

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (10): 2272.doi: 10.7503/cjcu20180170
• Physical Chemistry • Previous Articles Next Articles
YANG Yejin, YOU Jinglin*( ), WANG Jian, WANG Min, HE Yingxia, WU Zhidong
), WANG Jian, WANG Min, HE Yingxia, WU Zhidong
Received:2018-03-04
Online:2018-09-29
Published:2018-09-29
Contact:
YOU Jinglin
E-mail:jlyou@staff.shu.edu.cn
Supported by:CLC Number:
TrendMD:
YANG Yejin,YOU Jinglin,WANG Jian,WANG Min,HE Yingxia,WU Zhidong. In-situ High Temperature Raman Spectroscopic and Decomposition Thermodynamic Study of the Structure of Potassium Hydrogen Sulfate and Its Melt†[J]. Chem. J. Chinese Universities, 2018, 39(10): 2272.
| Raman shifta/cm-1 | Structureb | Vibrational mode | Raman shifta/cm-1 | Structureb | Vibrational mode | ||
|---|---|---|---|---|---|---|---|
| 412 | s | D | S—OH bending(βS—OH) | 871 | m | C | S—OH symmetric vibration(νs S—OH) |
| 444 | s | D | S—OH bending(βS—OH) | 1004 | s | C | S—O symmetric vibration(νs S—O) |
| 450 | s | C | S—OH bending(βS—OH) | 1028 | s | D | S—O symmetric vibration(νs S—O) |
| 570 | m | C+D | O—S—O deformation(δO—S—O) | 1164 | w | C | S—O deformation(δS—O) |
| 580 | s | D | O—S—O deformation(δO—S—O) | 1224 | w | D | S—O deformation(δS—O) |
| 588 | s | C | O—S—O deformation(δO—S—O) | 1239 | w | D | O—H deformation(δOH) |
| 597 | s | D | O—S—O deformation(δO—S—O) | 1250 | w | D | O—H bending(γOH) |
| 615 | m | C+D | O—S—O deformation(δO—S—O) | 1284 | w | D | O—S—O asymmetric vibration(νas O—S—O) |
| 854 | s | D | S—OH symmetric vibration(νs S—OH) | 1337 | w | C | O—S—O asymmetric vibration(νas O—S—O) |
Table 1 Assignment of major vibrational modes of KHSO4 at ambient temperature
| Raman shifta/cm-1 | Structureb | Vibrational mode | Raman shifta/cm-1 | Structureb | Vibrational mode | ||
|---|---|---|---|---|---|---|---|
| 412 | s | D | S—OH bending(βS—OH) | 871 | m | C | S—OH symmetric vibration(νs S—OH) |
| 444 | s | D | S—OH bending(βS—OH) | 1004 | s | C | S—O symmetric vibration(νs S—O) |
| 450 | s | C | S—OH bending(βS—OH) | 1028 | s | D | S—O symmetric vibration(νs S—O) |
| 570 | m | C+D | O—S—O deformation(δO—S—O) | 1164 | w | C | S—O deformation(δS—O) |
| 580 | s | D | O—S—O deformation(δO—S—O) | 1224 | w | D | S—O deformation(δS—O) |
| 588 | s | C | O—S—O deformation(δO—S—O) | 1239 | w | D | O—H deformation(δOH) |
| 597 | s | D | O—S—O deformation(δO—S—O) | 1250 | w | D | O—H bending(γOH) |
| 615 | m | C+D | O—S—O deformation(δO—S—O) | 1284 | w | D | O—S—O asymmetric vibration(νas O—S—O) |
| 854 | s | D | S—OH symmetric vibration(νs S—OH) | 1337 | w | C | O—S—O asymmetric vibration(νas O—S—O) |
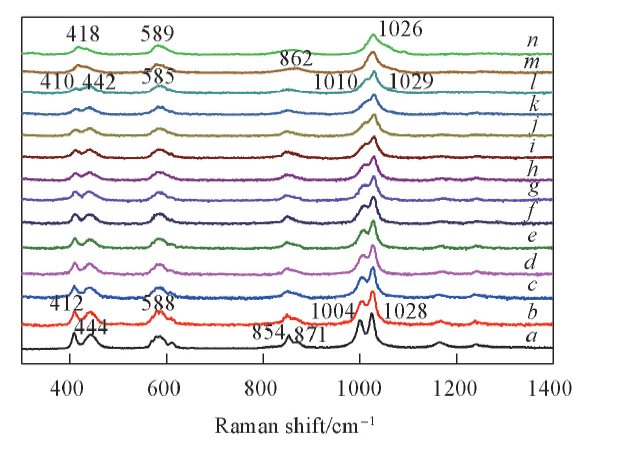
Fig.3 Temperature dependent Raman spectra of KHSO4 in the frequency range of 300—1400 cm-1 from ambient temperature to 220 ℃Temperature/℃: a. r. t.; b. 100; c. 110; d. 120; e. 130; f. 140; g. 150; h. 160; i. 170; j. 180; k. 190; l. 200; m. 210; n. 220.
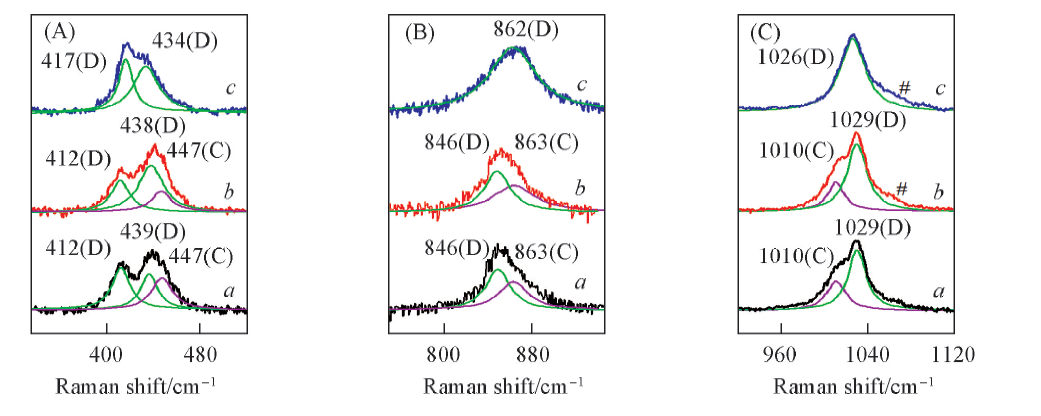
Fig.5 In-situ Raman spectra of the molten KHSO4 at 190 ℃(a), 200 ℃(b) and 210 ℃(c) along with the deconvolution of the bands of melts(D: dimer, C: chain)(A) βS—OH; (B) νs S—OH; (C) νs S—O. # Represents Raman characteristic band of K2S2O7 decomposed from trace KHSO4.
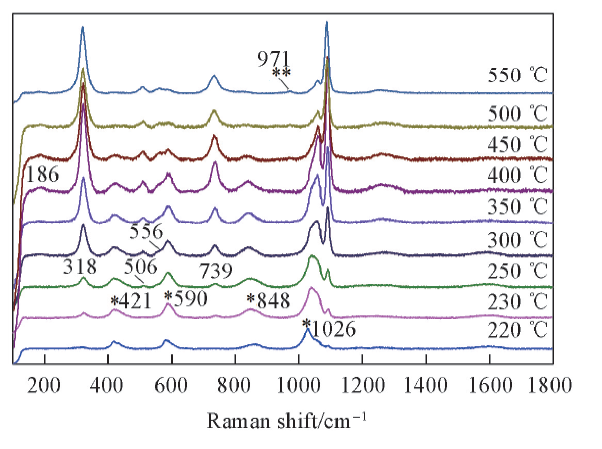
Fig.6 Temperature dependent Raman spectra of KHSO4-K2S2O7 in the frequency range of 100—1800 cm-1 from 220 ℃ to 550 ℃* and ** represent νs S—O of KHSO4 and νs S—O of K2SO4, respectively.
| Raman shift/cm-1 | Vibrational mode | Raman shift/cm-1 | Vibrational mode |
|---|---|---|---|
| 186w | S—O swing vibration(ρS—O) | 739m | S—O—S symmetric vibration(νs S—O—S) |
| 318s | S—O—S deformation(δS—O—S) | 971w** | S—O symmetric vibration(νs S—O) |
| 506w | S—O—S bending(βS—O—S) | 1060w* | S—O symmetric vibration(νs S—O) |
| 556w | S—O deformation(δO—S) | 1090s | S—O symmetric vibration(νs S—O) |
| 590w | S—O deformation(δS—O) |
Table 2 Raman vibrational mode assignments of K2S2O7-K2SO4 melt at 550 ℃
| Raman shift/cm-1 | Vibrational mode | Raman shift/cm-1 | Vibrational mode |
|---|---|---|---|
| 186w | S—O swing vibration(ρS—O) | 739m | S—O—S symmetric vibration(νs S—O—S) |
| 318s | S—O—S deformation(δS—O—S) | 971w** | S—O symmetric vibration(νs S—O) |
| 506w | S—O—S bending(βS—O—S) | 1060w* | S—O symmetric vibration(νs S—O) |
| 556w | S—O deformation(δO—S) | 1090s | S—O symmetric vibration(νs S—O) |
| 590w | S—O deformation(δS—O) |
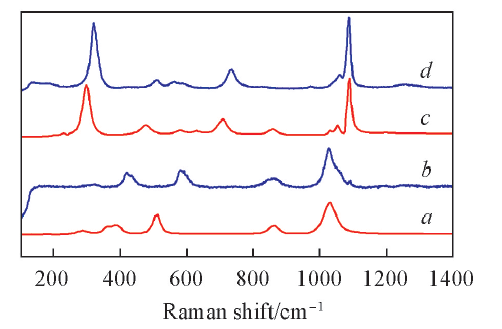
Fig.7 Experimental and calculated Raman spectra at 220 and 550 ℃The scaling factor is 0.92 and the Lorentzian smearing is 15 cm-1. a. Calculated Raman spectrum of KHSO4 cluster; b. KHSO4(l) experimental Raman spectrum at 220 ℃; c. calculated Raman spectrum of K2S2O7 cluster; d. K2S2O7(l) experimental Raman spectrum at 550 ℃.
| Temperature/℃ | lnK | ||||
|---|---|---|---|---|---|
| 230 | 13196 | 623 | 0.9762 | 0.0238 | -3.6884 |
| 250 | 23750 | 2448 | 0.9494 | 0.0506 | -2.8792 |
| 300 | 13689 | 5287 | 0.8335 | 0.1665 | -1.4282 |
| 350 | 11894 | 11403 | 0.6684 | 0.3316 | -0.2983 |
| 400 | 9676 | 15741 | 0.5430 | 0.4570 | 0.4383 |
| 450 | 5423 | 17596 | 0.3733 | 0.6267 | 1.5036 |
| 500 | 1180 | 6022 | 0.2748 | 0.7252 | 2.2624 |
| 550 | 834 | 8331 | 0.16208 | 0.8380 | 3.4632 |
Table 3 Raman characteristic band area at 1060, 1090 cm-1 (AHSO4-, AS2O72-), molar fraction of HSO4-, S2O72-(xHSO4-, xS2O72-) and lnK from 230 ℃ to 550 ℃
| Temperature/℃ | lnK | ||||
|---|---|---|---|---|---|
| 230 | 13196 | 623 | 0.9762 | 0.0238 | -3.6884 |
| 250 | 23750 | 2448 | 0.9494 | 0.0506 | -2.8792 |
| 300 | 13689 | 5287 | 0.8335 | 0.1665 | -1.4282 |
| 350 | 11894 | 11403 | 0.6684 | 0.3316 | -0.2983 |
| 400 | 9676 | 15741 | 0.5430 | 0.4570 | 0.4383 |
| 450 | 5423 | 17596 | 0.3733 | 0.6267 | 1.5036 |
| 500 | 1180 | 6022 | 0.2748 | 0.7252 | 2.2624 |
| 550 | 834 | 8331 | 0.16208 | 0.8380 | 3.4632 |
| System | Δ (kJ·mol-1) | Tfus/K | ΔfusH/ (kJ·mol-1) | Ttrans/K | ΔtransH/ (kJ·mol-1) | Cp/ (J·mol-1·K-1) | T/K | Cp/ (J·mol-1·K-1) | T/K |
|---|---|---|---|---|---|---|---|---|---|
| KHSO4 | -1163.00 | 495 | 18.00 | 442 | 2.10(α→β) | -62.50+0.56T | 298—488 | 287.00 | 488—545 |
| 453 | 4.00(β→γ) | ||||||||
| K2S2O7 | -1997.96 | 419 | 21.20 | 591 | 21.80(s1→s2) | 134.7+0.177T | 298—590 | 260.40 | 590—692 |
| H2O | 285.83 | 500 | 40.86 | — | — | 2.47×10-6T3- | 298—500 | -5.52×10-7T2 + | 500—1000 |
| 3.19×10-3T2+ | 0.016T+ | ||||||||
| 1.52T+ | 1107.27T-1- | ||||||||
| 3848757.66T-2- | 27999.32T-2+ | ||||||||
| 203.12 | 25.78 |
Table 4 Thermodynamic properties of KHSO4, K2S2O7 and H2O*
| System | Δ (kJ·mol-1) | Tfus/K | ΔfusH/ (kJ·mol-1) | Ttrans/K | ΔtransH/ (kJ·mol-1) | Cp/ (J·mol-1·K-1) | T/K | Cp/ (J·mol-1·K-1) | T/K |
|---|---|---|---|---|---|---|---|---|---|
| KHSO4 | -1163.00 | 495 | 18.00 | 442 | 2.10(α→β) | -62.50+0.56T | 298—488 | 287.00 | 488—545 |
| 453 | 4.00(β→γ) | ||||||||
| K2S2O7 | -1997.96 | 419 | 21.20 | 591 | 21.80(s1→s2) | 134.7+0.177T | 298—590 | 260.40 | 590—692 |
| H2O | 285.83 | 500 | 40.86 | — | — | 2.47×10-6T3- | 298—500 | -5.52×10-7T2 + | 500—1000 |
| 3.19×10-3T2+ | 0.016T+ | ||||||||
| 1.52T+ | 1107.27T-1- | ||||||||
| 3848757.66T-2- | 27999.32T-2+ | ||||||||
| 203.12 | 25.78 |
| [1] | Najafpour M.M., MoghaddamN. J., New Journal of Chemistry, 2017, 41(5), 1—16 |
| [2] | Busnardo R.G., Busnardo N. G., Salvato G. N., Afonso J. C., Hazardous Materials, 2007, 139(2), 391—398 |
| [3] | Paulino J.F., Busnardo N. G., Afonso J. C., Hazardous Materials, 2008, 150(3), 843—849 |
| [4] | Pérez M., Ruiz D., Autino J., Sathicq A., Romanelli G., Comptes Rendus Chimie, 2016, 19(5), 551—555 |
| [5] | Parry G.S., Glasser L., Crystalline, 1960, 113(1—6), 57—64 |
| [6] | Goypiron A., de Villepin J., Novak A., Raman Spectroscopy, 1980, (9), 297—303 |
| [7] | Bukleski M., Vladimir V., Vladimir P., Vibrational Spectroscopy, 2011, 57(1), 15—22 |
| [8] | Matsuo Y., Ferroelectrics, 2004, 302(1), 85—90 |
| [9] | Hamma H., Rasmussen S.B., Rogez J., Elbelghiti M. A., Eriksen K. M., Fehrmann R., Thermochemica Acta, 2006, 440(2), 200—204 |
| [10] | Hatem G., Eriksen K.M., Fehrmann R., Thermal Analysis and Calorimetry, 2002, 68(1), 25—30 |
| [11] | Swain D., Bhadram V.S., Pradhan G. K., Venkataprasad, Bhat S. V., Narayana C., Rao C. N. R., Physical Chemistry, 2010, 114(37), 10040—10044 |
| [12] | Periasamy A., Muruganand S., Palaniswamy M., Rasayan Journal of Chemistry, 2009, 2(4), 9781—9789 |
| [13] | Sharon M., Kalia A.K., Solid State Chemistry, 1980, 31(3), 295—303 |
| [14] | Fehramnn R., Hansen N.H., Bjerrum N. J., Inorganic Chemistry, 1983, 22(26), 4009—4014 |
| [15] | Walrafen G.E., Irish D. E., Young T. F., Chemical Physics, 1962, 37(3), 662—670 |
| [16] | Swain D., Row T.N., Inorganic Chemistry, 2008, 47(19), 8613—8615 |
| [17] | Knudsen C.B., Kalampounias A. G., Fehrmann R., Boghosian S., Physical Chemistry B, 2008, 112(38), 11996—12000 |
| [18] | Dey B., Jain Y.S., Verma A. L., Raman Spectroscopy, 1982, 13(3), 209—212 |
| [19] | Merinov B.V., Solid State Ionics, 1996, 84(1/2), 89—96 |
| [20] | Eriksen K.M., Fehrmann R., Hatem G., Escard M. G., Lapina O. B., Mastikhin V. M., Physical Chemistry, 1996, 100(25), 10771—10778 |
| [21] | You J.L., Novel High Temperature Raman Spectroscopic Techniques, Spectral Calculation and Their Application in Micro-structure Study of Inorganic Materials, Shanghai University,Shanghai, 2006 |
| (尤静林. 高温拉曼光谱创新技术、 光谱计算和在无机化合物微结构研究中的应用,上海: 上海大学, 2006) | |
| [22] | Li S.P., Wu G. M., Zheng X. M., Chem. J. Chinese Universities, 2004, 25(8), 1495—1498 |
| (李少鹏, 吴光明, 郑旭明. 高等学校化学学报, 2004, 25(8), 1495—1498) | |
| [23] | Kalampounias A.G., Boghosian S., Applied Spectroscopy, 2009, 63(9), 1050—1056 |
| [24] | Hatem G., Eriksen K.M., Fehrmann R., Thermal Analysis and Calorimetry, 2002, 68(1), 25—30 |
| [25] | Hatem G., Eriksen K.M., Escard M. G., Fehrmann R., Catalysis, 2002, 19(3), 323—331 |
| [1] | HE Hongrui, XIA Wensheng, ZHANG Qinghong, WAN Huilin. Density-functional Theoretical Study on the Interaction of Indium Oxyhydroxide Clusters with Carbon Dioxide and Methane [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220196. |
| [2] | WANG Kun, ZOU Xingli, CAO Zhanmin, LI Chonghe, LU Xionggang. Modified Quasichemical Model for Manifold Short-range Orders in Binary Solutions: Unity of Opposites for the Ordered Pairs [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220391. |
| [3] | WANG Yuanyue, AN Suosuo, ZHENG Xuming, ZHAO Yanying. Spectroscopic and Theoretical Studies on 5-Mercapto-1,3,4-thiadiazole-2-thione Microsolvation Clusters [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220354. |
| [4] | YING Fuming, JI Chenru, SU Peifeng, WU Wei. λ-DFCAS: A Hybrid Density Functional Complete Active Space Self Consistent Field Method [J]. Chem. J. Chinese Universities, 2021, 42(7): 2218. |
| [5] | HAN Yandong, HAN Mingyong, YANG Wensheng. Sol-gel Construction of Mesoporous Silica Nanomicrostructures [J]. Chem. J. Chinese Universities, 2021, 42(4): 965. |
| [6] | ZHANG Jiayi, DING Zhenyao, WANG Dandan, CHEN Liping, FENG Xinjian. Fabrication of Triphase Enzyme Electrode Based on Porous Gold Substrate for High-performance Electrochemical Biosensor [J]. Chem. J. Chinese Universities, 2021, 42(10): 3167. |
| [7] | JIANG Huayi,LIU Mei,QI Hongyuan,LIANG Aiguo,WANG Yulong,SUN Nana,WU Zhe. Fractal Characteristics of the Microstructures of Three Hydrophobic Surfaces with Steel Substrate and Their Effects on Wettability † [J]. Chem. J. Chinese Universities, 2020, 41(6): 1313. |
| [8] | CAO Hongyu,MA Zihui,ZHANG Wenqiong,TANG Qian,LI Ruyu,ZHENG Xuefang. Structures and Electronic Absorption Spectra of N/Neo-Confused, Doubly N-Confused and Neo-Confused N-Confused Porphyrin Isomers † [J]. Chem. J. Chinese Universities, 2020, 41(2): 341. |
| [9] | WEI Xin, DENG Yaoliang, ZHENG Xuming, ZHAO Yanying. Ground Structure and Excited State Proton Transfer Reaction of 2-Aminobenzothiazole [J]. Chem. J. Chinese Universities, 2019, 40(8): 1679. |
| [10] | HE Zijun,XIAO Ming,MA Xiangying,QIU Jiangyuan,XIAO Biyuan,QIN Fanghong,HUANG Zaiyin. Facet and Temperature Effects on Dissolution Thermodynamic Functions of Ag3PO4 Microcrystals† [J]. Chem. J. Chinese Universities, 2019, 40(5): 959. |
| [11] | ZHI Shasha,BAN Ying,XU Zhiguang,XU Xuan. Electron Transport of Metal String Complexes of [MM'M″(dpa)4(Cl)2](M=Co, Ni; M',M″=Co, Rh)† [J]. Chem. J. Chinese Universities, 2019, 40(5): 980. |
| [12] | LIU Qiuna,XU Wenwen,LIU Maozhu,WANG Huigang,ZHENG Xuming. Study on Raman Spectroscopy Non-coincidence Effect of Propionic Anhydride C=O Vibration Mode† [J]. Chem. J. Chinese Universities, 2019, 40(5): 932. |
| [13] |
ZHOU Xiaofeng,ZHOU Yanbing,TANG Chunmei.
Hydrogen Storage Capacity of the Alkaline Earth Metal Mg Exohedral Doped Boron Cage B40M |
| [14] | ZHOU Hegen,JIN Hua,GUO Huirui,LIN Jing,ZHANG Yongfan. Electronic Structures and Optical Properties of Cu-based Semiconductors with Chalcopyrite-type Structure† [J]. Chem. J. Chinese Universities, 2019, 40(3): 518. |
| [15] | XIAO Biyuan,QIU Jiangyuan,QIN Fanghong,WAN Ting,XU Yaqun,NONG Xiaohui,HUANG Zaiyin. Study on Particle Size Effect on Adsorption Thermodynamics and Kinetics of Cubic Nano-Cu2O † [J]. Chem. J. Chinese Universities, 2019, 40(10): 2214. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||