

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (4): 825.doi: 10.7503/cjcu20170482
• Polymer Chemistry • Previous Articles Next Articles
REN Jing1,2, WANG Shugang1, LI Yanchun2, YANG Qingbiao1,*( ), SONG Yan2,*(
), SONG Yan2,*( ), LI Yaoxian1
), LI Yaoxian1
Received:2017-07-19
Online:2018-04-10
Published:2018-03-27
Contact:
YANG Qingbiao,SONG Yan
E-mail:yangqb@jlu.edu.cn;songyan199809@163.com
Supported by:CLC Number:
TrendMD:
REN Jing, WANG Shugang, LI Yanchun, YANG Qingbiao, SONG Yan, LI Yaoxian. Preparation of AOPAN@PAN Coaxial Nanofiber Membrane and It’s Adsorption Property†[J]. Chem. J. Chinese Universities, 2018, 39(4): 825.
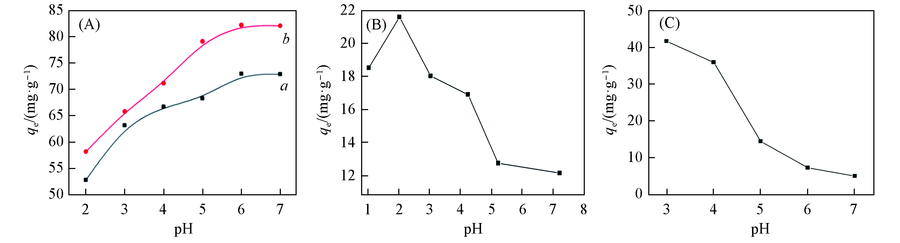
Fig.4 Effect of pH on adsorption capacityInitial concentrations of Cu2+(a), Pb2+(b)(A), Cr $O^{2-}_{4}$ (B) and MO(C) are 100, 100, 20 and 30 mg/L,respectively; adsorption time: 24 h; 30 ℃.
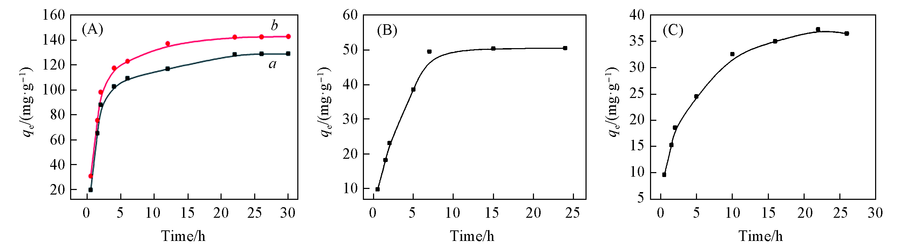
Fig.6 Effect of time on adsorption capacityInitial concentrations of Cu2+(a), Pb2+(b)(A), Cr$O^{2-}_{4}$ (B) and MO(C) are 100, 100, 20 and 30 mg/L, respectively.
| [1] | Liu D., Li Y., Ma J., Li C., Water Air & Soil Pollut., 2016, 227(5), 1-10 |
| [2] | Fu F., Wang Q., J. Environ. Manage, 2011, 92(3), 407-418 |
| [3] | Demirbas A., J. Hazard. Mater., 2008, 157(2/3), 220-229 |
| [4] | Suba V., Rathika G., J. Adv. Phys., 2016, 5(4), 277-294 |
| [5] | Wang L., Zhou J. B., Wang X., Anal. Bioanal. Chem., 2016, 408(16), 4445-4453 |
| [6] | Yan C. Q., Liu B., Lu G. X., Li Y. X., Yang Q. B., Song Y., Chem. J. Chinese Universities, 2016, 37(1), 189-194 |
| (闫春秋, 刘斌, 鲁冠秀, 杨清彪, 宋岩. 高等学校化学学报, 2016, 37(1), 189-194) | |
| [7] | Horzum N., Shahwan T., Parlak O., Demir M. M., Chem. Eng. J., 2012, 213, 41-49 |
| [8] | Chauque E. F. C., Dlamini L. N., Adelodun A. A., Greyling C. J., Ngila J. C., Appl. Surf. Sci. , 2016, 369, 19-28 |
| [9] | Hu L., Yan X. W., Yao C. G., Sep. Purif. Technol., 2016, 171, 44-51 |
| [10] | Saeed K., Khan I., Park S. Y., Desalin. Water Treat., 2015, 54(11), 3146-3151 |
| [11] | Abedi M., Chenar M. P., Sadeghi M., Fibers Polym., 2015, 16(4), 788-793 |
| [12] | Xie S., Liu X., Zhang B., Ma H., Ling C., J. Mater. Chem. A, 2015, 3(6), 2552-2558 |
| [13] | Kaerkitcha N., Chuangchote S., Sagawa T., Nanoscale Res. Lett., 2016, 11, 1-9 |
| [14] | Sun Z. C., Zussman E., Yarin A. L., Wendorff J. H., Adv. Mater. , 2003, 15(22), 1929-1932 |
| [15] | Zhang Y. X., Ye J., Xiong J., Paper Sci. & Technol., 2010, 29(1), 71-75 |
| (张燕兴, 叶君, 熊犍. 造纸科学与技术, 2010, 29(1), 71-75) | |
| [16] | Neghlani P. K., Rafizadeh M., Taromi F. A., J. Hazard. Mater., 2011, 186(1), 182-189 |
| [17] | Ding Y. Y., Wang Z. C., Wen X. F., Zhang X. P., Ye L., Zhang A. Y., Feng Z. G., Chem. J. Chinese Universities, 2013, 34(7), 1758-1764 |
| (丁耀莹, 王志成, 问县芳, 张鑫鹏, 叶霖, 张爱英, 冯增国. 高等学校化学学报, 2013, 34(7), 1758-1764) | |
| [18] | Wang J. N., He H., Li X. Y.,Acta Polym. Sinica, 2015, (7), 778-785 |
| (王娇娜, 贺欢, 李秀艳. 高分子学报, 2015, (7), 778-785) |
| [1] | TIAN Xiaokang, ZHANG Qingsong, YANG Shulin, BAI Jie, CHEN Bingjie, PAN Jie, CHEN Li, WEI Yen. Porous Materials Inspired by Microbial Fermentation: Preparation Method and Application [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220216. |
| [2] | MA Jianxin, LIU Xiaodong, XU Na, LIU Guocheng, WANG Xiuli. A Multi-functional Zn(II) Coordination Polymer with Luminescence Sensing, Amperometric Sensing, and Dye Adsorption Performance [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210585. |
| [3] | CHANG Shuqing, XIN Xu, HUANG Yaqi, ZHANG Xincong, FU Yanghe, ZHU Weidong, ZHANG Fumin, LI Xiaona. Pyroelectrically-induced Catalytic Performance of Zr-based MOF Under Cold-hot Alternation [J]. Chem. J. Chinese Universities, 2021, 42(8): 2558. |
| [4] | SU Yingli, REN Haisheng, LI Xiangyuan. Application of New Nonequilibrium Solvation Theory in Electronic Spectra of Organic Dyes [J]. Chem. J. Chinese Universities, 2021, 42(7): 2254. |
| [5] | YAN Yanhong, WU Simin, YAN Yilun, TANG Xihao, CAI Songliang, ZHENG Shengrun, ZHANG Weiguang, GU Fenglong. Sulfonic Acid-functionalized Spherical Covalent Organic Framework with Ultrahigh Capacity for the Removal of Cationic Dyes [J]. Chem. J. Chinese Universities, 2021, 42(3): 956. |
| [6] | PAN Jing, XU Minmin, YUAN Yaxian, YAO Jianlin. Rapid Detection of Banned Dyes in Textiles Based on Surface-enhanced Raman Spectroscopy [J]. Chem. J. Chinese Universities, 2021, 42(12): 3716. |
| [7] | GAO Xia,PAN Huibin,QIAO Chengfang,CHEN Fengying,ZHOU Yuan,YANG Wenhua. Construction of HRP Immobilized Enzyme Reactor Based on Hierarchically Porous Metal-organic Framework and Its Dye Degradation Application† [J]. Chem. J. Chinese Universities, 2020, 41(7): 1591. |
| [8] | ZHOU Jinlong, LIU Xiaolong, FU Yao. Visible-light-induced Selective Oxidation of Alcohols [J]. Chem. J. Chinese Universities, 2020, 41(11): 2435. |
| [9] | ZHANG Jubo,SUN Xiaohan,GAO Qiaojiao,WANG Haixin,LIANG Daxin,LIU Zhiming,HAN Guangting,JIANG Wei. Degradation of Organic Dyes over Regenerative Fe3O4/CuFeS2/Biomass Composite Column† [J]. Chem. J. Chinese Universities, 2019, 40(3): 425. |
| [10] | GAN Lu,DONG Yongchun. Photocatalytic Performance of Fe-complexes Prepared Using Cotton Fiber Modified with Different Dicarboxylic Acids † [J]. Chem. J. Chinese Universities, 2019, 40(10): 2205. |
| [11] | YU Chengxin, LIU Yangyang, ZHANG Xia. Preparation of Cu-BTC/PVDF Hybrid Membranes Using in situ Doping Method and Their Enhanced Dye Adsorption Properties† [J]. Chem. J. Chinese Universities, 2018, 39(7): 1384. |
| [12] |
WANG Bin,WU Yingga,LIU Zhelin,WANG Xiaohong,AN Zhihua,ZENG Jun,YANG Peng,LIU Zongrui.
Photocatalytic Activity of Keggin Type Polyoxometalates XW12 |
| [13] | LI Juan, ZHU Linfang, ZHAO Anting, LEI Guoming, GAO Li, XIA Wen, WANG Li. Catalytic Activity of Cucurbit[6]uril Modified Copper Flower Clusters† [J]. Chem. J. Chinese Universities, 2018, 39(3): 422. |
| [14] | ZHOU Yanfen,MENG Zhe,WANG Zelan,LI Jiguang,MEN Xiuqin,LIU Wanyi. Preparation and Selective Adsorption Performance of Polyaniline Silicon Magnetic Composite by Multilayer Assembly for Sulfonic Dye† [J]. Chem. J. Chinese Universities, 2018, 39(10): 2253. |
| [15] | LI Fu, DONG Yongchun, CHENG Bowen, KANG Weimin. Application of Hybrid Modified PAN Nanofibrous Membrane Fe Complexes with Adsorption-photocatalysis Bifunctions for Removal of Organic Dye from Water† [J]. Chem. J. Chinese Universities, 2018, 39(1): 115. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||