 6 | 6-Cl | 50.1±3.2 | 48.1±4.8 | 54.7±0.4 |
| 4p | 6 | 3-OH | 46.2±6.0 | 54.3±4.1 | 74.9±3.6 |
| Myricetin | 31.6±7.2 | 42.1±6.4 | 50.9±6.3 | ||
| Ribavirin | 39.9±3.7 | 51.8±6.0 | 73.3±2.9 | ||
| Ningnanmycin | 52.7±1.3 | 65.7±1.9 | 90.4±3.1 | ||
Table 6 Antiviral activities of the test compounds against TMV in vivo at 500 mg/L
| Compd. | n | R | Inhibition rate(%) | ||
|---|---|---|---|---|---|
| Curative | Protection | Inactivation | |||
| 4a | 3 | H | 39.2±4.5 | 40.1±3.9 | 63.2±6.6 |
| 4b | 3 | 3-CH3 | 38.6±4.7 | 40.2±4.9 | 60.0±5.5 |
| 4c | 3 | 6-Cl | 43.8±1.0 | 47.3±4.6 | 69.2±8.4 |
| 4d | 3 | 3-OH | 35.4±7.5 | 46.3±2.5 | 44.5±5.7 |
| 4e | 4 | H | 41.5±5.0 | 50.5±5.6 | 75.1±5.1 |
| 4f | 4 | 3-CH3 | 50.3±3.3 | 52.6±5.2 | 67.5±8.1 |
| 4g | 4 | 6-Cl | 51.6±6.6 | 59.8±5.1 | 77.7±6.4 |
| 4h | 4 | 3-OH | 33.7±3.5 | 43.3±4.4 | 65.8±4.2 |
| 4i | 5 | H | 47.9±0.7 | 49.1±8.5 | 66.4±7.3 |
| 4j | 5 | 3-CH3 | 44.4±5.1 | 40.7±4.5 | 62.8±3.6 |
| 4k | 5 | 6-Cl | 49.0±7.2 | 53.0±5.6 | 73.6±2.5 |
| 4l | 5 | 3-OH | 40.5±5.7 | 44.0±2.6 | 54.0±4.1 |
| 4m | 6 | H | 51.4±2.6 | 62.3±4.9 | 78.3±1.5 |
| 4n | 6 | 3-CH3 | 48.7±6.6 | 47.3±9.2 | 51.6±2.7 |
| 4o | 6 | 6-Cl | 50.1±3.2 | 48.1±4.8 | 54.7±0.4 |
| 4p | 6 | 3-OH | 46.2±6.0 | 54.3±4.1 | 74.9±3.6 |
| Myricetin | 31.6±7.2 | 42.1±6.4 | 50.9±6.3 | ||
| Ribavirin | 39.9±3.7 | 51.8±6.0 | 73.3±2.9 | ||
| Ningnanmycin | 52.7±1.3 | 65.7±1.9 | 90.4±3.1 | ||
| [1] | Li B. C., Lin X. R., Zhang Y. M., Zhang D. W., Xiao Y., Lin F., Chem. Res. Chinese Universities,2017, 33(1), 70—73 |
| [2] | Xu B. X., Ding X. D., Wu Y. C., Cui L., Qian P., Wang D., Zhao Y. F., Chem. Res. Chinese Universities,2018, 34(1), 51—56 |
| [3] | Huang M. G., Ruan X. H., Zhang J. P., Li Q., Wang Y. H., Chen L. J., Zhang C., Li P., Xue W., Chin. J. Org. Chem.,2017, 37(8), 2145—2152 |
| (黄民国, 阮祥辉, 张菊平, 李琴, 王一会, 陈丽娟, 张橙, 李普, 薛伟. 有机化学,2017, 37(8), 2145—2152) | |
| [4] | Zhou J. R., Mukheriee P., Gugger E. T., Tanaka T., Blackbum G. L., Clinton S. K., Cancer Res.,1998, 58(22), 5231—5238 |
| [5] | Zhang X. J., Huang Q. L., Ji Y. B., Tianjin Pharmacy,2008, 20(5), 57—60 |
| (张秀娟, 黄清玲, 季宇彬. 天津药学,2008, 20(5), 57—60) | |
| [6] | Grenier D., Chen H., Ben L. A., Fournier L. J., Pierre M. M., Plos One,2015, 10(6), e0131758 |
| [7] | Yu M. S., Lee J., Lee J. M., Kim Y., Chin Y. W., Jee J. G., Keum Y. S., Jeong Y. J., Bioorg. Med. Chem. Lett.,2012, 22(12), 4049—4054 |
| [8] | Ruan X. H., Zhao H. J., Zhang C., Chen L. J., Li P., Wang Y. H., He M., Xue W., Chem. J. Chinese Universities,2018, 39(6), 1197—1204 |
| (阮祥辉, 赵洪菊, 张橙, 陈丽娟, 李普, 王一会, 贺鸣, 薛伟. 高等学校化学学报,2018, 39(6), 1197—1204) | |
| [9] | Liu I. M., Tzeng T. F., Liou S. S., Lan T. W., Planta Med.,2007, 73(10), 1054—1060 |
| [10] | Guo J. J., Meng Y. H., Zhao Y., Hu Y. Y., Rena D. Y., Yang X. B., Food Funct.,2015, 6(5), 1620—1634 |
| [11] | Shimmyo Y., Kihara T., Akaike A., Niidome T., Sugimoto H. J., Neurosci. Res.,2008, 86(6), 1836—1845 |
| [12] | Xiao W., Ruan X. H., Li Q., Zhang J. P., Zhong X. M., Xie Y., Wang X. B., Huang M. G., Xue W., Chem. J. Chinese Universities,2017, 38(1), 35—40 |
| (肖维, 阮祥辉, 李琴, 张菊平, 钟新敏, 谢艳, 王晓斌, 黄民国, 薛伟. 高等学校化学学报,2017, 38(1), 35—40) | |
| [13] | Su X. W., D’Souza D. H., Food Environ. Virol.,2013, 5(2), 97—102 |
| [14] | Suguna M., Kumar N. S., Subbaiah M. V., Krishnaiah A. J., Chem. Pharm. Res.,2010, 2(1), 7—20 |
| [15] | Burguete A., Pontiki E., Hadjipavlou-Litina D., Villar R., Vicente E., Solano B., Ancizu S., Pérez-Silanes S., Aldana I., Monge A., Bioorg. Med. Chem. Lett.,2007, 17(23), 6439—6443 |
| [16] | Ganley B., Chowdhury G., Bhansali J., Daniels J. S., Gates K. S., Bioorg. Med. Chem.,2001, 9(9), 2395—2401 |
| [17] | Rodrigues F. A., Bomfim I. S., Cavalcanti B. C., Pessoa C. Ó., Wardell J. L., Wardell S. M., Pinheiro A. C., Kaiser C. R., Nogueira T. C., Low J. N., Gomes L. R., de Souza M. V., Bioorg. Med. Chem. Lett.,2014, 24(3), 934—939 |
| [18] | Khan S. A., Saleem K., Khan Z., Eur. J. Med. Chem.,2007, 42(1), 103—108 |
| [19] | Li Q., Synthesis and Biological Activity of 1,4-Pentadien-3-one(oxime) Ether Compounds Containing Quinazoline(Quinoxaline) Groups, Guizhou University, Guiyang, 2018 |
| (李琴. 含喹唑(喔)啉基团的1,4-戊二烯-3-酮(肟)醚类化合物的合成及生物活性研究, 贵阳: 贵州大学, 2018) | |
| [20] | Xue W., Song B. A., Zhao H. J., Qi X. B., Huang Y. J., Liu X. H., Eur. J. Med. Chem.,2015, 97, 155—163 |
| [21] | Li P., Yin J., Xu W. M., Wu J., He M., Hu D. Y., Yang S., Song B. A., Chem. Biol. Drug Des.,2013, 82(5), 546—556 |
| [22] | Xu W. M., Han F. F., He M., Hu D. Y., He J., Yang S., Song B. A., J. Agric. Food Chem.,2012, 60(4), 1036—1041 |
| [23] | Chen Y., Wang Z. B., Zhang X., Xia L. J., Gong H. Y., Zhao H. J., Xue W., Chin. J. Org. Chem.,2014, 34(8), 1662—1668 |
| (陈玉, 王忠波, 张贤, 夏丽娟, 龚华玉, 赵洪菊, 薛伟. 有机化学,2014, 34(8), 1662—1668) |
| [1] | HU Haocheng, LI Wenli, ZHANG Jianing, LIU Yubo. Extraction, Structure Characterization and Biological Activities of Oligosaccharides from Auricularia heimuer [J]. Chem. J. Chinese Universities, 2021, 42(8): 2465. |
| [2] | PAN Yixiao, LI Yanwen, HAN Jiahong, ZHAO Haoqiang, FENG Yu, DING Xiangyuan, XU Lijin, FAN Qinghua, SHI Qian. Synthesis of 1,2,3,4-Tetrahydroquinoxalines Through a One-pot Tandem Reaction Involving Cyclization and Hydrogenation of Imine and Amide Moieties [J]. Chem. J. Chinese Universities, 2020, 41(10): 2239. |
| [3] | MAO Long, LIU Yuejun, FAN Shuhong. Preparation and Properties of Polypyrrole Modified Layered Clay/poly(ε-caprolactone) Antibacterial Nanocomposites [J]. Chem. J. Chinese Universities, 2019, 40(8): 1726. |
| [4] | LI Bing,WANG Xuemin,BAI Fengying,LIU Shuqing. Synthesises, Structures and Antibacterial Activities of a Series of Rare Earth Nitrogen Heterocyclic Complexes† [J]. Chem. J. Chinese Universities, 2019, 40(4): 632. |
| [5] | WAN Jinlin, WU Shouqun, GAN Yiyuan, MENG Jiao, WANG Zhenchao*, OUYANG Guiping*. Synthesis and Antibacterial Activities Evaluation of Chalconesemicarbazone Derivatives Bearing 1,3,4-Thiadiazole Moiety† [J]. Chem. J. Chinese Universities, 2018, 39(8): 1683. |
| [6] | JIA Yunjing, SHI Wensi, HU Feiliu, ZHU Huajie, LIU Li, MA Zhengyue. Cytotoxic Activity of Trichothecene Compounds and Derivatives from Myrothecium sp.† [J]. Chem. J. Chinese Universities, 2018, 39(8): 1668. |
| [7] | RUAN Xianghui, ZHAO Hongju, ZHANG Cheng, CHEN Lijuan, LI Pu, WANG Yihui, HE Ming, XUE Wei. Syntheses and Bioactivities of Myricetin Derivatives Containing Piperazine Acidamide Moiety† [J]. Chem. J. Chinese Universities, 2018, 39(6): 1197. |
| [8] | ZHANG Bingyang, MA Yangyang, GUO Hua, ZHU Huajie, LI Wan. Absolute Configuration Determination of Two Drimane Sesquiterpenoids from the Endophytic Fungi Talaromyces Purpureogenus of Panax notoginseng† [J]. Chem. J. Chinese Universities, 2017, 38(6): 1046. |
| [9] | DONG Xiaoming, YUAN Baoming, CHEN Bingpeng, HE Chaoliang, WANG Jincheng, CHEN Xuesi. In vitro Antibacterial Activity of PLGA-PEG-PLGA Thermosensitive Hydrogels Loaded with Vancomycin [J]. Chem. J. Chinese Universities, 2017, 38(5): 866. |
| [10] | FU Ranran, JI Xiujie, LIU Chao, REN Yanfei, WANG Gang, CHENG Bowen. Fabrication of Cellulose/Nano Lamellar ZnO Composite Antibacterial Fibers Using Ionic Liquid† [J]. Chem. J. Chinese Universities, 2017, 38(12): 2344. |
| [11] | MA Yangyang, CAI Hui, DU Min, CAO Fei, ZHU Huajie. Fractionation of Azaphilones from Secondary Metabolites of Marine-Derived Fungus Penicillim pinophilum and Their Antibacterial Activity† [J]. Chem. J. Chinese Universities, 2017, 38(11): 1963. |
| [12] | XIAO Wei, RUAN Xianghui, LI Qin, ZHANG Juping, ZHONG Xinmin, XIE Yan, WANG Xiaobin, HUANG Minguo, XUE Wei. Synthesis and Antibacterial Activities of Myricetin Derivatives ontaining Acidamide Moiety† [J]. Chem. J. Chinese Universities, 2017, 38(1): 35. |
| [13] | ZOU Haimin, ZHOU Chen, SUN Chengjun, LI Yongxin, YANG Xiaosong, WEN Jun, ZENG Hongyan. Simultaneous Determination of 7 Components in Functional Food for Anti-hangover and Hepatoprotection by Capillary Electrophoresis† [J]. Chem. J. Chinese Universities, 2016, 37(7): 1276. |
| [14] | GAO Tong, CAI Siyuan, XU Lanlan, CAO Fei, ZHU Huajie. Citrinin Derivatives from Marine-derived Fungus Penicillim Grisefulvum and Antibacterial Activity† [J]. Chem. J. Chinese Universities, 2016, 37(7): 1282. |
| [15] | WANG Hongyun, LIU Jinbiao, LU Junrui, YING Ming, YANG Xuyun, YANG Shuxun, MA Yao. Synthesis and Antibacterial Activities of 5-Methyl-1,2,4-triazole-3-thione Glucosides Compounds† [J]. Chem. J. Chinese Universities, 2016, 37(2): 246. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||