

Chem. J. Chinese Universities ›› 2025, Vol. 46 ›› Issue (5): 20240548.doi: 10.7503/cjcu20240548
• Organic Chemistry • Previous Articles Next Articles
SHE Huixian, CHEN Yangmi, YANG Jiaqiang( )
)
Received:2024-12-18
Online:2025-05-10
Published:2025-02-20
Contact:
YANG Jiaqiang
E-mail:yjqcn@126.com
Supported by:CLC Number:
TrendMD:
SHE Huixian, CHEN Yangmi, YANG Jiaqiang. Design, Synthesis and Antibacterial Activities of Thioxime Amide Derivatives Containing Thiopeptide[J]. Chem. J. Chinese Universities, 2025, 46(5): 20240548.
| Compd. | Appearance | Yield(%) | m. p./℃ | Elemental analysis(%), calcd.(found) | ||
|---|---|---|---|---|---|---|
| C | H | N | ||||
| 5a | White solid | 35 | 124—126 | 49.03(49.22) | 3.87(3.81) | 20.18(20.05) |
| 5b | White solid | 55 | 135—137 | 50.22(50.36) | 4.21(4.16) | 19.52(19.70) |
| 5c | Oily liquid | 68 | — | 50.22(50.07) | 4.21(4.28) | 19.52(19.44) |
| 5d | White solid | 52 | 117—119 | 50.22(50.13) | 4.21(4.26) | 19.52(19.43) |
| 5e | Oily liquid | 66 | — | 47.00(47.19) | 3.48(3.42) | 19.34(19.26) |
| 5f | White solid | 65 | 202—203 | 47.00(46.85) | 3.48(3.50) | 19.34(19.22) |
| Compd. | Appearance | Yield(%) | m. p./℃ | Elemental analysis(%), calcd.(found) | ||
| C | H | N | ||||
| 5g | White solid | 66 | 140—142 | 47.00(46.87) | 3.48(3.45) | 19.34(19.46) |
| 5h | White solid | 39 | 232—234 | 45.13(45.02) | 3.12(3.17) | 18.57(18.67) |
| 5i | White solid | 57 | 124—125 | 45.28(45.14) | 3.35(3.30) | 18.64(18.75) |
| 5j | White solid | 43 | 131—132 | 45.28(45.46) | 3.35(3.41) | 18.64(18.55) |
| 5k | White solid | 46 | 134—136 | 45.28(45.42) | 3.35(3.29) | 18.64(18.71) |
| 5l | White solid | 62 | 144—146 | 42.07(42.18) | 2.91(3.00) | 17.32(17.21) |
| 5m | White solid | 51 | 124—126 | 41.22(41.14) | 3.05(3.12) | 16.97(16.88) |
| 5n | White solid | 65 | 134—135 | 41.22(41.37) | 3.05(2.99) | 16.97(17.06) |
| 5o | White solid | 46 | 140—142 | 41.22(41.35) | 3.05(3.00) | 16.97(16.91) |
| 5p | White solid | 52 | 159—161 | 48.42(48.53) | 4.06(4.12) | 18.82(18.90) |
| 5q | White solid | 53 | 114—115 | 48.42(48.26) | 4.06(4.11) | 18.82(18.77) |
| 5r | White solid | 59 | 221—222 | 48.42(48.58) | 4.06(4.12) | 18.82(18.74) |
| 5s | Yellow solid | 38 | 132—134 | 44.25(44.06) | 3.28(3.32) | 21.25(21.17) |
| 5t | Yellow solid | 36 | 165—167 | 44.25(44.38) | 3.28(3.22) | 21.25(21.35) |
| 5u | White solid | 51 | 182—184 | 39.71(39.58) | 3.09(3.03) | 23.15(23.24) |
| 5v | White solid | 70 | 126—128 | 44.20(44.33) | 4.24(4.29) | 22.09(22.15) |
| 5w | White solid | 77 | 125—127 | 42.38(42.50) | 4.38(4.41) | 22.81(22.88) |
| 5x | White solid | 70 | 154—155 | 40.67(40.83) | 3.98(4.04) | 23.71(23.60) |
Table 1 Physical properties of compounds 5a—5x
| Compd. | Appearance | Yield(%) | m. p./℃ | Elemental analysis(%), calcd.(found) | ||
|---|---|---|---|---|---|---|
| C | H | N | ||||
| 5a | White solid | 35 | 124—126 | 49.03(49.22) | 3.87(3.81) | 20.18(20.05) |
| 5b | White solid | 55 | 135—137 | 50.22(50.36) | 4.21(4.16) | 19.52(19.70) |
| 5c | Oily liquid | 68 | — | 50.22(50.07) | 4.21(4.28) | 19.52(19.44) |
| 5d | White solid | 52 | 117—119 | 50.22(50.13) | 4.21(4.26) | 19.52(19.43) |
| 5e | Oily liquid | 66 | — | 47.00(47.19) | 3.48(3.42) | 19.34(19.26) |
| 5f | White solid | 65 | 202—203 | 47.00(46.85) | 3.48(3.50) | 19.34(19.22) |
| Compd. | Appearance | Yield(%) | m. p./℃ | Elemental analysis(%), calcd.(found) | ||
| C | H | N | ||||
| 5g | White solid | 66 | 140—142 | 47.00(46.87) | 3.48(3.45) | 19.34(19.46) |
| 5h | White solid | 39 | 232—234 | 45.13(45.02) | 3.12(3.17) | 18.57(18.67) |
| 5i | White solid | 57 | 124—125 | 45.28(45.14) | 3.35(3.30) | 18.64(18.75) |
| 5j | White solid | 43 | 131—132 | 45.28(45.46) | 3.35(3.41) | 18.64(18.55) |
| 5k | White solid | 46 | 134—136 | 45.28(45.42) | 3.35(3.29) | 18.64(18.71) |
| 5l | White solid | 62 | 144—146 | 42.07(42.18) | 2.91(3.00) | 17.32(17.21) |
| 5m | White solid | 51 | 124—126 | 41.22(41.14) | 3.05(3.12) | 16.97(16.88) |
| 5n | White solid | 65 | 134—135 | 41.22(41.37) | 3.05(2.99) | 16.97(17.06) |
| 5o | White solid | 46 | 140—142 | 41.22(41.35) | 3.05(3.00) | 16.97(16.91) |
| 5p | White solid | 52 | 159—161 | 48.42(48.53) | 4.06(4.12) | 18.82(18.90) |
| 5q | White solid | 53 | 114—115 | 48.42(48.26) | 4.06(4.11) | 18.82(18.77) |
| 5r | White solid | 59 | 221—222 | 48.42(48.58) | 4.06(4.12) | 18.82(18.74) |
| 5s | Yellow solid | 38 | 132—134 | 44.25(44.06) | 3.28(3.32) | 21.25(21.17) |
| 5t | Yellow solid | 36 | 165—167 | 44.25(44.38) | 3.28(3.22) | 21.25(21.35) |
| 5u | White solid | 51 | 182—184 | 39.71(39.58) | 3.09(3.03) | 23.15(23.24) |
| 5v | White solid | 70 | 126—128 | 44.20(44.33) | 4.24(4.29) | 22.09(22.15) |
| 5w | White solid | 77 | 125—127 | 42.38(42.50) | 4.38(4.41) | 22.81(22.88) |
| 5x | White solid | 70 | 154—155 | 40.67(40.83) | 3.98(4.04) | 23.71(23.60) |
| Compd. | 1H NMR(400 MHz, DMSO⁃d6), δ | 13C NMR(101 MHz, DMSO⁃d6), δ |
|---|---|---|
| 5a | 12.07(s, 1H), 9.22(t, J=6.0 Hz, 1H), 7.82—7.78(m, 2H), 7.62(s, 1H), 7.57(s, 2H), 7.42(t, J=7.6 Hz, 2H), 7.32(t, J=7.4 Hz, 1H), 7.08(s, 1H), 4.13(d, J=5.8 Hz, 2H), 3.85(s, 3H) | 169.44, 168.60, 163.67, 158.46, 149.47, 149.31, 142.81, 134.45, 129.32, 128.41, 126.22, 109.22, 109.01, 62.58, 42.21 |
| 5b | 11.97(s, 1H), 9.30(t, J=6.0 Hz, 1H), 7.64(s, 2H), 7.45—7.42(m, 1H), 7.28(s, 1H), 7.28—7.21(m, 3H), 7.06(s, 1H), 4.14(d, J=5.8 Hz, 2H), 3.84(s, 3H), 2.35(s, 3H) | 169.78, 168.65, 163.79, 157.61, 149.42, 149.08, 142.65, 135.89, 134.58, 131.23, 129.71, 128.43, 126.42, 111.97, 108.02, 62.58, 42.26, 21.10 |
| 5c | 12.08(s, 1H), 9.27(s, 1H), 7.65(s, 3H), 7.61(d, J=8.2 Hz, 1H, 7.57(s, 1H), 7.32(t, J=7.6 Hz, 1H), 7.13(s, 2H), 4.21(d, J=6.3 Hz, 2H), 3.91(s, 3H), 2.67(s, 3H) | 169.64, 168.64, 165.11, 163.76, 158.46, 149.51, 142.81, 138.48, 134.43, 129.23, 129.08, 126.93, 123.43, 109.05, 108.83, 62.62, 42.31, 21.56 |
| 5d | 12.06(s, 1H), 9.24(t, J=6.0 Hz, 1H), 7.69(d, J=7.9 Hz, 2H), 7.62(s, 2H), 7.53(s, 1H), 7.22(d, J=7.9 Hz, 2H), 7.09(s, 1H), 4.15(d, J=5.8 Hz, 2H), 3.86(s, 3H), 2.29(s, 3H) | 169.53, 168.59, 163.69, 158.39, 149.46, 149.40, 142.83, 137.73, 131.82, 129.88, 126.20, 108.87, 108.38, 62.57, 42.23, 21.24 |
| 5e | 12.19(s, 1H), 9.30(t, J=5.9 Hz, 1H), 7.97(dt, J=8.6, 4.2 Hz, 1H), 7.62(d, J=2.3 Hz, 1H), 7.57(s, 2H), 7.47(qt, J=5.2, 2.9 Hz, 1H), 7.37(t, J=7.0 Hz, 2H), 7.16(s, 1H), 4.22(d, J=5.8 Hz, 2H), 3.94(s, 3H) | 169.45, 168.68, 163.72, 157.96, 149.47, 142.98, 142.83, 130.23(d, J=8.5 Hz), 129.68, 125.40, 116.81, 116.59, 113.48, 113.35, 109.17, 62.61, 42.23 |
| 5f | 12.12(s, 1H), 9.19(t, J=5.9 Hz, 1H), 7.74(s, 1H), 7.66(dt, J=7.8, 1.2 Hz, 1H), 7.64—7.58(m, 1H), 7.51(s, 2H), 7.49—7.41(m, 1H), 7.14(td, J=8.6, 2.6 Hz, 1H), 7.08(s, 1H), 4.15(d, J=5.8 Hz, 2H), 3.86(s, 3H) | 169.39, 168.66, 164.22, 163.64, 161.80, 158.55, 149.55, 148.01(d, J=2.7 Hz), 142.93, 136.86(d, J=8.2 Hz), 131.35(d, J=8.3 Hz), 122.24(d, J=2.7 Hz), 115.07(d, J=21.0 Hz), 112.81(d, J=22.9 Hz), 110.50, 62.56, 42.22 |
| 5g | 12.08(s, 1H), 9.21(t, J=6.0 Hz, 1H), 7.88—7.81(m, 2H), 7.60(s, 1H), 7.54(s, 2H), 7.24(t, J=8.9 Hz, 2H), 7.08(s, 1H), 4.14(d, J=5.8 Hz, 2H), 3.85(s, 3H) | 169.48, 168.62, 163.69, 163.49, 161.06, 158.57, 149.51, 148.33, 142.86, 131.11(d, J=3.1 Hz), 128.29(d, J=8.3 Hz), 116.15(d, J=21.6 Hz), 109.62—108.43(m), 62.58, 42.23 |
| 5h | 12.12(s, 1H), 9.21(t, J=5.9 Hz, 1H), 7.90(td, J=8.8, 6.6 Hz, 1H), 7.50(s, 2H), 7.49(s, 1H), 7.38—7.28(m, 1H), 7.16 (td, J=8.4, 2.5 Hz, 1H), 7.07(s, 1H), 4.15(d, J=5.9 Hz, 2H), 3.85(s, 3H) | 169.42, 168.67, 163.66, 161.18(d, J=12.9 Hz), 160.77(d, J=12.6 Hz), 158.67(d, J=12.8 Hz), 158.02, 149.54, 142.92, 142.16(d, J=2.3 Hz), 130.89, 119.06, 112.90, 109.21(d, J=11.6 Hz), 105.15(d, J=10.4 Hz), 62.55, 42.22 |
| Compd. | 1H NMR(400 MHz, DMSO⁃d6), δ | 13C NMR(101 MHz, DMSO⁃d6), δ |
| 5i | 12.00(s, 1H), 9.30(t, J=6.0 Hz, 1H), 7.63(dd, J=7.3, 2.5 Hz, 1H), 7.61(s, 2H), 7.54(s, 1H), 7.51(d, J=2.1 Hz, 1H), 7.38(td, J=4.7, 2.2 Hz, 2H), 7.05(s, 1H), 4.14(d, J=5.9 Hz, 2H), 3.84(s, 3H) | 169.79, 168.74, 163.87, 157.74, 149.30, 145.90, 142.53, 133.25, 131.54, 130.72, 130.13, 127.92, 113.82, 108.18, 62.62, 42.24 |
| 5j | 12.18(s, 1H), 9.18(t, J=5.9 Hz, 1H), 7.94—7.86(m, 1H), 7.79(s, 2H), 7.53—7.34(m, 4H), 7.07(d, J=4.4 Hz, 1H), 4.14(d, J=6.0 Hz, 2H), 3.85(s, 3H) | 169.27, 168.61, 163.60, 158.53, 149.57, 147.70, 143.01, 136.52, 134.10, 131.23, 128.11, 125.93, 124.70, 110.54, 109.61, 62.53, 42.19 |
| 5k | 12.08(s, 1H), 9.21(t, J=6.0 Hz, 1H), 7.84—7.78(m, 2H), 7.66(s, 1H), 7.53(s, 2H), 7.49—7.43(m, 2H), 7.07(s, 1H), 4.14(d, J=5.8 Hz, 2H), 3.85(s, 3H) | 169.44, 168.65, 163.69, 158.63, 149.48, 148.09, 142.84, 133.33, 132.89, 129.33, 127.96, 109.91, 109.10, 62.60, 42.22 |
| 5l | 12.19(s, 1H), 9.17(t, J=5.9 Hz, 1H), 8.07(d, J=2.1 Hz, 1H), 7.83(s, 1H), 7.80(dd, J=8.4, 2.1 Hz, 1H), 7.67(d, J=8.4 Hz, 1H), 7.43(s, 2H), 7.06(s, 1H), 4.13(d, J=5.7 Hz, 2H), 3.84(s, 3H) | 169.22, 168.62, 163.58, 158.65, 149.57, 146.72, 143.03, 135.07, 132.08, 131.52, 130.64, 127.91, 126.21, 111.08, 109.66, 62.52, 42.17 |
| 5m | 12.02(s, 1H), 9.34(t, J=6.0 Hz, 1H), 7.75(d, J=8.0 Hz, 1H), 7.66(s, 2H), 7.59(dd, J=7.7, 1.8 Hz, 1H), 7.52(s, 1H), 7.48(t, J=7.6 Hz, 1H), 7.35(dt, J=7.8, 3.9 Hz, 1H), 7.09(d, J=4.3 Hz, 1H), 4.18(d, J=5.9 Hz, 2H), 3.89 (s, 3H) | 169.85, 168.77, 163.84, 157.78, 149.41, 147.62, 142.62, 135.62, 133.86, 131.83, 130.45, 128.37, 121.87, 113.50, 107.90, 62.63, 42.30 |
| 5n | 12.17(s, 1H), 9.17(t, J=5.9 Hz, 1H), 8.04(t, J=1.9 Hz, 1H), 7.82(dt, J=7.7, 1.3 Hz, 1H), 7.77(s, 1H), 7.50(dd, J=8.2, 2.0 Hz, 1H), 7.44(s, 2H), 7.38(t, J=7.9 Hz, 1H), 7.07(s, 1H), 4.13(d, J=5.8 Hz, 2H), 3.84(s, 3H) | 169.26, 168.59, 163.60, 158.52, 149.56, 147.58, 143.00, 136.74, 131.50, 131.00, 128.82, 125.05, 122.70, 110.51, 109.66, 62.54, 42.18 |
| 5o | 12.11(s, 1H), 9.22(t, J=6.0 Hz, 1H), 7.77—7.71(m, 2H), 7.67(d, J=1.3 Hz, 1H), 7.60(d, J=8.6 Hz, 2H), 7.55(s, 2H), 7.08(s, 1H), 4.15(d, J=5.8 Hz, 2H), 3.85(s, 3H) | 169.44, 168.66, 163.68, 158.64, 149.50, 148.14, 142.88, 133.69, 132.23, 128.26, 121.50, 109.97, 109.12, 62.58, 42.22 |
| 5p | 12.00(s, 1H), 9.24(t, J=6.0 Hz, 1H), 7.91—7.84(m, 1H), 7.63(s, 1H), 7.57(s, 2H), 7.33(t, J=7.8 Hz, 1H), 7.12(d, J=8.6 Hz, 2H), 7.04(t, J=7.5 Hz, 1H), 4.18(d, J=5.9 Hz, 2H), 3.89(s, 6H, 2OCH3) | 169.59, 168.53, 163.73, 156.99, 156.94, 149.52, 145.32, 142.80, 129.55, 129.41, 122.89, 121.02, 112.64, 112.13, 108.80, 62.61, 55.88, 42.29 |
| 5q | 12.20(s, 1H), 9.29(t, J=5.8 Hz, 1H), 7.62(s, 1H), 7.41(s, 1H), 7.39(s, 2H), 7.31(d, J=8.0 Hz, 1H), 7.14(s, 1H), 6.90—6.86(m, 1H), 6.86(s, 1H), 4.18(d, J=5.8 Hz, 2H), 3.89(s, 3H), 3.76(s, 3H) | 170.18, 168.40, 162.78, 160.05, 158.24, 149.22, 148.17, 139.93, 135.88, 130.32, 118.52, 114.19, 111.34, 109.77, 109.33, 62.79, 55.42, 42.24 |
| 5r | 12.02(s, 1H), 9.22(t, J=6.0 Hz, 1H), 7.73(d, J=8.5 Hz, 2H), 7.59(s, 2H), 7.45(s, 1H), 7.08(s, 1H), 7.00—6.91(m, 2H), 4.13(d, J=5.8 Hz, 2H), 3.85(s, 3H), 3.76(s, 3H) | 169.49, 168.53, 163.66, 159.49, 158.32, 149.51, 149.22, 142.82, 127.60, 127.29, 114.65, 108.90, 107.28, 62.57, 55.59, 42.22 |
| 5s | 12.07(s, 1H), 9.17(t, J=5.9 Hz, 1H), 7.90(d, J=8.0 Hz, 1H), 7.76—7.70(m, 2H), 7.63—7.59(m, 1H), 7.53(s, 1H), 7.39(s, 2H), 7.04(s, 1H), 4.13(d, J=5.8 Hz, 2H), 3.83(s, 3H) | 169.28, 168.67, 163.60, 158.27, 149.59, 148.96, 145.28, 143.02, 133.20, 131.42, 129.92, 128.66, 124.58, 112.77, 109.61, 62.52, 42.14 |
| 5t | 12.20(s, 1H), 9.18(t, J=6.0 Hz, 1H), 8.61(t, J=2.0 Hz, 1H), 8.24(d, J=7.9 Hz, 1H), 8.15(dd, J=8.3, 2.3 Hz, 1H), 7.88(s, 1H), 7.70(t, J=8.0 Hz, 1H), 7.39(s, 2H), 7.06(s, 1H), 4.13(d, J=5.8 Hz, 2H), 3.84(s, 3H) | 169.31, 168.65, 163.73, 158.81, 149.42, 148.73, 146.88, 142.86, 135.91, 132.30, 130.98, 122.91, 120.58, 111.53, 109.70, 60.31, 42.18 |
| 5u | 12.28(s, 1H), 9.20(t, J=5.8 Hz, 1H), 7.91(d, J=3.4 Hz, 1H), 7.84(s, 1H), 7.78(d, J=3.3 Hz, 1H), 7.48(s, 2H), 7.08(s, 1H), 4.16(d, J=5.8 Hz, 2H), 3.88(s, 3H) | 169.34, 168.82, 163.65, 162.33, 159.00, 149.55, 144.35, 143.54, 142.96, 120.93, 111.81, 109.42, 62.58, 42.21 |
| 5v | 11.76(s, 1H), 9.23(t, J=6.0 Hz, 1H), 7.74(s, 2H), 7.05(s, 1H), 6.81(s, 1H), 4.06(d, J=5.9 Hz, 2H), 3.83(s, 3H), 1.92(t, J=4.7 Hz, 1H), 0.84(d, J=8.4, 2.3 Hz, 2H), 0.66(d, J=4.9, 2.1 Hz, 2H) | 169.68, 168.26, 163.69, 157.84, 152.71, 149.40, 142.65, 108.29, 106.27, 62.56, 42.15, 12.22(d, J=10.0 Hz), 7.89 |
| Compd. | 1H NMR(400 MHz, DMSO⁃d6), δ | 13C NMR(101 MHz, DMSO⁃d6), δ |
| 5w | 11.70(s, 1H), 9.31(t, J=6.1 Hz, 1H), 8.03(s, 2H), 7.06(s, 1H), 6.83(s, 1H), 4.08(d, J=6.1 Hz, 2H), 3.84(s, 3H), 2.57(d, J=7.6 Hz, 2H), 1.15(t, J=7.5 Hz, 3H) | 170.14, 168.44, 163.88, 158.04, 152.64, 149.34, 142.49, 107.81, 107.00, 62.64, 42.25, 24.35, 13.76 |
| 5x | 11.70(s, 1H), 9.36(t, J=6.1 Hz, 1H), 8.09(s, 2H), 7.10(s, 1H), 6.84(s, 1H), 4.12(d, J=6.1 Hz, 2H), 3.87(s, 3H), 2.24(s, 3H) | 170.29, 168.42, 164.00, 157.91, 149.26, 146.44, 142.41, 109.04, 106.87, 62.68, 42.25, 16.68 |
Table 2 1H NMR and 13C NMR data of compounds 5a—5x
| Compd. | 1H NMR(400 MHz, DMSO⁃d6), δ | 13C NMR(101 MHz, DMSO⁃d6), δ |
|---|---|---|
| 5a | 12.07(s, 1H), 9.22(t, J=6.0 Hz, 1H), 7.82—7.78(m, 2H), 7.62(s, 1H), 7.57(s, 2H), 7.42(t, J=7.6 Hz, 2H), 7.32(t, J=7.4 Hz, 1H), 7.08(s, 1H), 4.13(d, J=5.8 Hz, 2H), 3.85(s, 3H) | 169.44, 168.60, 163.67, 158.46, 149.47, 149.31, 142.81, 134.45, 129.32, 128.41, 126.22, 109.22, 109.01, 62.58, 42.21 |
| 5b | 11.97(s, 1H), 9.30(t, J=6.0 Hz, 1H), 7.64(s, 2H), 7.45—7.42(m, 1H), 7.28(s, 1H), 7.28—7.21(m, 3H), 7.06(s, 1H), 4.14(d, J=5.8 Hz, 2H), 3.84(s, 3H), 2.35(s, 3H) | 169.78, 168.65, 163.79, 157.61, 149.42, 149.08, 142.65, 135.89, 134.58, 131.23, 129.71, 128.43, 126.42, 111.97, 108.02, 62.58, 42.26, 21.10 |
| 5c | 12.08(s, 1H), 9.27(s, 1H), 7.65(s, 3H), 7.61(d, J=8.2 Hz, 1H, 7.57(s, 1H), 7.32(t, J=7.6 Hz, 1H), 7.13(s, 2H), 4.21(d, J=6.3 Hz, 2H), 3.91(s, 3H), 2.67(s, 3H) | 169.64, 168.64, 165.11, 163.76, 158.46, 149.51, 142.81, 138.48, 134.43, 129.23, 129.08, 126.93, 123.43, 109.05, 108.83, 62.62, 42.31, 21.56 |
| 5d | 12.06(s, 1H), 9.24(t, J=6.0 Hz, 1H), 7.69(d, J=7.9 Hz, 2H), 7.62(s, 2H), 7.53(s, 1H), 7.22(d, J=7.9 Hz, 2H), 7.09(s, 1H), 4.15(d, J=5.8 Hz, 2H), 3.86(s, 3H), 2.29(s, 3H) | 169.53, 168.59, 163.69, 158.39, 149.46, 149.40, 142.83, 137.73, 131.82, 129.88, 126.20, 108.87, 108.38, 62.57, 42.23, 21.24 |
| 5e | 12.19(s, 1H), 9.30(t, J=5.9 Hz, 1H), 7.97(dt, J=8.6, 4.2 Hz, 1H), 7.62(d, J=2.3 Hz, 1H), 7.57(s, 2H), 7.47(qt, J=5.2, 2.9 Hz, 1H), 7.37(t, J=7.0 Hz, 2H), 7.16(s, 1H), 4.22(d, J=5.8 Hz, 2H), 3.94(s, 3H) | 169.45, 168.68, 163.72, 157.96, 149.47, 142.98, 142.83, 130.23(d, J=8.5 Hz), 129.68, 125.40, 116.81, 116.59, 113.48, 113.35, 109.17, 62.61, 42.23 |
| 5f | 12.12(s, 1H), 9.19(t, J=5.9 Hz, 1H), 7.74(s, 1H), 7.66(dt, J=7.8, 1.2 Hz, 1H), 7.64—7.58(m, 1H), 7.51(s, 2H), 7.49—7.41(m, 1H), 7.14(td, J=8.6, 2.6 Hz, 1H), 7.08(s, 1H), 4.15(d, J=5.8 Hz, 2H), 3.86(s, 3H) | 169.39, 168.66, 164.22, 163.64, 161.80, 158.55, 149.55, 148.01(d, J=2.7 Hz), 142.93, 136.86(d, J=8.2 Hz), 131.35(d, J=8.3 Hz), 122.24(d, J=2.7 Hz), 115.07(d, J=21.0 Hz), 112.81(d, J=22.9 Hz), 110.50, 62.56, 42.22 |
| 5g | 12.08(s, 1H), 9.21(t, J=6.0 Hz, 1H), 7.88—7.81(m, 2H), 7.60(s, 1H), 7.54(s, 2H), 7.24(t, J=8.9 Hz, 2H), 7.08(s, 1H), 4.14(d, J=5.8 Hz, 2H), 3.85(s, 3H) | 169.48, 168.62, 163.69, 163.49, 161.06, 158.57, 149.51, 148.33, 142.86, 131.11(d, J=3.1 Hz), 128.29(d, J=8.3 Hz), 116.15(d, J=21.6 Hz), 109.62—108.43(m), 62.58, 42.23 |
| 5h | 12.12(s, 1H), 9.21(t, J=5.9 Hz, 1H), 7.90(td, J=8.8, 6.6 Hz, 1H), 7.50(s, 2H), 7.49(s, 1H), 7.38—7.28(m, 1H), 7.16 (td, J=8.4, 2.5 Hz, 1H), 7.07(s, 1H), 4.15(d, J=5.9 Hz, 2H), 3.85(s, 3H) | 169.42, 168.67, 163.66, 161.18(d, J=12.9 Hz), 160.77(d, J=12.6 Hz), 158.67(d, J=12.8 Hz), 158.02, 149.54, 142.92, 142.16(d, J=2.3 Hz), 130.89, 119.06, 112.90, 109.21(d, J=11.6 Hz), 105.15(d, J=10.4 Hz), 62.55, 42.22 |
| Compd. | 1H NMR(400 MHz, DMSO⁃d6), δ | 13C NMR(101 MHz, DMSO⁃d6), δ |
| 5i | 12.00(s, 1H), 9.30(t, J=6.0 Hz, 1H), 7.63(dd, J=7.3, 2.5 Hz, 1H), 7.61(s, 2H), 7.54(s, 1H), 7.51(d, J=2.1 Hz, 1H), 7.38(td, J=4.7, 2.2 Hz, 2H), 7.05(s, 1H), 4.14(d, J=5.9 Hz, 2H), 3.84(s, 3H) | 169.79, 168.74, 163.87, 157.74, 149.30, 145.90, 142.53, 133.25, 131.54, 130.72, 130.13, 127.92, 113.82, 108.18, 62.62, 42.24 |
| 5j | 12.18(s, 1H), 9.18(t, J=5.9 Hz, 1H), 7.94—7.86(m, 1H), 7.79(s, 2H), 7.53—7.34(m, 4H), 7.07(d, J=4.4 Hz, 1H), 4.14(d, J=6.0 Hz, 2H), 3.85(s, 3H) | 169.27, 168.61, 163.60, 158.53, 149.57, 147.70, 143.01, 136.52, 134.10, 131.23, 128.11, 125.93, 124.70, 110.54, 109.61, 62.53, 42.19 |
| 5k | 12.08(s, 1H), 9.21(t, J=6.0 Hz, 1H), 7.84—7.78(m, 2H), 7.66(s, 1H), 7.53(s, 2H), 7.49—7.43(m, 2H), 7.07(s, 1H), 4.14(d, J=5.8 Hz, 2H), 3.85(s, 3H) | 169.44, 168.65, 163.69, 158.63, 149.48, 148.09, 142.84, 133.33, 132.89, 129.33, 127.96, 109.91, 109.10, 62.60, 42.22 |
| 5l | 12.19(s, 1H), 9.17(t, J=5.9 Hz, 1H), 8.07(d, J=2.1 Hz, 1H), 7.83(s, 1H), 7.80(dd, J=8.4, 2.1 Hz, 1H), 7.67(d, J=8.4 Hz, 1H), 7.43(s, 2H), 7.06(s, 1H), 4.13(d, J=5.7 Hz, 2H), 3.84(s, 3H) | 169.22, 168.62, 163.58, 158.65, 149.57, 146.72, 143.03, 135.07, 132.08, 131.52, 130.64, 127.91, 126.21, 111.08, 109.66, 62.52, 42.17 |
| 5m | 12.02(s, 1H), 9.34(t, J=6.0 Hz, 1H), 7.75(d, J=8.0 Hz, 1H), 7.66(s, 2H), 7.59(dd, J=7.7, 1.8 Hz, 1H), 7.52(s, 1H), 7.48(t, J=7.6 Hz, 1H), 7.35(dt, J=7.8, 3.9 Hz, 1H), 7.09(d, J=4.3 Hz, 1H), 4.18(d, J=5.9 Hz, 2H), 3.89 (s, 3H) | 169.85, 168.77, 163.84, 157.78, 149.41, 147.62, 142.62, 135.62, 133.86, 131.83, 130.45, 128.37, 121.87, 113.50, 107.90, 62.63, 42.30 |
| 5n | 12.17(s, 1H), 9.17(t, J=5.9 Hz, 1H), 8.04(t, J=1.9 Hz, 1H), 7.82(dt, J=7.7, 1.3 Hz, 1H), 7.77(s, 1H), 7.50(dd, J=8.2, 2.0 Hz, 1H), 7.44(s, 2H), 7.38(t, J=7.9 Hz, 1H), 7.07(s, 1H), 4.13(d, J=5.8 Hz, 2H), 3.84(s, 3H) | 169.26, 168.59, 163.60, 158.52, 149.56, 147.58, 143.00, 136.74, 131.50, 131.00, 128.82, 125.05, 122.70, 110.51, 109.66, 62.54, 42.18 |
| 5o | 12.11(s, 1H), 9.22(t, J=6.0 Hz, 1H), 7.77—7.71(m, 2H), 7.67(d, J=1.3 Hz, 1H), 7.60(d, J=8.6 Hz, 2H), 7.55(s, 2H), 7.08(s, 1H), 4.15(d, J=5.8 Hz, 2H), 3.85(s, 3H) | 169.44, 168.66, 163.68, 158.64, 149.50, 148.14, 142.88, 133.69, 132.23, 128.26, 121.50, 109.97, 109.12, 62.58, 42.22 |
| 5p | 12.00(s, 1H), 9.24(t, J=6.0 Hz, 1H), 7.91—7.84(m, 1H), 7.63(s, 1H), 7.57(s, 2H), 7.33(t, J=7.8 Hz, 1H), 7.12(d, J=8.6 Hz, 2H), 7.04(t, J=7.5 Hz, 1H), 4.18(d, J=5.9 Hz, 2H), 3.89(s, 6H, 2OCH3) | 169.59, 168.53, 163.73, 156.99, 156.94, 149.52, 145.32, 142.80, 129.55, 129.41, 122.89, 121.02, 112.64, 112.13, 108.80, 62.61, 55.88, 42.29 |
| 5q | 12.20(s, 1H), 9.29(t, J=5.8 Hz, 1H), 7.62(s, 1H), 7.41(s, 1H), 7.39(s, 2H), 7.31(d, J=8.0 Hz, 1H), 7.14(s, 1H), 6.90—6.86(m, 1H), 6.86(s, 1H), 4.18(d, J=5.8 Hz, 2H), 3.89(s, 3H), 3.76(s, 3H) | 170.18, 168.40, 162.78, 160.05, 158.24, 149.22, 148.17, 139.93, 135.88, 130.32, 118.52, 114.19, 111.34, 109.77, 109.33, 62.79, 55.42, 42.24 |
| 5r | 12.02(s, 1H), 9.22(t, J=6.0 Hz, 1H), 7.73(d, J=8.5 Hz, 2H), 7.59(s, 2H), 7.45(s, 1H), 7.08(s, 1H), 7.00—6.91(m, 2H), 4.13(d, J=5.8 Hz, 2H), 3.85(s, 3H), 3.76(s, 3H) | 169.49, 168.53, 163.66, 159.49, 158.32, 149.51, 149.22, 142.82, 127.60, 127.29, 114.65, 108.90, 107.28, 62.57, 55.59, 42.22 |
| 5s | 12.07(s, 1H), 9.17(t, J=5.9 Hz, 1H), 7.90(d, J=8.0 Hz, 1H), 7.76—7.70(m, 2H), 7.63—7.59(m, 1H), 7.53(s, 1H), 7.39(s, 2H), 7.04(s, 1H), 4.13(d, J=5.8 Hz, 2H), 3.83(s, 3H) | 169.28, 168.67, 163.60, 158.27, 149.59, 148.96, 145.28, 143.02, 133.20, 131.42, 129.92, 128.66, 124.58, 112.77, 109.61, 62.52, 42.14 |
| 5t | 12.20(s, 1H), 9.18(t, J=6.0 Hz, 1H), 8.61(t, J=2.0 Hz, 1H), 8.24(d, J=7.9 Hz, 1H), 8.15(dd, J=8.3, 2.3 Hz, 1H), 7.88(s, 1H), 7.70(t, J=8.0 Hz, 1H), 7.39(s, 2H), 7.06(s, 1H), 4.13(d, J=5.8 Hz, 2H), 3.84(s, 3H) | 169.31, 168.65, 163.73, 158.81, 149.42, 148.73, 146.88, 142.86, 135.91, 132.30, 130.98, 122.91, 120.58, 111.53, 109.70, 60.31, 42.18 |
| 5u | 12.28(s, 1H), 9.20(t, J=5.8 Hz, 1H), 7.91(d, J=3.4 Hz, 1H), 7.84(s, 1H), 7.78(d, J=3.3 Hz, 1H), 7.48(s, 2H), 7.08(s, 1H), 4.16(d, J=5.8 Hz, 2H), 3.88(s, 3H) | 169.34, 168.82, 163.65, 162.33, 159.00, 149.55, 144.35, 143.54, 142.96, 120.93, 111.81, 109.42, 62.58, 42.21 |
| 5v | 11.76(s, 1H), 9.23(t, J=6.0 Hz, 1H), 7.74(s, 2H), 7.05(s, 1H), 6.81(s, 1H), 4.06(d, J=5.9 Hz, 2H), 3.83(s, 3H), 1.92(t, J=4.7 Hz, 1H), 0.84(d, J=8.4, 2.3 Hz, 2H), 0.66(d, J=4.9, 2.1 Hz, 2H) | 169.68, 168.26, 163.69, 157.84, 152.71, 149.40, 142.65, 108.29, 106.27, 62.56, 42.15, 12.22(d, J=10.0 Hz), 7.89 |
| Compd. | 1H NMR(400 MHz, DMSO⁃d6), δ | 13C NMR(101 MHz, DMSO⁃d6), δ |
| 5w | 11.70(s, 1H), 9.31(t, J=6.1 Hz, 1H), 8.03(s, 2H), 7.06(s, 1H), 6.83(s, 1H), 4.08(d, J=6.1 Hz, 2H), 3.84(s, 3H), 2.57(d, J=7.6 Hz, 2H), 1.15(t, J=7.5 Hz, 3H) | 170.14, 168.44, 163.88, 158.04, 152.64, 149.34, 142.49, 107.81, 107.00, 62.64, 42.25, 24.35, 13.76 |
| 5x | 11.70(s, 1H), 9.36(t, J=6.1 Hz, 1H), 8.09(s, 2H), 7.10(s, 1H), 6.84(s, 1H), 4.12(d, J=6.1 Hz, 2H), 3.87(s, 3H), 2.24(s, 3H) | 170.29, 168.42, 164.00, 157.91, 149.26, 146.44, 142.41, 109.04, 106.87, 62.68, 42.25, 16.68 |
| Compd. | R | MIC/(μg·mL-1) | |||
|---|---|---|---|---|---|
| S. aureus | E. coli | MRSA | FREC | ||
| 5a | 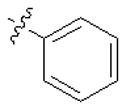 | 64 | 128 | 64 | >128 |
| 5b | 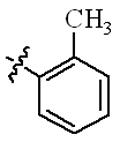 | 64 | >128 | 128 | >128 |
| 5c | 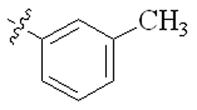 | 128 | 128 | 128 | >128 |
| 5d | 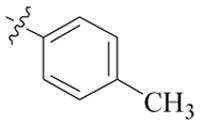 | >128 | >128 | >128 | >128 |
| 5e | 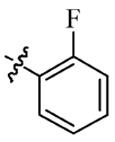 | 16 | 128 | 32 | 128 |
| 5f | 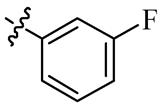 | 32 | 128 | 64 | >128 |
| 5g | 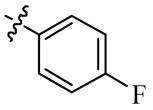 | 64 | 128 | 128 | >128 |
| 5h | 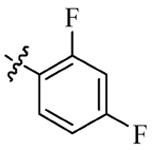 | 2 | 64 | 4 | 128 |
| 5i | 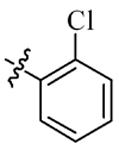 | 32 | 128 | 32 | 128 |
| 5j | 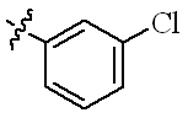 | 64 | >128 | 64 | >128 |
| 5k | 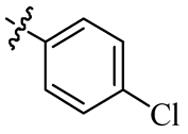 | 128 | >128 | >128 | >128 |
| 5l | 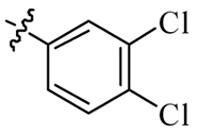 | 64 | 128 | 128 | 128 |
| 5m | 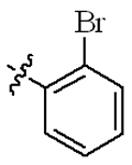 | 32 | 128 | 64 | 128 |
| 5n | 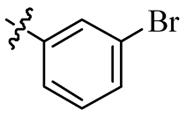 | 64 | 128 | 64 | >128 |
| 5o | 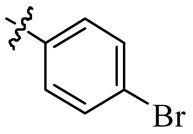 | >128 | >128 | >128 | >128 |
| 5p | 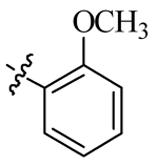 | 32 | 64 | 32 | 128 |
| Compd. | R | MIC/(μg·mL-1) | |||
| S. aureus | E. coli | MRSA | FREC | ||
| 5q | 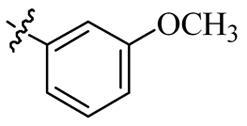 | 64 | >128 | 64 | >128 |
| 5r | 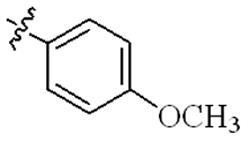 | 64 | 128 | 64 | >128 |
| 5s | 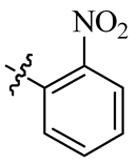 | 4 | 64 | 8 | 128 |
| 5t | 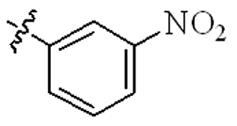 | 16 | 64 | 32 | >128 |
| 5u | 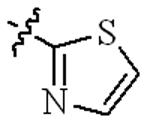 | 0.25 | 32 | 2 | 64 |
| 5v | 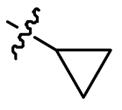 | 0.5 | 32 | 2 | 64 |
| 5w | C2H5 | 32 | 128 | 64 | 128 |
| 5x | CH3 | 64 | >128 | 128 | >128 |
| Oxacillin | 0.5 | 16 | >128 | 64 | |
Table 3 MIC values of different target compounds
| Compd. | R | MIC/(μg·mL-1) | |||
|---|---|---|---|---|---|
| S. aureus | E. coli | MRSA | FREC | ||
| 5a |  | 64 | 128 | 64 | >128 |
| 5b |  | 64 | >128 | 128 | >128 |
| 5c |  | 128 | 128 | 128 | >128 |
| 5d |  | >128 | >128 | >128 | >128 |
| 5e |  | 16 | 128 | 32 | 128 |
| 5f |  | 32 | 128 | 64 | >128 |
| 5g |  | 64 | 128 | 128 | >128 |
| 5h |  | 2 | 64 | 4 | 128 |
| 5i |  | 32 | 128 | 32 | 128 |
| 5j |  | 64 | >128 | 64 | >128 |
| 5k |  | 128 | >128 | >128 | >128 |
| 5l |  | 64 | 128 | 128 | 128 |
| 5m |  | 32 | 128 | 64 | 128 |
| 5n |  | 64 | 128 | 64 | >128 |
| 5o |  | >128 | >128 | >128 | >128 |
| 5p |  | 32 | 64 | 32 | 128 |
| Compd. | R | MIC/(μg·mL-1) | |||
| S. aureus | E. coli | MRSA | FREC | ||
| 5q |  | 64 | >128 | 64 | >128 |
| 5r |  | 64 | 128 | 64 | >128 |
| 5s |  | 4 | 64 | 8 | 128 |
| 5t |  | 16 | 64 | 32 | >128 |
| 5u |  | 0.25 | 32 | 2 | 64 |
| 5v |  | 0.5 | 32 | 2 | 64 |
| 5w | C2H5 | 32 | 128 | 64 | 128 |
| 5x | CH3 | 64 | >128 | 128 | >128 |
| Oxacillin | 0.5 | 16 | >128 | 64 | |
| 1 | Shao W. H., Hu X., Shang J., Lin F., Jin L. M., Quan C. S., Zhang Y. M., Li J., Chem. J. Chinese Universities, 2022, 43(10), 20220132 |
| 邵文惠, 胡欣, 尚静, 林峰, 金黎明, 权春善, 张艳梅, 李军. 高等学校化学学报, 2022, 43(10), 20220132 | |
| 2 | Darby E. M., Trampari E., Siasat P., Gaya M. S., Alav I., Webber M. A., Blair J. M., Nat. Rev. Microbiol., 2023, 21(5), 280—295 |
| 3 | Ungureanu D., Tiperciuc B., Nastasă C., Ionut I., Marc G., Oniga I., Oniga O., Pharmaceutics, 2024, 16(1), 89 |
| 4 | Sharma P. C., Bansal K. K., Sharma A., Sharma D., Deep A., Eur. J. Med. Chem., 2020, 188, 112016 |
| 5 | Singh A., Malhotra D., Singh K., Chadha R., Bedi P. M. S., J. Mol. Struct., 2022, 1266, 133479 |
| 6 | Das D., Sikdar P., Bairagi M., Eur. J. Med. Chem., 2016, 109, 89—98 |
| 7 | Chen Y. C., Li Z., Lin T., Li Z., Chen D. J., Xu X. Y., Eur. J. Med. Chem., 2024, 279, 116879 |
| 8 | Chen J. P., Battini N., Ansari M. F., Zhou C. H., Eur. J. Med. Chem., 2021, 217, 113340 |
| 9 | Dhuguru J., Zviagin E., Skouta R., Pharmaceuticals, 2022, 15(1), 66 |
| 10 | Bagley M. C., Dale J. W., Merritt E. A., Xiong X., Chem. Rev., 2005, 105(2), 685—714 |
| 11 | Mocek U., Knaggs A. R., Tsuchiya R., Nguyen T., Beale J. M., Floss H. G., J. Am. Chem. Soc., 1993, 115(17), 7557—7568 |
| 12 | Zipperer A., Konnerth M. C., Laux C., Berscheid A., Janek D., Weidenmaier C., Burian M., Schilling N. A., Slavetinsky C., Marschal M., Schittek B., Br tz⁃Oesterhelt H., Grond S., Peschel A., Krismer B., Nature, 2016, 535(7613), 511—516 |
| 13 | Zhang E., Chen D. D., Wang S. F., Liu W., Chin. J. Org. Chem., 2020, 40(10), 3120—3131 |
| 张鄂, 陈单丹, 王守锋, 刘文. 有机化学, 2020, 40(10), 3120—3131 | |
| 14 | Chan D. C. K., Burrows L. L., J. Antibiot., 2021, 74(3), 161—175 |
| 15 | Just⁃Baringo X., Albericio F., Álvarez M., Mar. Drugs, 2014, 12(1), 317—351 |
| 16 | Bailly C., Eur. J. Pharmacol., 2022, 914, 174661 |
| 17 | Vinogradov A. A., Suga H., Cell Chem. Biol., 2020, 27(8), 1032—1051 |
| 18 | Just⁃Baringo X., Bruno P., Pitart C., Vila J., Albericio F., Alvarez M., J. Med. Chem., 2014, 57(10), 4185—4195 |
| 19 | Zhu F. Q., Wang W. G., Qu X. D., Wang S. F., Acta Chim. Sinica, 2022, 80(10), 1448—1462 |
| 朱凤巧, 王文贵, 瞿旭东, 王守锋. 化学学报, 2022, 80(10), 1448—1462 | |
| 20 | Fabbretti A., He C. G., Gaspari E., Maffioli S., Brandi L., Spurio R., Sosio M., Jabes D., Donadio S., Antimicrob. Agents Ch., 2015, 59(8), 4560—4568 |
| 21 | Chang J. P, Zhao J. R., Chen S. J., Meng K., Shi W. N., Li R. F., Chem. J. Chinese Universities, 2019, 40(4), 705—711 |
| 常俊朋, 赵佳瑞, 陈思佳, 孟凯, 石微妮, 李瑞芳. 高等学校化学学报, 2019, 40(4), 705—711 | |
| 22 | Zhong C., Zhang F. Y., Zhu N. Y., Zhu Y. W., Yao J., Gou S. H., Xie J. Q., Ni J. M., Eur. J. Med. Chem., 2021, 212, 113138 |
| 23 | Xu J. J., Hu A. X., Fine Chemicals, 2007, 24(4), 385—386, 396 |
| 徐娟娟, 胡艾希. 精细化工, 2007, 24(4), 385—386, 396 | |
| 24 | Yang J. Q., Wu X. J., Lu Z. C., Chen Y. M., She H. X., Liu H. J., Chin. J. Org. Chem., 2024, 44(11), 3541—3549 |
| 杨家强, 吴学姣, 卢子聪, 陈阳密, 佘慧娴, 刘海军. 有机化学, 2024, 44(11), 3541—3549 | |
| 25 | Shen G. X., Microbiology and Immunology, People’s Medical Publishing House, Beijing, 2007, 326—328 |
| 沈关心. 微生物与免疫学, 北京: 人民卫生出版社, 2007, 326—328 | |
| 26 | Humphries R., Bobenchik A. M., Hindler J. A., Schuetz A. N., J. Clin. Microbiol., 2021, 59(12), e0021321 |
| [1] | LIU Dongmei, XU Yuanqiang, XIA Chao, ZHENG Jingjing, SU Xianbin. Continuous Flow Liquid Phase Synthesis of Thymopentin Using Fluoride-labile Hydrophobic Tag [J]. Chem. J. Chinese Universities, 2025, 46(4): 20240544. |
| [2] | SUN Wei, LI Fusen, HAN Cunxin, DENG Yue, WEI Wenlin, LI Bing, SHI Dongfang. Photocatalytic Preparation and Antibacterial Activity of GO@Au Nanocomposites [J]. Chem. J. Chinese Universities, 2025, 46(3): 20240461. |
| [3] | LIU Yixuan, HU Huimin, FAN Xiaoqiang, YU Xuehua, KONG Lian, XIAO Xia, XIE Zean, ZHAO Zhen. Preparation of Pt/Mn-silicalite-1 Catalysts and Their Catalytic Performance for Propane Dehydrogenation [J]. Chem. J. Chinese Universities, 2025, 46(3): 20240460. |
| [4] | WANG Lu. Ionic Liquid-assisted Hydrothermal Synthesis of 1T-MoS2 and Its Zinc Ion Storage Performance [J]. Chem. J. Chinese Universities, 2024, 45(9): 20240145. |
| [5] | XU Ling, YIN Panpan, LU Xianfu, LI Yiming. Development and Applications of Ligation-Desulfurization Strategy in Protein Chemical Synthesis [J]. Chem. J. Chinese Universities, 2024, 45(8): 20240196. |
| [6] | YANG Jiaqiang, WU Xuejiao, LU Zicong, CHEN Yangmi, SHE Huixian, LIU Ouling. Synthesis and Antibacterial Activities of Osthole Derivatives Containing (Hetero)arylsulfonylpiperazine [J]. Chem. J. Chinese Universities, 2024, 45(7): 20240124. |
| [7] | LIU Hao, LIU Dongmei, SUN Haotian, XIA Chao, SU Xianbin. Continuous Flow Liquid-phase Vialox Peptide Synthesis Using Hydrophobic Silyl Tag [J]. Chem. J. Chinese Universities, 2024, 45(7): 20240024. |
| [8] | LONG Lei, WEI Wei, LUO Yunjun, LI Xiaoyu. Research Progress in Efficient Azide Methods [J]. Chem. J. Chinese Universities, 2024, 45(5): 20230511. |
| [9] | ZHAO Xiaoguang, WANG Yunlong, YIN Haibo, QU Yakun, SU Haiwei, FANG Wei. Research Progress of Electrocatalytic Ammonia Synthesis from Different Nitrogen Sources [J]. Chem. J. Chinese Universities, 2024, 45(3): 20230527. |
| [10] | HU Wenxin, ZHAO Ying, DU Danyang, ZHANG Hongdan, CHENG Peng. Preparation of ZSM-5 Encapsulated Pt-La Bimetallic Catalysts and Their Catalytic Performance for iso-Butane Cracking [J]. Chem. J. Chinese Universities, 2024, 45(10): 20240244. |
| [11] | WANG Gang, LIANG Shuang, SHAN Zhonggang, YING Junwu, LYU Liang, LI Bin, YANG Huibin. Design, Synthesis and Fungicidal Activity of Pyrazinamide Analogs [J]. Chem. J. Chinese Universities, 2024, 45(10): 20240369. |
| [12] | ZHANG Xinghong, GENG Peng, XIANG Juanjuan, YAN Jiaying, MAO Miaofu, XIAO Shuzhang. One-pot Synthesis of Room Temperature Phosphorescent Boron-difluoride Derivative for Printing [J]. Chem. J. Chinese Universities, 2024, 45(1): 20230432. |
| [13] | YANG Wei, LIU Jiaqi, TANG Cuiman, ZHANG Haonan, WANG Bingbing, SUN Zhong, XU Xiaohui. Copper-catalyzed Synthesis of Amides from Alcohols and Aminopyridines Under Aerobic Conditions [J]. Chem. J. Chinese Universities, 2023, 44(8): 20230029. |
| [14] | YANG Yingnan, SUN Qiming. Synthesis of EAB Zeolite and Its Application in Methanol-to-olefin Reaction [J]. Chem. J. Chinese Universities, 2023, 44(8): 20230119. |
| [15] | FENG Ruiqin, FANG Yun, FAN Ye, XIA Yongmei. Facile Synthesis of Gold Nanoflowers and the Catalytic Reduction of p-Nitrophenol with Sodium Borohydride [J]. Chem. J. Chinese Universities, 2023, 44(8): 20230027. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||