

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (6): 1207.doi: 10.7503/cjcu20190022
• Physical Chemistry • Previous Articles Next Articles
XIA Xiaoli, TAN Jingjing( ), WEI Caiyun, ZHAO Yongxiang(
), WEI Caiyun, ZHAO Yongxiang( )
)
Received:2019-01-09
Online:2019-06-10
Published:2019-04-18
Supported by:CLC Number:
TrendMD:
XIA Xiaoli,TAN Jingjing,WEI Caiyun,ZHAO Yongxiang. Molybdenum Modified Nickel Phyllosilicates Catalyst for Maleic Anhydride Hydrogenation†[J]. Chem. J. Chinese Universities, 2019, 40(6): 1207.
| Catalyst | Mass fractiona(%) | ce/(μmol·g-1) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mo | Ni | |||||||
| 0MoNi-PS | 0 | 31.50 | 347.09 | 0.61 | 7.66 | 3.8 | 0.80 | 1348.84 |
| 0.5MoNi-PS | 0.29 | 31.21 | 302.18 | 0.65 | 5.79 | 3.9 | 0.83 | 1304.68 |
| 1MoNi-PS | 1.21 | 29.97 | 270.20 | 0.51 | 5.39 | 4.0 | 0.88 | 1276.51 |
| 3MoNi-PS | 2.97 | 29.05 | 213.98 | 0.32 | 5.0 | 4.5 | 0.96 | 1240.47 |
| 5MoNi-PS | 5.18 | 26.25 | 182.02 | 0.26 | 4.74 | 4.7 | 0.57 | 836.38 |
| 7MoNi-PS | 7.82 | 23.70 | 123.86 | 0.17 | 6.66 | 5.1 | 0.54 | 777.80 |
Table 1 Textural properties of the catalysts
| Catalyst | Mass fractiona(%) | ce/(μmol·g-1) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mo | Ni | |||||||
| 0MoNi-PS | 0 | 31.50 | 347.09 | 0.61 | 7.66 | 3.8 | 0.80 | 1348.84 |
| 0.5MoNi-PS | 0.29 | 31.21 | 302.18 | 0.65 | 5.79 | 3.9 | 0.83 | 1304.68 |
| 1MoNi-PS | 1.21 | 29.97 | 270.20 | 0.51 | 5.39 | 4.0 | 0.88 | 1276.51 |
| 3MoNi-PS | 2.97 | 29.05 | 213.98 | 0.32 | 5.0 | 4.5 | 0.96 | 1240.47 |
| 5MoNi-PS | 5.18 | 26.25 | 182.02 | 0.26 | 4.74 | 4.7 | 0.57 | 836.38 |
| 7MoNi-PS | 7.82 | 23.70 | 123.86 | 0.17 | 6.66 | 5.1 | 0.54 | 777.80 |
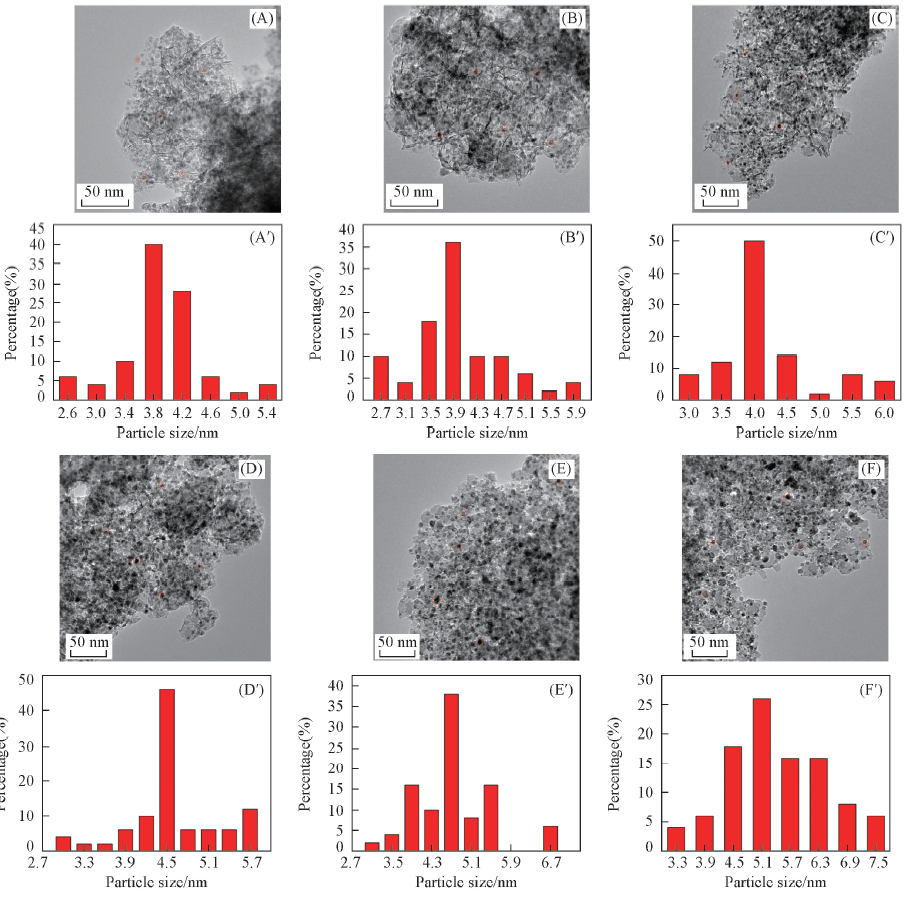
Fig.3 TEM images(A—F) and corresponding size distributions(A'—F') of catalysts (A, A') 0MoNi-PS; (B, B') 0.5MoNi-PS; (C, C') 1MoNi-PS; (D, D') 3MoNi-PS; (E, E') 5MoNi-PS; (F, F') 7MoNi-PS.
| Catalyst | Acid content(%) | Total acid/(mmol NH3·g-1) | ||
|---|---|---|---|---|
| Weak acid, α | Medium strong acid, (β+γ) | Strong acid, η | ||
| 0MoNi-PS | 5.13 | 81.30 | 13.57 | 1.71 |
| 0.5MoNi-PS | 5.55 | 78.35 | 16.10 | 1.69 |
| 1MoNi-PS | 4.02 | 78.95 | 17.03 | 1.68 |
| 3MoNi-PS | 14.44 | 66.03 | 19.53 | 1.66 |
| 5MoNi-PS | 9.04 | 74.72 | 16.24 | 1.29 |
| 7MoNi-PS | 25.51 | 49.62 | 24.87 | 0.69 |
Table 2 Distribution of different acid strength and the total acid content for different catalysts
| Catalyst | Acid content(%) | Total acid/(mmol NH3·g-1) | ||
|---|---|---|---|---|
| Weak acid, α | Medium strong acid, (β+γ) | Strong acid, η | ||
| 0MoNi-PS | 5.13 | 81.30 | 13.57 | 1.71 |
| 0.5MoNi-PS | 5.55 | 78.35 | 16.10 | 1.69 |
| 1MoNi-PS | 4.02 | 78.95 | 17.03 | 1.68 |
| 3MoNi-PS | 14.44 | 66.03 | 19.53 | 1.66 |
| 5MoNi-PS | 9.04 | 74.72 | 16.24 | 1.29 |
| 7MoNi-PS | 25.51 | 49.62 | 24.87 | 0.69 |
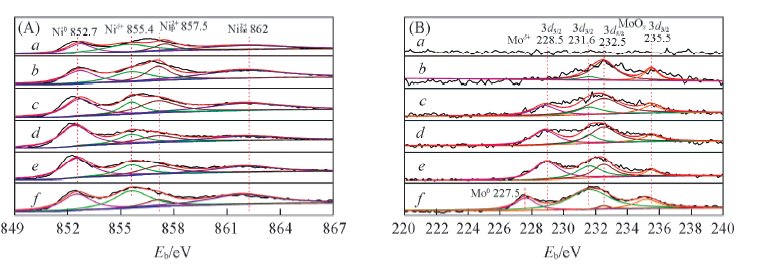
Fig.6 In situ XPS spectra of the catalysts (A) N i 2 p ; (B) M o 3 d . a. 0MoNi-PS; b. 0.5MoNi-PS; c. 1MoNi-PS; d. 3MoNi-PS; e. 5MoNi-PS; f. 7MoNi-PS.
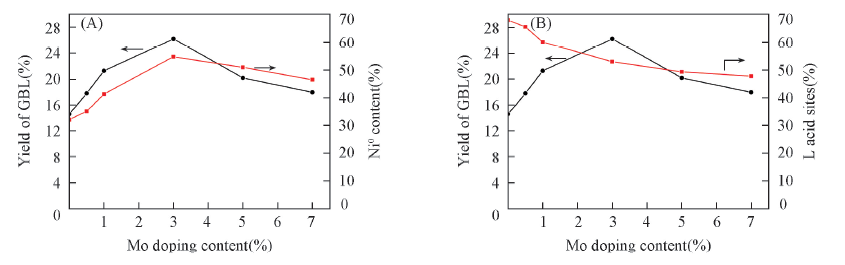
Fig.7 Effect of Mo doping content on the yield of GBL and the Ni0(A) and Lewis acid sites(B) on the surface of the catalysts Reaction conditions: MA: 4.9 g; catalyst: 0.3 g; hydrogen pressure: 5 MPa; 40 mL THF as solvent; t=160 ℃; reaction time: 3 h. MA: maleic anhydride, GBL:γ-butyrolactone, THF: tetrahydrofuran.
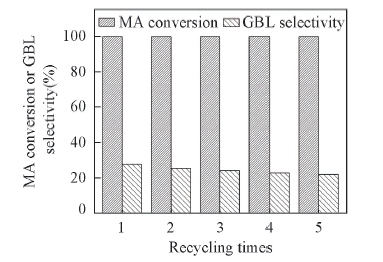
Fig.9 Stability of 3MoNi-PS catalyst Reaction conditions: MA: 4.9 g; catalyst: 0.3 g; hydrogen pressure: 5 MPa; 40 mL THF as solvent; temperature: 160 ℃; reaction time: 3 h.
| [1] | Feng Y. H., Yin H. B., Wang A. L., Xie T., Jiang T. S., Appl. Catal. A:Gen.,2012, 425/426, 205-212 |
| [2] | Ung G. H., Hai W. P., Joongwon L., Sunhwan H., In K. S., J. Ind. Eng. Chem.,2012, 18, 462-468 |
| [3] | Huo W. T., Zhang C. L., Yuan H. J., Jia M. J., Ning C. L., Tang Y., Zhang Y., Luo J. H., Wang Z. L., Zhang W. X., J. Ind. Eng. Chem., 2014, 20(6), 4140-4145 |
| [4] | Yuan H. J., Zhang C. L., Huo W. T., Ning C. L., Tang Y., Zhang Y., Cong D. Q., Zhang W. X., Luo J. H., Li S., Wang Z. L., J. Chem.Sci., 2014, 126(1), 141-145 |
| [5] | Huang Y. Q., Ma Y., Cheng Y. W., Wang L. J., Li X., Appl. Catal. A: Gen.,2015, 495, 124-130 |
| [6] | Yu Y., Guo Y. L., Zhan W. C., Guo Y., Wang Y. Q., Wang Y. S., Zhang Z. G., Lu G. Z., J. Mol. Catal. A: Chem.,2011, 337, 77-81 |
| [7] | Zhang B., Zhu Y. L., Ding G. Q., Zheng H. Y., Li Y. W., Appl. Catal. A: Gen.,2012, 443/444, 191-201 |
| [8] | Yan L. Y., Jia Z. Q., Li D., Zhao Y. X., Chem.Bioengin.,2015, 32(12), 21-24 |
| (闫丽云,贾志奇,李丹,赵永祥.化学与生物工程, 2015, 32 (12), 21-24) | |
| [9] | Li J., Tian W. P., Wang X., Shi L., Chem. Eng. J.,2011, 175, 417-422 |
| [10] | Ma Y., Huang Y. Q., Cheng Y. W., Wang L. J., Li X., Catal. Commun.,2014, 57, 40-44 |
| [11] | Liao X., Zhang Y., Hill M., Xia X., Zhao Y. X., Jiang Z., Appl. Catal. A: Gen.,2014, 488, 256-264 |
| [12] | Xu A. J., Liang E. Y., Wang J., Zhang Y., Zhao Y. X., Ind.Catal.,2018, 26(2), 28-32 |
| (徐爱军,梁二艳,王杰,张因,赵永祥.工业催化, 2018, 26(2), 28-32) | |
| [13] | Li J., Tian W. P., Shi L., Ind. Eng. Chem. Res., 2010, 49, 11837-11840 |
| [14] | Song W. J., Zhao C., Johannes A. L., Chem. Eur. J.,2013, 19(30), 9833-9842 |
| [15] | Peng B. X., Zhao C., Kasakov S., Foraita S., Lercher J. A., Chem. Eur. J., 2013, 19(15), 4732-4741 |
| [16] | Foraita S., Fulton J. L., Chase Z. A., Vjunov A., Xu P. H., Barath E., Camaioni D. M., Zhao C., Lercher L. A., Chem. Eur. J., 2015, 21, 2423-2434 |
| [17] | Ma B., Hu J. B., Wang Y. M., Zhao C., Green Chem., 2015, 17, 4610-4617 |
| [18] | Zhang J. J., Zhao C., ACS Catal.,2016, 6, 4512-4525 |
| [19] | Ma B., Yi X. F., Chen L., Zheng A. M., Zhao C., J. Mater. Chem. A,2016, 4, 11330-11341 |
| [20] | Maluf S. S., Assaf E. M., Fuel,2009, 88, 1547-1553 |
| [21] | Cui J. L., Tan J. J., Zhu Y. L., Cheng F. Q., Chem. Sus. Chem,2018, 11, 1316-1320 |
| [22] | Zhang C. X., Yue H. R., Huang Z. Q., Li S. R., Wu G. W., Ma X. B., Gong J. L., ACS Sustainable Chem. Eng.,2012, 1, 161-173 |
| [23] | Kong X., Zhu Y. F., Zheng H. Y., Li X. Q., Zhu Y. L., Li Y. W., ACS Catal., 2015, 5, 5914-5920 |
| [24] | Zhang X., He D. H., Zhang Q. J., Xu B. Q., Zhu Q. M., Topics in Catalysis,2005, 32(3/4), 215-223 |
| [25] | Ding R.R., Wu Y. L., Chen Y., Chen H., Wang J. L., Shi Y. C., Yang M. D., Catal. Sci. Technol., 2016, 6, 2065—2076 |
| [26] | Shi Y. C., Cao Y. Y., Duan Y. N., Chen H., Chen Y., Yang M. D., Wu Y. L., Green Chemistry,2016, 18(17), 4633-4648 |
| [27] | Liu H. M., Xu Y. D., Chinese J. Catal.,2006, 27(4), 319-323 |
| (刘红梅, 徐奕德. 催化学报, 2006, 27(4), 319-323) | |
| [28] | Yuan Q., Yin A. X., Luo C., Sun L. D., Zhang Y. W., Duan W. T., Liu H. C., Yan C. H., J. Am. Chem. Soc.,2008, 130, 3465-3472 |
| [29] | Layman K. A., Ivey M. M., Hemminger J. C., J. Phys. Chem. B.,2003, 107, 8538-8546 |
| [30] | Das S., Ashok J., Bian Z., Dewangan N., Wai M. H., Du Y., Borgna A., Hidajat K., Kawi S., Appl. Catal. B: Environ.,2018, 230, 220-236 |
| [31] | Zhao X. L., Wang Y., Wu H. Y., Fang L. C., Liang J. J., Fan Q. H., Li P., J. Mol. Liq.,2017, 248, 1030-1038 |
| [32] | Lehmann T., Wolff T., Hamel C., Veit P., Garke B., Seidel-Morgenstern A., Microporous and Mesoporous Materials,2012, 151, 113-125 |
| [33] | Kim K. S., Winograd N., Surf. Sci.,1974, 43, 625-643 |
| [34] | Li Z. W., Jiang B., Wang Z. G., Kawi S., J. CO2 Utilization,2018, 27, 238-246 |
| [35] | Sawhill S. J., Layman K. A., van-Wyk D. R., Engelhard M. H., Wang C. M., Bussell M. E., J.Catal.,2005, 231, 300-313 |
| [1] | DING Yang, WANG Wanhui, BAO Ming. Recent Progress in Porous Framework-immobilized Molecular Catalysts for CO2 Hydrogenation to Formic Acid [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220309. |
| [2] | ZHANG Xinxin, XU Di, WANG Yanqiu, HONG Xinlin, LIU Guoliang, YANG Hengquan. Effect of Mn Promoter on CuFe-based Catalysts for CO2 Hydrogenation to Higher Alcohols [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220187. |
| [3] | ZHOU Zixuan, YANG Haiyan, SUN Yuhan, GAO Peng. Recent Progress in Heterogeneous Catalysts for the Hydrogenation of Carbon Dioxide to Methanol [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220235. |
| [4] | HUANG Xiaoshun, MA Haiying, LIU Shujuan, WANG Bin, WANG Hongli, QIAN Bo, CUI Xinjiang, SHI Feng. Recent Advances on Indirect Conversion of Carbon Dioxide to Chemicals [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220222. |
| [5] | ZHOU Leilei, CHENG Haiyang, ZHAO Fengyu. Research Progress of CO2 Hydrogenation over Pd-based Heterogeneous Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220279. |
| [6] | SONG Youwei, AN Jiangwei, WANG Zheng, WANG Xuhui, QUAN Yanhong, REN Jun, ZHAO Jinxian. Effects of Ag,Zn,Pd-doping on Catalytic Performance of Copper Catalyst for Selective Hydrogenation of Dimethyl Oxalate [J]. Chem. J. Chinese Universities, 2022, 43(6): 20210842. |
| [7] | HU Huimin, CUI Jing, LIU Dandan, SONG Jiaxin, ZHANG Ning, FAN Xiaoqiang, ZHAO Zhen, KONG Lian, XIAO Xia, XIE Zean. Influence of Different Transition Metal Decoration on the Propane Dehydrogenation Performance over Pt/M-DMSN Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210815. |
| [8] | MENG Xiangyu, ZHAN Qi, WU Yanan, MA Xiaoshuang, JIANG Jingyi, SUN Yueming, DAI Yunqian. Photothermal Enhanced Photocatalytic Hydrogenation Performance of Au/RGO/Na2Ti3O7 [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210655. |
| [9] | LI Xueyu, WANG Zhao, CHEN Ya, LI Keke, LI Jianquan, JIN Shunjing, CHEN Lihua, SU Baolian. Enhanced Catalytic Performance of Supported Nano-gold by the Localized Surface Plasmon Resonance for Selective Hydrogenation of Butadiene [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220174. |
| [10] | YANG Yingjie, ZHANG Xiaorong, SUN Yuxue, LIU Jun, XIE Haiming. Synthesis of a Dual-lithium-salt Comb Polymer Electrolyte and Its Electrochemical Performance [J]. Chem. J. Chinese Universities, 2021, 42(9): 2861. |
| [11] | YAN Pengquan, WANG Jingrong, SHEN Yaxing, ZUO Zhijun, GAO Zhihua, HUANG Wei. Effect of CuAl2O4 Spinel Structure on CO Hydrogenation in Slurry Reactor [J]. Chem. J. Chinese Universities, 2021, 42(6): 1846. |
| [12] | FAN Ye, HAN Huihui, FANG Yun, FENG Ruiqin, XIA Yongmei. Facile Synthesis of Hollow Nickel Submicrospheres with Hierarchical Nano-structure and Its Catalytic Hydrogenation of Phenol [J]. Chem. J. Chinese Universities, 2021, 42(6): 1801. |
| [13] | LIU Hanlin, YIN Linlin, CHEN Xifeng, LI Guodong. Recent Advances in Indium Oxide Based Nanocatalysts for Selective Hydrogenation of CO2 [J]. Chem. J. Chinese Universities, 2021, 42(5): 1430. |
| [14] | XIAO Zhaozhong, MA Zhi, PIAO Lingyu. Co-catalytic Effect of Ni2P on Photocatalytic Formic Acid Dehydrogenation over Different Semiconductors [J]. Chem. J. Chinese Universities, 2021, 42(12): 3692. |
| [15] | YU Xia, SONG Chenhai, GUO Xiangke, XUE Nianhua, DING Weiping. Cooperative Catalysis of Adjacent Acid Sites in Zeolite HZSM-5 [J]. Chem. J. Chinese Universities, 2021, 42(1): 239. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||