

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (1): 78.doi: 10.7503/cjcu20170245
• Organic Chemistry • Previous Articles Next Articles
FAN Zhongquan1,2, CHEN Chen1,2, SHEN Zhenlu1, LI Meichao1,2,*( )
)
Received:2017-04-19
Online:2018-01-10
Published:2017-12-21
Contact:
LI Meichao
E-mail:limc@zjut.edu.cn
Supported by:CLC Number:
TrendMD:
FAN Zhongquan, CHEN Chen, SHEN Zhenlu, LI Meichao. One-pot Electrochemical Oxidation Synthesis of Nitriles from Alcohols with Hexamethyldisilazane as the Nitrogen Source†[J]. Chem. J. Chinese Universities, 2018, 39(1): 78.
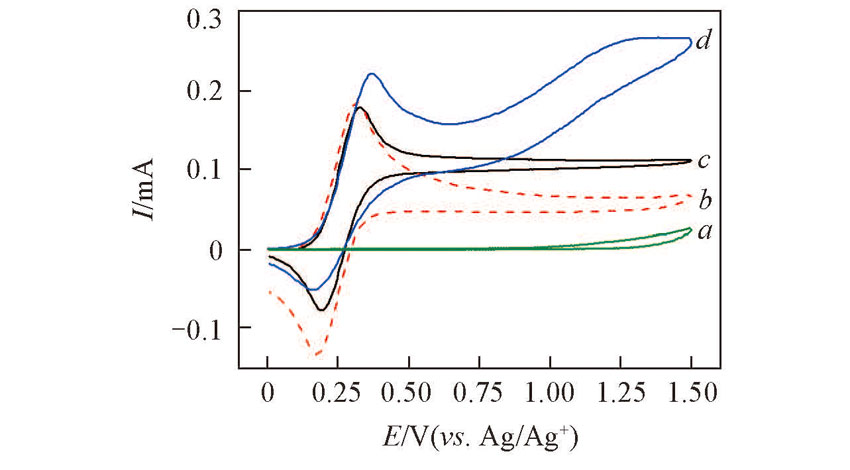
Fig.1 Cyclic voltammograms recorded in 0.1 mol/L NaClO4-CH3CN solution in the presence of 1.0 mmol benzyl alcohol(a), 0.15 mmol TEMPO(b), 0.15 mmol TEMPO+1.0 mmol benzyl alcohol(c) and 0.15 mmol TEMPO+1.0 mmol benzyl alcohol+2.5 mmol HMDS+5.0 mmol AcOH(d)
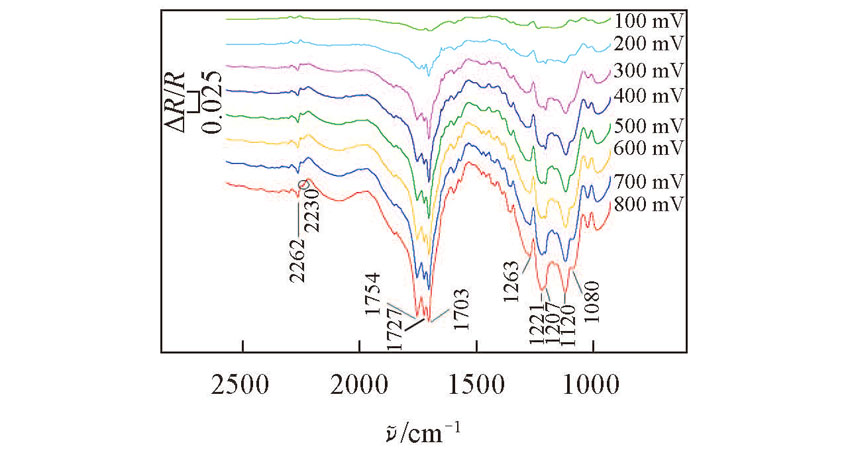
Fig.2 In situ FTIR spectra collected on Pt electrode during the oxidation of 1mmol benzyl alcohol with 0.15 mmol TEMPO, 2.5 mmol HMDS and 5.0 mmol AcOH in 0.1 mol/L NaClO4-CH3CN solution
| Entry | nHMDS/mmol | xTEMPO(molar fraction, %) | nAcOH/mmol | Potential/V | Yield of benzonitrileb(%) |
|---|---|---|---|---|---|
| 1 | 2.5 | 15 | 3.0 | 1.5 | 27 |
| 2 | 2.5 | 15 | 4.0 | 1.5 | 88 |
| 3 | 2.5 | 15 | 5.0 | 1.5 | 95 |
| 4 | 1.0 | 15 | 5.0 | 1.5 | 62 |
| 5 | 3.0 | 15 | 5.0 | 1.5 | 97 |
| 6 | 2.5 | 5 | 5.0 | 1.5 | 58 |
| 7 | 2.5 | 10 | 5.0 | 1.5 | 84 |
| 8 | 2.5 | 15 | 0 | 1.5 | 0 |
| 9 | 2.5 | 15 | 5.0 | 1.2 | 12 |
| 10 | 2.5 | 15 | 5.0 | 1.8 | 60 |
Table 1 Optimization of electrochemical synthesis of benzonitrilea
| Entry | nHMDS/mmol | xTEMPO(molar fraction, %) | nAcOH/mmol | Potential/V | Yield of benzonitrileb(%) |
|---|---|---|---|---|---|
| 1 | 2.5 | 15 | 3.0 | 1.5 | 27 |
| 2 | 2.5 | 15 | 4.0 | 1.5 | 88 |
| 3 | 2.5 | 15 | 5.0 | 1.5 | 95 |
| 4 | 1.0 | 15 | 5.0 | 1.5 | 62 |
| 5 | 3.0 | 15 | 5.0 | 1.5 | 97 |
| 6 | 2.5 | 5 | 5.0 | 1.5 | 58 |
| 7 | 2.5 | 10 | 5.0 | 1.5 | 84 |
| 8 | 2.5 | 15 | 0 | 1.5 | 0 |
| 9 | 2.5 | 15 | 5.0 | 1.2 | 12 |
| 10 | 2.5 | 15 | 5.0 | 1.8 | 60 |
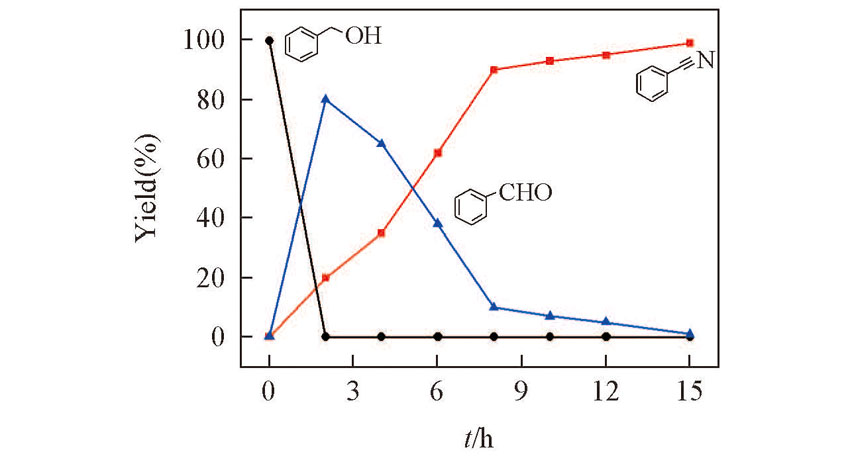
Fig.3 Reaction profiles for electrochemical synthesis of benzonitrile from benzyl alcohol in 0.1 mol/L NaClO4-CH3CN solution containing 0.15 mmol TEMPO, 2.5 mmol HMDS and 5.0 mmol AcOH
| Entry | Alcohol | Nitrile | Time/h | Conversionb(%) | Yieldb(%) |
|---|---|---|---|---|---|
| 1 | 15 | >99 | 99 | ||
| 2 | 12 | >99 | 98 | ||
| 3 | 12 | >99 | 95 | ||
| 4 | 12 | >99 | >99 | ||
| 5 | 12 | >99 | 96 | ||
| 6 | 12 | >99 | 90 | ||
| 7 | 12 | >99 | 92 | ||
| 8 | 12 | >99 | 94 | ||
| 9 | 12 | >99 | 97 | ||
| 10c | 20 | >99 | 68 | ||
| 11c | 15 | 18 | |||
| 12c | 15 | >99 | 9 |
Table 2 Electrochemical conversion of various alcohols to nitrilesa
| Entry | Alcohol | Nitrile | Time/h | Conversionb(%) | Yieldb(%) |
|---|---|---|---|---|---|
| 1 | 15 | >99 | 99 | ||
| 2 | 12 | >99 | 98 | ||
| 3 | 12 | >99 | 95 | ||
| 4 | 12 | >99 | >99 | ||
| 5 | 12 | >99 | 96 | ||
| 6 | 12 | >99 | 90 | ||
| 7 | 12 | >99 | 92 | ||
| 8 | 12 | >99 | 94 | ||
| 9 | 12 | >99 | 97 | ||
| 10c | 20 | >99 | 68 | ||
| 11c | 15 | 18 | |||
| 12c | 15 | >99 | 9 |
| [1] | Anbarasan P., Schareina T., Beller M., Chem. Soc. Rev., 2011, 40, 5049—5067 |
| [2] | Ragab F. A., Abdel Gawad N. M., Georgey H. H., Said M. F., Eur. J. Med. Chem., 2013, 63, 645—654 |
| [3] | Berteotti A., Vacondio F., Lodola A., Bassi M., Silva C., Mor M., Cavalli A., ACS Med. Chem. Lett., 2014, 5, 501—505 |
| [4] | Kumar S., Dixit S. K., Awasthi S. K., Tetrahedron Lett., 2014, 55, 3802—3804 |
| [5] | Mukherjee A., Srimani D., Chakraborty S., Ben-David Y., Milstein D., J. Am. Chem. Soc., 2015, 137, 8888—8891 |
| [6] | Battilocchio C., Hawkins J. M., Ley S. V., Org. Lett., 2014, 16, 1060—1063 |
| [7] | Kamitanaka T., Yamamoto K., Matsuda T., Harada T., Tetrahedron, 2008, 64, 5699—5702 |
| [8] | Ortiz-Marciales M., Tirado L. M., Colon R., Ufret M. L., Figueroa R., Lebron M., DeJesus M., Martnez J., Malave T., Synth. Commun., 1998, 28, 4067—4075 |
| [9] | Miller J. S., Manson J. L., Acc. Chem. Res., 2001, 34, 563—570 |
| [10] | Beletskaya I. P., Sigeev A. S., Peregudov A. S., Petrovskii P. V., J. Organomet. Chem., 2004, 689, 3810—3812 |
| [11] | Lindley J., Tetrahedron, 1984, 40, 1433—1456 |
| [12] | Enthaler S., Inoue S., Chem. Asian J., 2012, 7(1), 169—175 |
| [13] | Zhou S., Addis D., Das S., Junge K., Seller M., Chem. Commun., 2009, 32, 4883—4885 |
| [14] | Oskooie H. A., Heravi M. M., Jaddi Z., Ghassemzadeh M., Phosphorus Sulfur and Silicon and the Related Elements, 2005, 180(9), 1993—1996 |
| [15] | Ciriminna R., Pagliaro M., Org. Process Re. Dev., 2010, 14, 245—251 |
| [16] | Vogler T., Studer A., Synthesis, 2008, 13, 1979—1993 |
| [17] | Tao C., Liu F., Zhu Y., Liu W., Cao Z., Org. Biomol. Chem., 2013, 11, 3349—3354 |
| [18] | Yin W., Wang C., Huang Y., Org. Lett., 2013, 15, 1850—1853 |
| [19] | Kelly C. B., Lambert K. M., Mercadante M. A., Angew. Chem. Int. Ed., 2015, 54(14), 4241—4245 |
| [20] | Chen Q. G., Fang C. J., Shen Z. L., Li M. C., Electrochem. Commun., 2016, 64, 51—55 |
| [21] | Liaigre D., Breton T., Belgsir E. M., Electrochem. Commun., 2005, 7(3), 312—316 |
| [22] | Francke R., Little R. D., Chem. Soc. Rev., 2014, 43, 2492—2521 |
| [23] | Nicholson R. S., Shain I., Anal. Chem., 1964, 36, 706—723 |
| [24] | Keresszegi C., Ferri D., Mallat T., Baiker A., J. Phys. Chem. B, 2005, 109(2), 958—967 |
| [25] | Meier D. M., Urakawa A., Baiker A., J. Phys. Chem. C, 2009,113(52), 21849—21855 |
| [26] | Lampert H., Mikenda W., Karpfen A., J. Phys. Chem. A, 1997, 101, 2254—2263 |
| [27] | Tjahjono M., Cheng S., Li C. Z., Garland M., J. Phys. Chem. A, 2010, 114, 12168—12175 |
| [28] | Pai P. G., Chao S. S., Takagi Y., Lucovsky G., J.Vac. Sci. Technol. A, 1986, 4(3), 689—694 |
| [29] | Nogueira S., Da Silva M. L. P., Tan I. H., Rev. Bras. Apl. Vácuo, 2008, 25(1), 45—53 |
| [30] | Dheivamalar S., Silambarasan V., Spectrochim. Acta Part A, 2012, 96, 480—484 |
| [31] | Erkabaeva A. M. Yaroslavtsevaa T. V., Popovb S. E., Bushkovaa O. V., Vib. Spectrosc., 2014, 75, 19—25 |
| [32] | Kadam S. T., Kim S. S., J. Organomet. Chem., 2009, 694, 2562—2566 |
| [33] | Hagiwara H., Ono H., Komatsubara N., Tetrahedron Lett., 1999, 40(36), 6627—6630 |
| [1] | HUANG Xiaoshun, MA Haiying, LIU Shujuan, WANG Bin, WANG Hongli, QIAN Bo, CUI Xinjiang, SHI Feng. Recent Advances on Indirect Conversion of Carbon Dioxide to Chemicals [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220222. |
| [2] | ZHANG Xinxin, XU Di, WANG Yanqiu, HONG Xinlin, LIU Guoliang, YANG Hengquan. Effect of Mn Promoter on CuFe-based Catalysts for CO2 Hydrogenation to Higher Alcohols [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220187. |
| [3] | DAI Wei, HOU Hua, WANG Baoshan. Theoretical Investigations on the Electronic Structures and Reactivity of Heptafluoro-iso-butyronitrile Anion [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220044. |
| [4] | ZHANG Yong, XU Jun, BAO Yu, CUI Shuxun. Quantifying the Degree of Weakening Effect of Nonpolar Organic Solvent on the Strength of Intramolecular Hydrogen Bonding by Single-molecule Force Spectroscopy [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210863. |
| [5] | LI Qiao, ZHAO Yang, WANG Enju. Moisture Absorption Reaction and Fluorescence Property of Highly Active Michael System Based on Arylidenemalononitrile [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210690. |
| [6] | ZHANG Shiyu, HE Runhe, LI Yongbing, WEI Shijun, ZHANG Xingxiang. Fabrication of Lithium-sulfur Battery Cathode with Radiation Crosslinked Low Molecular Weight of Polyacrylonitrile and the Mechanism of Sulfur Storage [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210632. |
| [7] | YU Jing, WU Chao, LI Chenyang, CHEN Danfeng, DING Liuyue, MA Xiantao. Catalyst-free and Highly Efficient O-Silylation of Alcohols and Phenols [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210588. |
| [8] | YANG Zhaohua, CHENG Hongjing, YANG Yi, LIU Hui, DU Feipeng, ZHANG Yunfei. Preparation of Silver-loaded Polyvinyl Alcohol Sponge and Its Interfacial Photothermal Driven Water Evaporation Performance [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220181. |
| [9] | SUN Jinshi, CHEN Peng, JING Liping, SUN Fuxing, LIU Jia. Synthesis of Hierarchical Porous Aromatic Frameworks for Immobilization of Thiourea Catalyst [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220171. |
| [10] | BAI Jingqi, BAI Shan, REN Lixia, ZHU Kongying, ZHAO Yunhui, LI Xiaohui, YUAN Xiaoyan. Trehalose-modified Poly(vinyl alcohol) and Their Antifogging/Antifrosting Coatings [J]. Chem. J. Chinese Universities, 2021, 42(8): 2683. |
| [11] | XU Yan, YANG Hongguo, NIU Huibin, TIAN Hailin, PIAO Hongguang, HUANG Yingping, FANG Yanfen. Preparation Mechanism and Application of Alcohol⁃modified Fe3O4 Magnetic Nanoparticles [J]. Chem. J. Chinese Universities, 2021, 42(8): 2564. |
| [12] | YAN Pengquan, WANG Jingrong, SHEN Yaxing, ZUO Zhijun, GAO Zhihua, HUANG Wei. Effect of CuAl2O4 Spinel Structure on CO Hydrogenation in Slurry Reactor [J]. Chem. J. Chinese Universities, 2021, 42(6): 1846. |
| [13] | WANG Jie, LI Ying, SHAO Liang, BAI Yang, MA Zhonglei, MA Jianzhong. Preparation and Properties of Poly(vinyl alcohol)/polypyrrole Composite Conductive Hydrogel Strain Sensor [J]. Chem. J. Chinese Universities, 2021, 42(3): 929. |
| [14] | WANG Ruxin, ZHAO Zhongjun, HE Feiyao, YUE Hanlu, DENG Fulong, LI Hong, LI Wenwen, DUAN Yixiang. Characteristic Analysis of C1—C3n-Aldehydes and n-Alcohols in Proton Transfer Reaction Time-of-flight Mass Spectrometry [J]. Chem. J. Chinese Universities, 2021, 42(12): 3632. |
| [15] | NI Qingsheng, DU Miao, SHAN Guorong, SONG Yihu, WU Ziliang, ZHENG Qiang. Regulation of Rheological Behavior of Polyvinyl Alcohol Aqueous Solution by One-dimensional Particles [J]. Chem. J. Chinese Universities, 2021, 42(12): 3738. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||