

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (5): 983.doi: 10.7503/cjcu20170629
Previous Articles Next Articles
LI Jianwei, LI Xiang, ZHANG Jie*, LEI Zhigang
Received:2017-09-20
Online:2018-04-19
Published:2018-04-19
Contact:
ZHANG Jie
Supported by:CLC Number:
TrendMD:
LI Jianwei,LI Xiang,ZHANG Jie,LEI Zhigang. Mechanism of the Interaction Between Ionic Liquid [Bmim][DBP] and Methanol for Seperation of Mixed C4/Methanol†[J]. Chem. J. Chinese Universities, 2018, 39(5): 983.
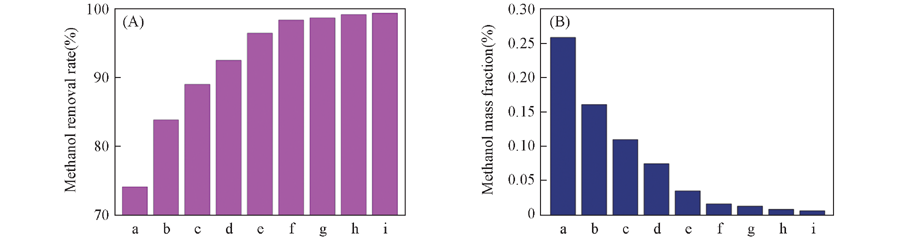
Fig.1 Extraction performance of different ILs for CH3OH Note:(A) Extraction efficiency of different ILs; (B) the contents of CH3OH after experiments. t=25 ℃; m(ILs)∶m(oil)=2∶5. a. [Bmim] [Tos]; b. [Bmim] [NTf2]; c. [Bmim] [BF4]; d. [Bmim] [DEP]; e. [Bmim] [HSO4]; f. [Bmim] [TA]; g. [Bmim] [DCA]; h. [Bmim] [Lac]; i. [Bmim] [DBP].
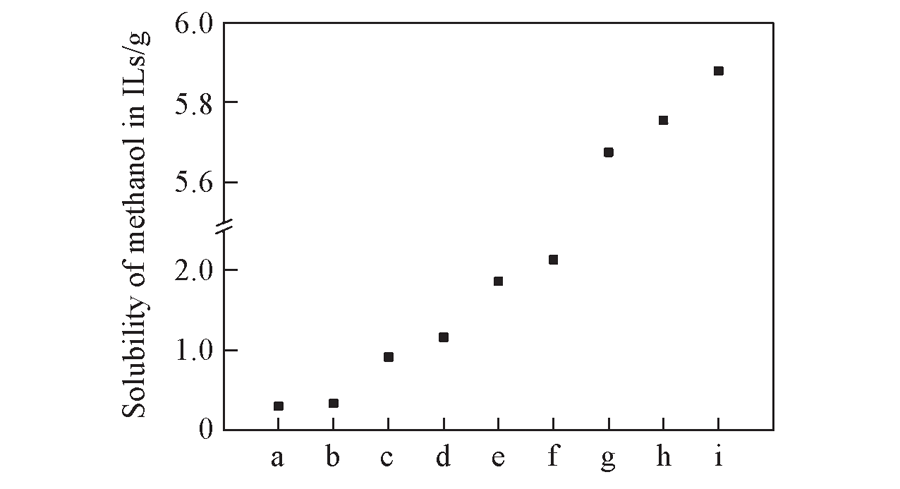
Fig.2 Prediction of solubility of CH3OH in ILs Note:a. [Bmin][Tos]; b. [Bmin][NTf2]; c. [Bmin][BF4]; d. [Bmin][DEP]; e. [Bmin][HSO4]; f. [Bmin][TA]; g. [Bmin][DCA]; h. [Bmin][Lac]; i. [Bmin][DBP].
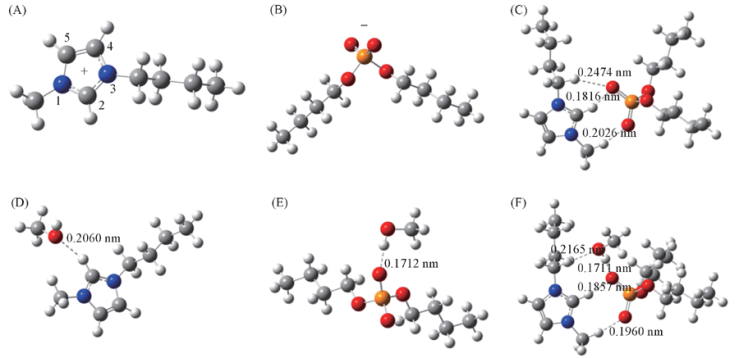
Fig.3 Optimized geometries at the B3LYP/6-31++G(d,p) level for the extraction systems Note:(A) [Bmim]+; (B) [DBP]-; (C) [Bmim][DBP]; (D) [Bmim]+-CH3OH; (E) [DBP]--CH3OH; (F )[Bmim][DBP]-CH3OH.
| Compd. | Total energy/(kJ·mol-1) | Compd. | Total energy/(kJ·mol-1) |
|---|---|---|---|
| CH3OH | 3.04×105 | [Bmim]+ and CH3OH | 1.42×106 |
| [Bmim]+ | 1.11×106 | [DBP]- and CH3OH | 2.82×106 |
| [DBP]- | 2.52×106 | [Bmim][DBP] and CH3OH | 3.93×106 |
| [Bmim][DBP] | 3.63×106 |
Table 1 Total energy for CH3OH and [Bmim][DBP]
| Compd. | Total energy/(kJ·mol-1) | Compd. | Total energy/(kJ·mol-1) |
|---|---|---|---|
| CH3OH | 3.04×105 | [Bmim]+ and CH3OH | 1.42×106 |
| [Bmim]+ | 1.11×106 | [DBP]- and CH3OH | 2.82×106 |
| [DBP]- | 2.52×106 | [Bmim][DBP] and CH3OH | 3.93×106 |
| [Bmim][DBP] | 3.63×106 |
| [1] | Collignon F., Loenders R., Martens J.A., [J]. Catal., 1999, 182, 302—312 |
| [2] | Mike T. John S.; Translated by Du H. S., Shan J. X., Value Stream Mapping, China Communications Press, Beijing, 1999, 35—39 |
| (杜宏生, 单金秀[译]. 价值流图析, 北京: 人民交通出版社, 1999, 35—39) | |
| [3] | Matouq M., Tagawa T., Goto S., [J]. Chem. Eng. Jpn., 1993, 26, 254—258 |
| [4] | Ma X., Hu C., Guo R., Fang X., Wu H., Jiang Z.Y., Sep. Purif. Technol., 2008, 59, 34—42 |
| [5] | Bitar L.S., Hazbun E. A., Fiel W. [J]., Hydrocarb. Proc., 1984, 63, 63—66 |
| [6] | Wang B., Liu C.J., Wang J. D., Lei Z. K., Hu D. L., Chem. [J]. Chinese Universities, 2012, 33(1), 76—81 |
| (王斌, 刘晨江, 王吉德, 雷振凯, 胡东林. 高等学校化学学报, 2012, 33(1), 76—81) | |
| [7] | Zhang H., Zhang H.M., Wang L. J, Shen J. Y., Chem. [J]. Chinese Universities, 2016, 37(9), 1660—1668 |
| (张慧, 张红梅, 王连军, 沈锦优. 高等学校化学学报, 2016, 37(9), 1660—1668) | |
| [8] | Zhang S.J., Xu C. M., Lü X. M., Zhou Q., Ionic Liquids and Green Chemistry, Science Press, Beijing, 2009, 78—99 |
| (张锁江, 徐春明, 吕兴梅, 周清. 离子液体与绿色化学, 北京: 科学出版社, 2009, 78—99) | |
| [9] | Tong J., Zheng X., Tong J., Qu Y., Liu L., Li H., Chem. Res. Chinese Universities, 2017, 33(5), 828—832 |
| [10] | Zhao Y.M., Cui H. M., Zheng C. Z., Chen X. G., Li C. Y., Chem. Res. Chinese Universities, 2016, 32(1), 112—117 |
| [11] | Huddleston J.G., Rogers R. D., Chem. Commun., 1998, 16, 1765—1766 |
| [12] | Fadeev A.G., Meagher M. M., Chem. Commun., 2001, 3, 295—296 |
| [13] | Khachatryan K.S., Smirnova S. V., Torocheshnikova I. I., Shvedene N. V., Formanovsky A. A., Anal. Bioanal. Chem., 2005, 381, 464—470 |
| [14] | Smirnova S.V., Torocheshnikova I. I., Formanovsky A. A., Pletnev I. V., Anal. Bioanal. Chem., 2004, 378, 1369—1375 |
| [15] | Huddleston J.G., ACS Symposium, 2002, 818, 270—288 |
| [16] | Pereiro A.B., Deive F. J., Esperança J. M. S. S., Rodríguez A., Fluid Phase Equilibr., 2010, 294, 49—53 |
| [17] | Revelli A.L., Mutelet F., Jaubert J., J. Chem. Eng. Data, 2011, 56, 3873—3880 |
| [18] | Marciniak A., Królikowski M., Fluid Phase Equilibr., 2012, 321, 59—63 |
| [19] | Wlazło M., Marciniak A., Fluid Phase Equilibr., 2014, 338, 253—256 |
| [20] | Cai F., Xiao G., [J]. Chem. Thermodyn., 2015, 87, 110—116 |
| [21] | Cammarata L., Kazarian S.G., Salterb P. A., Welton T., Phys. Chem. Chem. Phys., 2001, 3, 5192—5200 |
| [22] | Porter A.R., Liem S. Y., Popelier P. L. A., Phys. Chem. Chem. Phys., 2008, 10, 4240—4248 |
| [23] | Zhang Q.G., Wang N. N., Yu Z. W., J. Phys. Chem. B, 2010, 114, 47—54 |
| [24] | Köddermann T., Wertz C., Heintz A., Ludwig R., ChemPhysChem, 2010, 7, 1944—1949 |
| [25] | Dhumal N.R., Kim H. J., Kiefer J., J. Phys. Chem. A, 2009, 113, 10397—10404 |
| [26] | Becke A.D., [J]. Chem. Phys., 1993, 98, 5648—5653 |
| [27] | Ditchfield R., Hehre W.J., Pople J. A., [J]. Chem. Phys., 1971, 54, 724—728 |
| [1] | CUI Wei, ZHAO Deyin, BAI Wenxuan, ZHANG Xiaodong, YU Jiang. CO2 Absorption in Composite of Aprotic Solvent and Iron-based Ionic Liquid [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220120. |
| [2] | ZHOU Zixuan, YANG Haiyan, SUN Yuhan, GAO Peng. Recent Progress in Heterogeneous Catalysts for the Hydrogenation of Carbon Dioxide to Methanol [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220235. |
| [3] | PENG Kuilin, LI Guilin, JIANG Chongyang, ZENG Shaojuan, ZHANG Xiangping. Research Progress for the Role of Electrolytes in the CO2 Electrochemical Reduction [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220238. |
| [4] | JI Shuangqi, JIN Zhao, GUAN Wenna, PAN Xiangyu, GUAN Tong. Preparation and Chromatographic Performance of Mixed-mode Silica Stationary Phase Modified by Double Cationic Ionic Liquid and Octadecyl Group [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220008. |
| [5] | BI Gening, XIAO Xiaohua, LI Gongke. Development and Validation of Multiple Physical Fields Coupling Model for Microwave-assisted Extraction [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210739. |
| [6] | LIU Jie, LI Jinsheng, BAI Jingsen, JIN Zhao, GE Junjie, LIU Changpeng, XING Wei. Constructing a Water-blocking Interlayer Containing Sulfonated Carbon Tubes to Reduce Concentration Polarization in Direct Methanol Fuel Cells [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220420. |
| [7] | CHANG Sihui, CHEN Tao, ZHAO Liming, QIU Yongjun. Thermal Degradation Mechanism of Bio-based Polybutylactam Plasticized by Ionic Liquids [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220353. |
| [8] | XU Xiaolong, FANG Lining, LIU Changyu, LIU Minchao, JIA Jianbo. Preparation of Z-type g-C3N4/Pt/TiO2 Nanotube Array Composite Electrode and Its Performance of Photoelectric Oxidation of Methanol [J]. Chem. J. Chinese Universities, 2021, 42(9): 2926. |
| [9] | LI Jian, YU Mingming, SUN Yuan, FENG Wenhua, FENG Zhaochi, WU Jianfeng. Effect of Aqueous Solution pH on the Oxidation of Methane to Methanol at Low Temperature [J]. Chem. J. Chinese Universities, 2021, 42(3): 776. |
| [10] | WAN Ren, SONG Fan, PENG Changjun, LIU Honglai. Group Contribution Method for Infinite Dilution Molar Conductivity of Unconventional Ions in Water [J]. Chem. J. Chinese Universities, 2021, 42(12): 3672. |
| [11] | WANG Man, WANG Xin, ZHOU Jing, GAO Guohua. Efficient Synthesis of Dimethyl Carbonate via Transesterification of Methanol and Ethylene Carbonate Catalyzed by Poly(ionic liquid)s [J]. Chem. J. Chinese Universities, 2021, 42(12): 3701. |
| [12] | GUO Shujia, WANG Sen, ZHANG Li, QIN Zhangfeng, WANG Pengfei, DONG Mei, WANG Jianguo, FAN Weibin. Regulating the Acid Sites Distribution in ZSM-5 Zeolite and Its Catalytic Performance in the Conversion of Methanol to Olefins [J]. Chem. J. Chinese Universities, 2021, 42(1): 227. |
| [13] | ZHOU Molin, JIANG Xin, YI Ting, YANG Xiangguang, ZHANG Yibo. Improvement of Interface Stability Between Sulfide Solid Electrolyte Li10GeP2S12 and Lithium Metal [J]. Chem. J. Chinese Universities, 2020, 41(8): 1810. |
| [14] | GAO Chong,YU Fengli,XIE Congxia,YU Shitao. Baeyer-Villiger Oxidation of Cyclic Ketones Catalyzed by Amino Alcohol Heteropoly Acid Ionic Liquid † [J]. Chem. J. Chinese Universities, 2020, 41(5): 1101. |
| [15] | GAO Naiwei, MA Qiang, HE Yonglin, WANG Yapei. Green Electronic Devices Based on Ionic Liquids † [J]. Chem. J. Chinese Universities, 2020, 41(5): 901. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||