

Chem. J. Chinese Universities ›› 2017, Vol. 38 ›› Issue (10): 1804.doi: 10.7503/cjcu20170242
• Physical Chemistry • Previous Articles Next Articles
JIANG Rongjun1, LUO Jianhui2, BAI Ruibing1, JIANG Bo1, ZHOU Ge1,*( )
)
Received:2017-04-19
Online:2017-10-10
Published:2017-09-22
Contact:
ZHOU Ge
E-mail:zhougekk@scu.edu.cn
Supported by:CLC Number:
TrendMD:
JIANG Rongjun, LUO Jianhui, BAI Ruibing, JIANG Bo, ZHOU Ge. Molecular Dynamics Simulation on Behavior of Common Surfactants at the Oil/Water Interface in Complex Systems†[J]. Chem. J. Chinese Universities, 2017, 38(10): 1804.
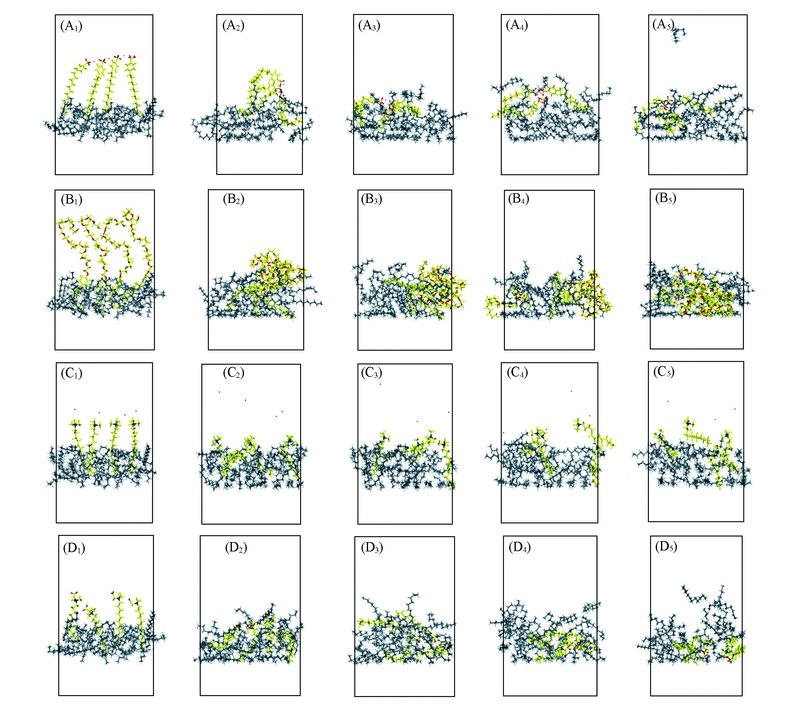
Fig.4 Behavior variation of different surfactants systems(highlight for surfactants)(A1—A5) SDBS; (B1—B5) NPE; (C1—C5) DTAB; (D1—D5) Betaine. (A1—D1) 0 ps;(A2—D2) 250 ps; (A3—D3) 500 ps; (A4—D4) 1000 ps; (A5—D5) 2000 ps.
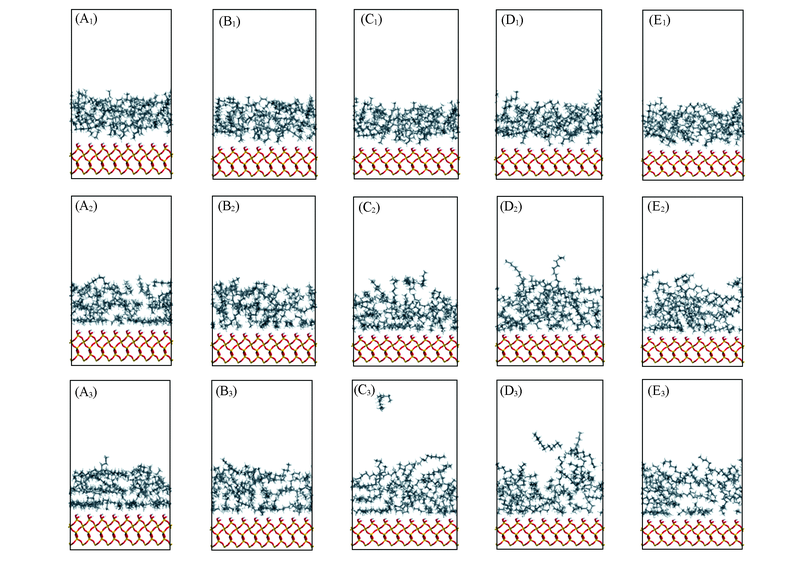
Fig.7 Snaps of different surfactants systems at 0 ps(A1—E1), 500 ps(A2—E2), 2000 ps(A3—E3), respectively(A1—A3) None; (B1—B3) DTAB; (C1—C3) SDBS; (D1—D3) Betaine; (E1—E3) NPE.
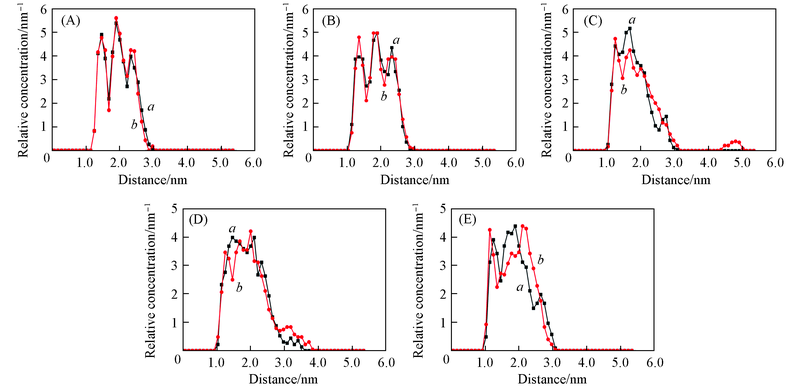
Fig.8 Relative concentrations of oil molecules of different surfactants systems at 500 ps(a) and 2000 ps(b)(A) None; (B) DTAB; (C) SDBS, (D) Betaine; (E) NPE.
| [1] | Zhang Y.,Chem. Eng. & Equip, 2011, (4), 119—120 |
| (张毅. 化学工程与装备, 2011, (4), 119—120) | |
| [2] | Nan H. M., Dai. Chem. Sci., 2009, 32(3), 18—20 |
| (南海明. 日用化学品科学, 2009, 32(3), 18—20) | |
| [3] | Xiao B., Cheng J. C., Jiang B., Oilfield Chem., 2010, 27(3), 291—294 |
| (肖波, 程杰成, 江波. 油田化学, 2010, 27(3), 291—294) | |
| [4] | Chen Y. P., Ding W., Yu T., Qu G. M., Liu G. Y., Gao X., Acta Petrolei. Sinica(Petroleum Processing Section), 2012, 28(4), 591—597 |
| (陈玉萍, 丁伟, 于涛, 曲广淼, 刘国宇, 高翔. 石油学报(石油加工), 2012, 28(4), 591—597) | |
| [5] | Smit B., Schlijper A. G., Rupert L. A. M., Nmv O., J. Phys. Chem., 1990, 94(18), 558—565 |
| [6] | Chanda J., Bandyopadhyay S., J. Chem. Theory Comput., 2005, 1(5), 963—971 |
| [7] | Baaden M., Burgard M., Wipff G., J. Phys. Chem. B,2001, 105(45), 11131—11141 |
| [8] | Li Z. Q., He X. J., Li Y., Ma B. M., Cao X. L., Acta Chim. Sinica,2007, 65(24), 2803—2808 |
| (李振泉, 何秀娟, 李英, 马保民, 曹绪龙. 化学学报, 2007, 65(24), 2803—2808) | |
| [9] | Gao J., Ge W., Li J. H., Sci. Chin.: B,2005, 35(3), 252—257 |
| (高健, 葛蔚, 李静海. 中国科学: B 辑, 2005, 35(3), 252—257) | |
| [10] | Liu M. T., Pu M. F., Ma H. W., Chem. J. Chinese Universities,2012, 33(6), 1319—1325 |
| (刘梅堂, 浦敏锋, 马鸿文. 高等学校化学学报, 2012, 33(6), 1319—1325) | |
| [11] | Fodi B., Hentschke R., Langmuir,2000, 16(4), 1626—1633 |
| [12] | Karaborni S., Van Os N. M., Esselink K., Paj H., Langmuir,1993, 9(5), 1175—1178 |
| [13] | Li Z. Q., Guo X. L., Wang H. Y., Li Q. H., Yuan S. L., Acta Physico-Chimica Sinica,2009, 25(1), 6—12 |
| (李振泉, 郭新利, 王红艳, 李青华, 苑世领. 物理化学学报, 2009, 25(1), 6—12) | |
| [14] | Ding W., Shi P., Yu T., Liu H. B., Chen J. C., Comp. & Appl. Chem., 2011, 28(3), 333—337 |
| (丁伟, 史鹏, 于涛, 刘宏彬, 程杰成. 计算机与应用化学, 2011, 28(3), 333—337) | |
| [15] | Zhang X. Q., Yuan S. L., Chen Y. J., Xu G. Y., J. Shandong Univ.(Natural Science), 2005, 4, 97—101 |
| (张秀青, 苑世领, 陈贻建, 徐桂英. 山东大学学报(理学版), 2005, 4, 97—101) | |
| [16] | Jang S. S., Lin S. T., Maiti P. K., Blanco M., Goddard W. A., J. Phys. Chem. B,2004, 108(32), 12130—12140 |
| [17] | Jang S. S., Wag L., J. Phys. Chem. B,2006, 110(15), 7992—8001 |
| [18] | Buckley J., Liu Y., Monsterleet S., SPE Journal,1998, 3(1), 54—61 |
| [19] | Liu Q., Dong M., Asghari K., Tu Y., Nat. Sci., 2010, 2(5), 450—456 |
| [20] | Cauchy M. A., Seances Acad. Sci., 1847, 177(19), 2903—2906 |
| [21] | Hesteness M. R., Stiefel E. J., Res. Nat. Bur. Standards,1952, 49, 409—412 |
| [22] | Fox R. L., Optmization Methods for Engineering Design Addison-Wesley, Translated by Zhang J. Z., Zhu M. F., Science Press, Beijing, 1984, 45—51 |
| (张建中, 诸梅芳[译]. 工程设计的优化方法, 北京: 科学出版社, 1984, 45—51) | |
| [23] | Nosé S., Mol. Phys., 1984, 52, 255—268 |
| [24] | Berendsen H. J. C., Postma J. P. M., van Gunsteren W. F., DiNola A., Haak J. R., J. Chem. Phy., 1984, 81, 3684—3690 |
| [25] | Chen J., Du L. B., Zhang Y. X., Thieo H. E., Jiang M., Polym. Int., 2001, 50, 148—152 |
| [26] | Albert Einstein, Collected Works of Albert Einstein(Volume Second), Translated by Fan D. N., Zhao Z. L., Xu L. Y., Commercial Press, Beijing, 1977, 72—83 |
| (范岱年, 赵中立, 许良英[译]. 爱因斯坦文集(第2卷), 北京: 商务印书馆, 1977, 72—83) | |
| [27] | Rao M., Berne B. J., Mol. Phys., 1979, 37, 455—461 |
| [28] | Hill T. L., Introduction to Statistical Mechanics, Dover, New York, 1986 |
| [1] | REN Shijie, QIAO Sicong, LIU Chongjing, ZHANG Wenhua, SONG Li. Synchrotron Radiation X-Ray Absorption Spectroscopy Research Progress on Platinum Single-atom Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220466. |
| [2] | PENG Kuilin, LI Guilin, JIANG Chongyang, ZENG Shaojuan, ZHANG Xiangping. Research Progress for the Role of Electrolytes in the CO2 Electrochemical Reduction [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220238. |
| [3] | ZHOU Leilei, CHENG Haiyang, ZHAO Fengyu. Research Progress of CO2 Hydrogenation over Pd-based Heterogeneous Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220279. |
| [4] | GAO Zhiwei, LI Junwei, SHI Sai, FU Qiang, JIA Junru, AN Hailong. Analysis of Gating Characteristics of TRPM8 Channel Based on Molecular Dynamics [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220080. |
| [5] | HU Huimin, CUI Jing, LIU Dandan, SONG Jiaxin, ZHANG Ning, FAN Xiaoqiang, ZHAO Zhen, KONG Lian, XIAO Xia, XIE Zean. Influence of Different Transition Metal Decoration on the Propane Dehydrogenation Performance over Pt/M-DMSN Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210815. |
| [6] | ZENG Xianyang, ZHAO Xi, HUANG Xuri. Mechanism of Inhibition of Glucose and Proton Cotransport Protein GlcPSe by Cytochalasin B [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210822. |
| [7] | CHEN Hanxiang, BIAN Shaoju, HU Bin, LI Wu. Molecular Simulation of the Osmotic Pressures for LiCl-NaCl-KCl-H2O Solution System [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210727. |
| [8] | HU Bo, ZHU Haochen. Dielectric Constant of Confined Water in a Bilayer Graphene Oxide Nanosystem [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210614. |
| [9] | ZHANG Mi, TIAN Yafeng, GAO Keli, HOU Hua, WANG Baoshan. Molecular Dynamics Simulation of the Physicochemical Properties of Trifluoromethanesulfonyl Fluoride Dielectrics [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220424. |
| [10] | LIU Yang, LI Wangchang, ZHANG Zhuxia, WANG Fang, YANG Wenjing, GUO Zhen, CUI Peng. Theoretical Exploration of Noncovalent Interactions Between Sc3C2@C80 and [12]Cycloparaphenylene Nanoring [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220457. |
| [11] | JIANG Shan, SHEN Qianqian, LI Qi, JIA Husheng, XUE Jinbo. Pd-loaded Defective TiO2 Nanotube Arrays for Enhanced Photocatalytic Hydrogen Production Performance [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220206. |
| [12] | XU Wenzhe, ZHANG Hao. Supramolecular Interactions-mediated Nanodrug Nucleation [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220264. |
| [13] | ZHANG Lingyu, ZHANG Jilong, QU Zexing. Dynamics Study of Intramolecular Vibrational Energy Redistribution in RDX Molecule [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220393. |
| [14] | LI Congcong, LIU Minghao, HAN Jiarui, ZHU Jingxuan, HAN Weiwei, LI Wannan. Theoretical Study of the Catalytic Activity of VmoLac Non-specific Substrates Based on Molecular Dynamics Simulations [J]. Chem. J. Chinese Universities, 2021, 42(8): 2518. |
| [15] | PAN Xiaojun, BAO Rongrong, PAN Caofeng. Research Progress of Flexible Tactile Sensors Applied to Wearable Electronics [J]. Chem. J. Chinese Universities, 2021, 42(8): 2359. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||