

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (10): 2265.doi: 10.7503/cjcu20180226
• Physical Chemistry • Previous Articles Next Articles
HU Xueyan1, WANG Na2, HAO Yuting2, XU Zhiqing2, WANG Minghui2, SHI Gaiqin2, YANG Huimin1,*( ), LIANG Zhenhai1,2,*(
), LIANG Zhenhai1,2,*( )
)
Received:2018-03-22
Online:2018-09-29
Published:2018-09-29
Contact:
YANG Huimin,LIANG Zhenhai
E-mail:yanghuimin@tyut.edu.cn;liangzhenhai@tyut.edu.cn
Supported by:CLC Number:
TrendMD:
HU Xueyan,WANG Na,HAO Yuting,XU Zhiqing,WANG Minghui,SHI Gaiqin,YANG Huimin,LIANG Zhenhai. Preparation of Cu Doped SnO2 Cathode Material for Electroreduction of Carbon Dioxide at Low Overpotential†[J]. Chem. J. Chinese Universities, 2018, 39(10): 2265.
| Sample | Dhkl/nm | d/nm | a/nm | c/nm |
|---|---|---|---|---|
| SC0 | 22.2344 | 0.2587 | 0.4743 | 0.3191 |
| SC1 | 9.3089 | 0.2586 | 0.4740 | 0.3191 |
| SC1.5 | 8.0839 | 0.2586 | 0.4739 | 0.3191 |
| SC2 | 11.8905 | 0.2593 | 0.4766 | 0.3199 |
| SC3 | 12.2344 | 0.2587 | 0.4745 | 0.3192 |
Table 1 XRD parameters of different samples
| Sample | Dhkl/nm | d/nm | a/nm | c/nm |
|---|---|---|---|---|
| SC0 | 22.2344 | 0.2587 | 0.4743 | 0.3191 |
| SC1 | 9.3089 | 0.2586 | 0.4740 | 0.3191 |
| SC1.5 | 8.0839 | 0.2586 | 0.4739 | 0.3191 |
| SC2 | 11.8905 | 0.2593 | 0.4766 | 0.3199 |
| SC3 | 12.2344 | 0.2587 | 0.4745 | 0.3192 |
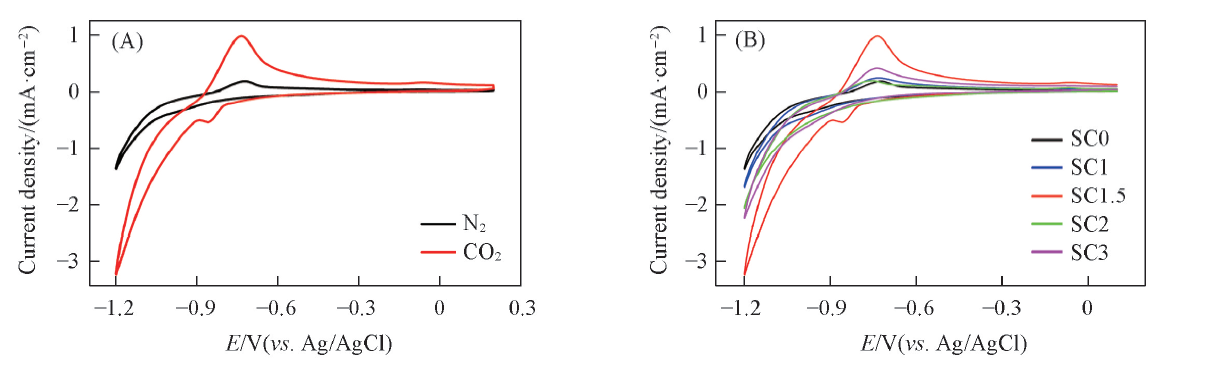
Fig.6 Cyclic voltammograms of sample SC1.5 in N2 and CO2 saturated 0.5 mol/L NaHCO3 solution(A) and different samples in CO2 saturated 0.5 mol/L NaHCO3 solution(B)
| Electrode | Electrolyte | Overpotential | Reference |
|---|---|---|---|
| SnO2 nanowires | 0.1 mol/L KHCO3 | 0.35 V(vs. RHE) | [ |
| Sn electrode | 0.1 mol/L NaHCO3 | 0.34 V(vs. RHE) | [ |
| Cu doped SnO2 | 0.5 mol/L NaHCO3 | 0.30 V(vs. RHE) | This work |
Table 2 Overpotential comparison of different catalysts for electrocatalytic reduction of CO2
| Electrode | Electrolyte | Overpotential | Reference |
|---|---|---|---|
| SnO2 nanowires | 0.1 mol/L KHCO3 | 0.35 V(vs. RHE) | [ |
| Sn electrode | 0.1 mol/L NaHCO3 | 0.34 V(vs. RHE) | [ |
| Cu doped SnO2 | 0.5 mol/L NaHCO3 | 0.30 V(vs. RHE) | This work |
| [1] | Yang D.W., Li L., Wang Q., Wang X. C., Li Q. Y., Shi J., Chem. J. Chinese Universities, 2016, 37(1), 94—99 |
| (杨冬伟, 李露, 王琴, 王晓春, 李青远, 施锦. 高等学校化学学报, 2016, 37(1), 94—99) | |
| [2] | Zhao K., Liu Y.M., Quan X., Chen S., Yu H. T., ACS Appl. Mater. Inter., 2017, 9(6), 5302—5311 |
| [3] | Kumar B., Atla V., Brian J.P., Kumari S., Nguyen T. Q., Sunkara M., Spurgeon J. M., Angew. Chem. Int. Ed., 2017, 56, 3645—3649 |
| [4] | Lee S., Ocon J.D., Son Y., Lee J., J. Phys. Chem.C, 2015, 119(9), 4884—4890 |
| [5] | Baek G., Kim J., Lee S.Y., Lee C. S., Bioresource Technol., 2017, 241, 1201—1207 |
| [6] | Dong C.H., Ji M. S., Yang X. Z., Yao J. N., Chen H., Catalysts, 2016, 7(1), 5—17 |
| [7] | Li Q., Fu J.J., Zhu W. L., Chen Z. Z., Shen B., Wu L. H., Xi Z., Wang T. Y., Lu G., Zhu J. J., Sun S. H., J. Am. Chem. Soc., 2017, 139(12), 4290—4293 |
| [8] | Ensafi A.A., Alinajafi H. A., Rezaei B., J. Electroanal. Chem., 2016, 783, 82—89 |
| [9] | Manthiram K., Beberwyck B.J., Alivisatos A. P., J. Am. Chem. Soc., 2014, 136(38), 13319—13325 |
| [10] | Lum Y.W., Ager J. W., Angew. Chem. Int. Ed., 2018, 57(2), 551—554 |
| [11] | Machunda R.L., Ju H. K., Lee J. Y., Curr. Appl. Phys., 2011, 11(4), 986—988 |
| [12] | Sheng Z., Peng K., Thomas J.M., J. Am. Chem. Soc., 2014, 136(5), 1734—1737 |
| [13] | Ju W., Bagger A., Hao G.P., Varela1 A. S., Sinev I., Bon V., Cuenya B. R., Kaskel S., Rossmeisl J., Strasser P., Nat. Commun., 2017, 8, 944—953 |
| [14] | Royer M. E., Compt. Rend. Hebd. Seances Acad. Sci., 1870, 70, 731—732 |
| [15] | Chiu H.C., Yeh C. S., J. Phys. Chem.C, 2007, 111(20), 7256—7259 |
| [16] | Zhang H.L., Wang D. Z., Hu C. G., Kang X. L., Liu H., Sensor. Actuat. B: Chem., 2013, 184, 288—294 |
| [17] | Li Q.K., Wang Z., Zhang M., Hou P. F., Kang P., Appl. Catal. B: Environ., 2017, 26(5), 825—829 |
| [18] | Bhardwaj N., Pandey A., Satpati B., Tomar M., Gupta V., Mohapatra S., Phys. Chem. Chem. Phys., 2016, 18(28), 18846—18854 |
| [19] | Mishra R.K., Kushwaha A., Sahay P. P., RSC Adv., 2014, 4(8), 3904—3912 |
| [20] | Thomas B., Skariah B., J. Alloy. Compd., 2015, 625, 231—240 |
| [21] | Punginsang M., Wisitsoraat A., Sriprachuabwong C., Phokharatkul D., Tuantranont A., Phanichphant S., Liewhiran C., Appl. Surf. Sci., 2017, 425, 351—366 |
| [22] | Harish S., Navaneethan M., Archana J., Ponnusamy S., Muthamizhchelvan C., Hayakaw Y., Mater. Lett., 2014, 121, 129—132 |
| [23] | Deng J.Q., Liu Y. G., Liu S. B., Zeng G. M., Tan X. F., Huang B. Y., Tang X. J., Wang S. F., Hua Q., Yan Z. L., J. Colloid Interf. Sci., 2017, 506, 355—364 |
| [24] | Liu Y.Y., Fan M. Y., Zhang X., Zhang Q., Guay D., Qiao J. L., Electrochim.Acta, 2017, 248, 123—132 |
| [25] | Chen Y.H., Kanan M. W., J. Am. Chem. Soc., 2012, 134(4), 1986—1989 |
| [26] | Chen Y.H., Li C. W., Kanan M. W., J. Am. Chem. Soc., 2012, 134(49), 19969—19972 |
| [27] | Lee S., Ju H.K., Machund R., Uhm S., Lee J. K., Lee H. J., Lee. J., J. Mater. Chem.A, 2015, 3(6), 3029—3035 |
| [28] | Yu J.L., Liu H. Y., Song S. Q., Wang Y., Tsiakaras P., Appl. Catal. A, Gen., 2017, 545, 159—166 |
| [29] | Zhang X., Lei T., Liu Y.Y., Qiao J. L., Appl. Catal. B: Environ., 2017, 218, 46—50 |
| [30] | Zhu D.D., Liu J. L., Qiao S. Z., Adv. Mater., 2016, 28(18), 3423—3452 |
| [31] | Shi M.L., AC Impedance Spectroscopy Principles and Application, Transluted by Wu X. F., National Defense Industry Press, Beijing, 2001 |
| (吴宣方译, 交流阻抗谱原理及应用, 北京: 国防工业出版社, 2001) | |
| [32] | Gattrell M., Gupta N., Co A., J. Electroanal. Chem., 2006, 594(1), 1—19 |
| [33] | Nada H., Ikeda S., Yamamoto A., Einaga H., Ito K., Bull. Chem. Soc.Jpn., 1995, 68(7), 1889—1895 |
| [34] | Wang H.X., Chen Y. B., Hou X. L., Ma C. Y., Tan T. W., Green Chem., 2016, 18(11), 3250—3258 |
| [35] | Zhelev V., Petkov P., Shindov P., Bineva I., Vasilev S., Ilcheva V., Petkova T., Thin Solid Films, 2018, 653, 19—23 |
| [36] | Hu J., Wang T., Wang Y.J., Huang D., He G. L., Han Y. T., Hu N. T., Su Y. J., Zhou Z. H., Zhang Y. F., Yang Z., Sensor Actuat B: Chem., 2018, 263, 120—128 |
| [1] | LIN Gaoxin, WANG Jiacheng. Progress and Perspective on Molybdenum Disulfide with Single-atom Doping Toward Hydrogen Evolution [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220321. |
| [2] | WANG Sicong, PANG Beibei, LIU Xiaokang, DING Tao, YAO Tao. Application of XAFS Technique in Single-atom Electrocatalysis [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220487. |
| [3] | WU Yu, LI Xuan, YANG Hengpan, HE Chuanxin. Construction of Cobalt Single Atoms via Double-confinement Strategy for High-performance Electrocatalytic Reduction of Carbon Dioxide [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220343. |
| [4] | QIN Yongji, LUO Jun. Applications of Single-atom Catalysts in CO2 Conversion [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220300. |
| [5] | YAO Qing, YU Zhiyong, HUANG Xiaoqing. Progress in Synthesis and Energy-related Electrocatalysis of Single-atom Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220323. |
| [6] | HAN Fuchao, LI Fujin, CHEN Liang, HE Leiyi, JIANG Yunan, XU Shoudong, ZHANG Ding, QI Lu. Enhance of CoSe2/C Composites Modified Separator on Electrochemical Performance of Li-S Batteries at High Sulfur Loading [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220163. |
| [7] | ZHAO Runyao, JI Guipeng, LIU Zhimin. Efficient Electrocatalytic CO2 Reduction over Pyrrole Nitrogen-coordinated Single-atom Copper Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220272. |
| [8] | WANG Ruhan, JIA Shunhan, WU Limin, SUN Xiaofu, HAN Buxing. CO2-involved Electrochemical C—N Coupling into Value-added Chemicals [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220395. |
| [9] | WANG Lijun, LI Xin, HONG Song, ZHAN Xinyu, WANG Di, HAO Leiduan, SUN Zhenyu. Efficient Electrocatalytic CO2 Reduction to CO by Tuning CdO-Carbon Black Interface [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220317. |
| [10] | YANG Lijun, YU Yang, ZHANG Lei. Construction of Dual-functional 2D/3D Hydrid Co2P-CeO x Heterostructure Integrated Electrode for Electrocatalytic Urea Oxidation Assisted Hydrogen Production [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220082. |
| [11] | SONG Yingying, HUANG Lin, LI Qingsen, CHEN Limiao. Preparation of CuO/BiVO4 Photocatalyst and Research on Carbon Dioxide Reduction [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220126. |
| [12] | ZHANG Hongwei, CHEN Wen, ZHAO Meiqi, MA Chao, HAN Yunhu. Research Progress of Single Atom Catalysts in Electrochemistry [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220129. |
| [13] | XIA Tian, WAN Jiawei, YU Ranbo. Progress of the Structure-property Correlation of Heteroatomic Coordination Structured Carbon-based Single-atom Electrocatalysts [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220162. |
| [14] | TAO Yu, OU Honghui, LEI Yongpeng, XIONG Yu. Research Progress of Single-atom Catalysts in Photocatalytic Reduction of Carbon Dioxide [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220143. |
| [15] | WU Jun, HE Guanchao, FEI Huilong. Self-supported Film Electrodes Decorated with Single Atoms for Energy Electrocatalysis [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220051. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||