

Chem. J. Chinese Universities ›› 2017, Vol. 38 ›› Issue (10): 1772.doi: 10.7503/cjcu20170164
• Organic Chemistry • Previous Articles Next Articles
SUN Taiqiang, LI Bin, NIE Yao*( ), WANG Dong*(
), WANG Dong*( ), XU Yan
), XU Yan
Received:2017-03-20
Online:2017-10-10
Published:2017-09-21
Contact:
NIE Yao,WANG Dong
E-mail:ynie@jiangnan.edu.cn;dwang@jiangnan.edu.cn
Supported by:CLC Number:
TrendMD:
SUN Taiqiang, LI Bin, NIE Yao, WANG Dong, XU Yan. Asymmetric Synthesis of (S)-N,N-Dimethyl-3-hydroxy-3-(2-thienyl)-1-propanamine by Cell-free System of Carbonyl Reductase CR2†[J]. Chem. J. Chinese Universities, 2017, 38(10): 1772.
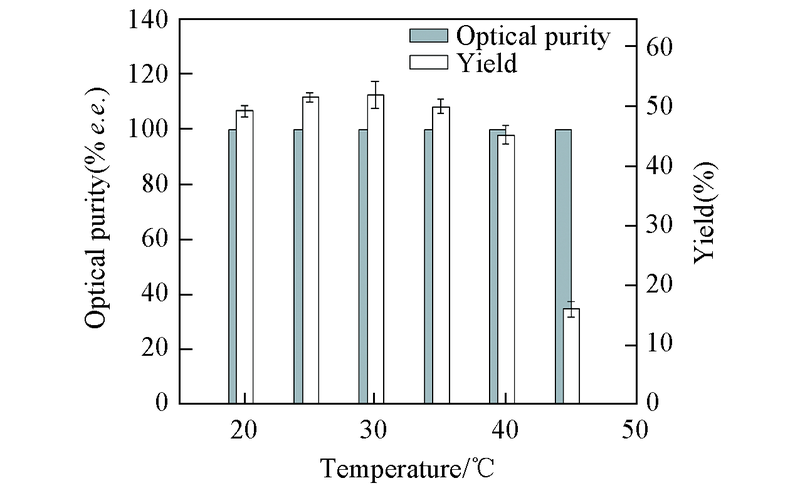
Fig.1 Effect of reaction temperature on asymmetric synthesis of (S)-DHTP by CR2 cell-free systemAll reactions were carried out in 10 mL TEA buffer(0.1 mol/L) comprising DKTP(10 g/L) with addition of glucose(100 g/L) and NADP+(0.2 mmol/L). All the results were the average of three parallel replicates.
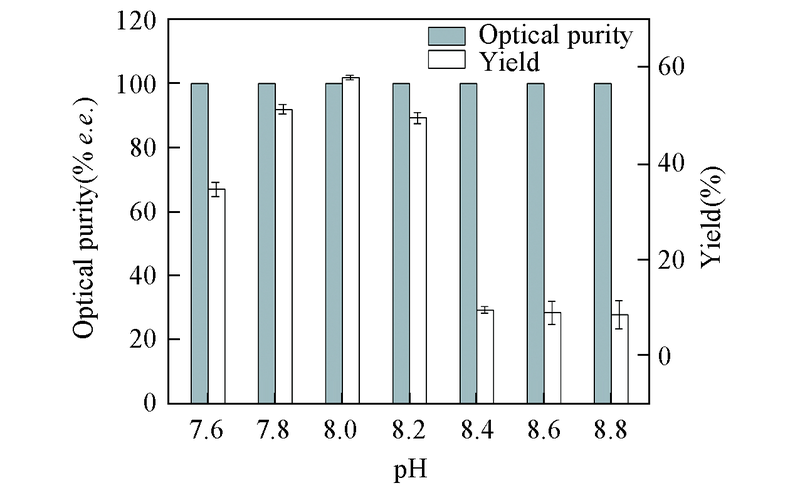
Fig.2 Effect of initial pH on asymmetric synthesis of (S)-DHTP by CR2 cell-free systemAll reactions were carried out in 10 mL TEA buffer(0.1 mol/L) comprising DKTP(10 g/L) with addition of glucose(100 g/L) and NADP+(0.2 mmol/L). All the results were the average of three parallel replicates.
| NADP+/(mmol·L-1) | e.e.(%) | Yield(%) | TTN | NADP+/(mmol·L-1) | e.e.(%) | Yield(%) | TTN |
|---|---|---|---|---|---|---|---|
| 0.200 | >99.9 | 45 | 103 | 0.010 | >99.9 | 38 | 1512 |
| 0.100 | >99.9 | 53 | 241 | 0.005 | >99.9 | 33 | 3436 |
| 0.050 | >99.9 | 50 | 325 | 0 | | ||
| 0.020 | >99.9 | 49 | 1129 |
Table 1 Effect of cofactor concentration on reduction of DKTP by CR2 cell-free system*
| NADP+/(mmol·L-1) | e.e.(%) | Yield(%) | TTN | NADP+/(mmol·L-1) | e.e.(%) | Yield(%) | TTN |
|---|---|---|---|---|---|---|---|
| 0.200 | >99.9 | 45 | 103 | 0.010 | >99.9 | 38 | 1512 |
| 0.100 | >99.9 | 53 | 241 | 0.005 | >99.9 | 33 | 3436 |
| 0.050 | >99.9 | 50 | 325 | 0 | | ||
| 0.020 | >99.9 | 49 | 1129 |
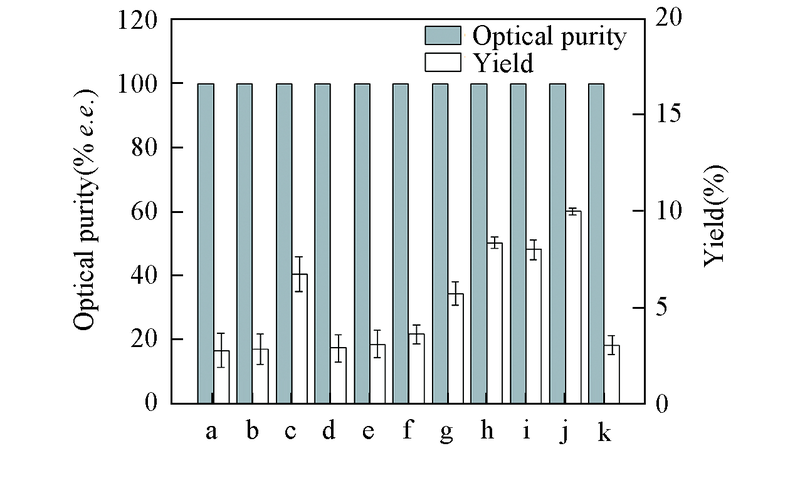
Fig.3 Effect of co-substrates on reduction of DKTP by CR2 cell-free systemAll reactions were carried out in 10 mL TEA buffer(0.1 mol/L, pH=8.0) comprising DKTP(10 g/L) with addition of cosubstrate(10 g/L) and NADP+(0.1 mmolL) at 30 ℃. All the results were the average of three parallel replicates. a. Glycerol; b. ethanol; c. 2-propanol; d. sorbitol; e. mannitol; f. sucrose; g. fructose; h. lactose; i. maltose; j. glucose; k. xylose.
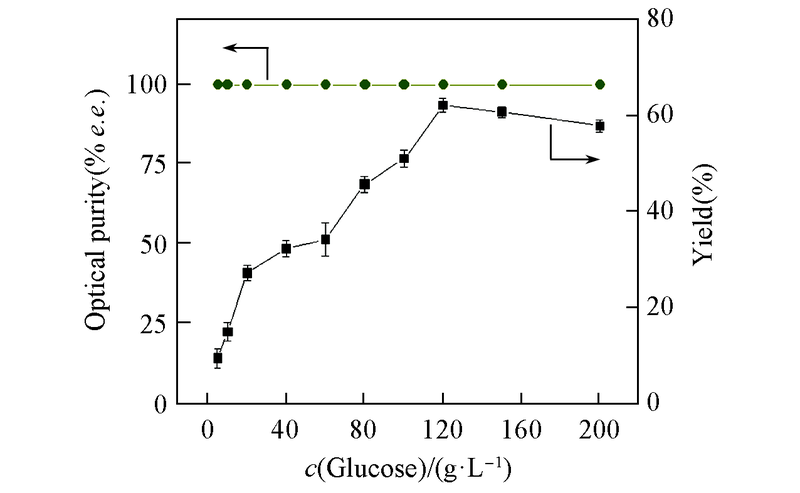
Fig.4 Effect of glucose concentration on reduction of DKTP by CR2 cell-free systemAll reactions were carried out in 10 mL TEA buffer(0.1 mol/L, pH=8.0) comprising DKTP(10 g/L) and NADP+(0.1 mmol/L) at 30 ℃. All the results were the average of three parallel replicates.
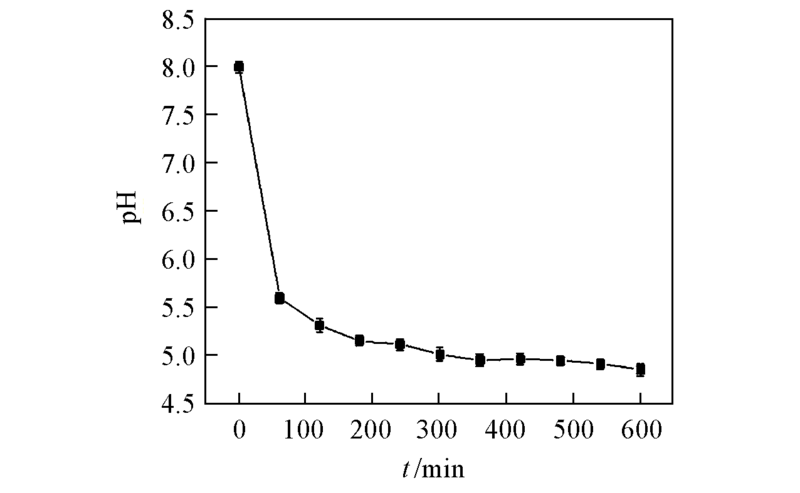
Fig.5 Curves of variation of pH value in the asymmetric reaction processAll reactions were carried out in 10 mL TEA buffer(0.1 mol/L, pH=8.0) comprising DKTP(10 g/L) with addition of glucose(120 g/L) and NADP+(0.1 mmol/L) at 30 ℃. All the results were the average of three parallel replicates.
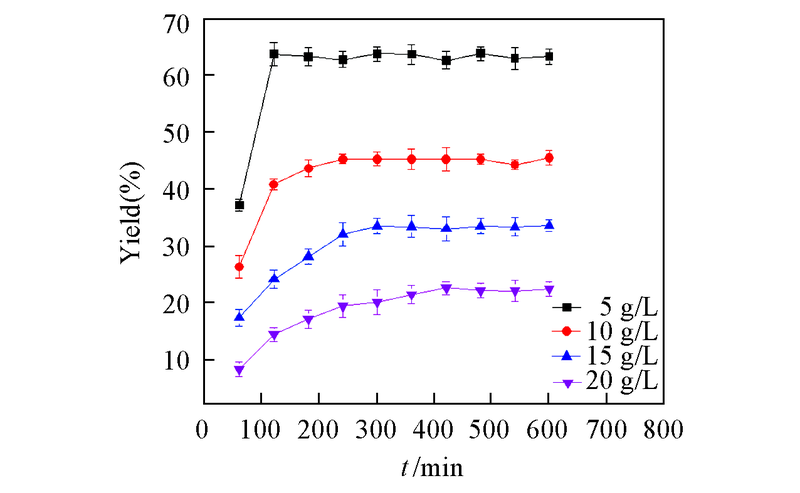
Fig.6 Reaction process of CR2 cell-free system catalyzing asymmetric reduction of DKTP under different concentrationsAll reactions were carried out in 10 mL TEA buffer(0.1 mol/L, pH=8.0) comprising DKTP with addition of glucose(100 g/L) and NADP+(0.2 mmol/L) at 30 ℃. All the results were the average of three parallel replicates.
| [1] | Gamenara D., de Maria P. D., Biotechnol. Adv., 2009, 27(3), 278—285 |
| [2] | Patel R. N., Coord. Chem. Rev., 2008, 252(5), 659—701 |
| [3] | Zilbeyaz K., Taskin M., Kurbanoglu E. B., Kurbanoglu N. I., Kilic H., Chirality,2010, 22(6), 543—547 |
| [4] | Ye Q., Cao H., Mi L., Yan M., Wang Y., He Q. T., Li J. A., Xu L., Chen Y., Xiong J. A., Ouyang P. K., Ying H. J., Bioresour. Technol., 2010, 101(22), 8911—8914 |
| [5] | Wohlgemuth R., Curr. Opin. Microbiol., 2010, 13(3), 283—292 |
| [6] | Pollard D. J., Woodley J. M., Trends Biotechnol., 2007, 25(2), 66—73 |
| [7] | Liu J. Z., Xiong J. B., Zhao G. H., Liu Q., Jiao Q. C., Chem. J. Chinese Universities,2010, 31(11), 2234—2238 |
| (刘均忠, 熊吉滨, 赵根海, 刘茜, 焦庆才. 高等学校化学学报, 2010, 31(11), 2234—2238) | |
| [8] | Hall M., Chem. Rev., 2011, 111(7), 4088—4110 |
| [9] | Fox R. J., Davis S. C., Mundorff E. C., Newman L. M., Gavrilovic V., Ma S. K., Chung L. M., Ching C., Tam S., Muley S., Grate J., Gruber J., Whitman J. C., Sheldon R. A., Huisman G.W., Nat. Biotechnol., 2007, 25(3), 338—344 |
| [10] | Tao J. H., Xu J. H., Curr. Opin. Chem. Biol., 2009, 13(1), 43—50 |
| [11] | Bymaster F. P., Dreshfield-Ahmad L. J., Threlkeld P. G., Shaw J. L., Thompson L., Nelson D. L., Hemrick-Luecke S. K., Wong D. T., Neuropsychopharmacology,2001, 25(6), 871—880 |
| [12] | Traff A., Lihammar R., Backvall J. E., J. Org. Chem., 2011, 76(10), 3917—3921 |
| [13] | Liu H. L., Hoff B. H., Anthonsen T., Chirality,2000, 12(1), 26—29 |
| [14] | Soni P., Banerjee U., Appl. Microbiol. Biotechnol., 2005, 67(6), 771—777 |
| [15] | Tang C. G., Lin H., Zhang C., Liu Z. Q., Yang T., Wu Z. L., Biotechnol. Lett., 2011, 33(7), 1435—1440 |
| [16] | Ou Z. M., Sun X. Y., Shi H. B., Bi H. X., Appl. Biochem. Biotechnol., 2012, 168(8), 2297—2308 |
| [17] | Ou Z. M., Zhao H. B., Tang L., Zhang W., Yang G. S., Bioproc. Biosyst. Eng., 2014, 37(11), 2243—2250 |
| [18] | Ren Z. Q., Liu Y., Pei X. Q., Wang H. B., Wu Z. L., J. Mol. Catal. B: Enzym., 2015, 113, 76—81 |
| [19] | Li B., Nie Y., Mu X. Q., Xu Y., J. Mol. Catal. B: Enzym., 2016, 129, 21—28 |
| [20] | Silva V. D., Stambuk B. U., Nascimento M. D., J. Mol. Catal. B: Enzym., 2012, 77, 98—104 |
| [21] | van der Donk W. A., Zhao H. M., Curr. Opin. Biotechnol., 2003, 14(4), 421—426 |
| [22] | Zhang J. L., Tao S. S., Zhang B. J., Wu X. R., Chen Y. J., ACS Catal., 2014, 4(5),1584—1587 |
| [1] | HE Beibei, YANG Kuihua, LYU Rui. Construction of Mn-Cu Bimetal Containing Phyllosilicate Nanozyme and Evaluation of the Enzyme-like Properties [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220150. |
| [2] | SHA Meng, XU Weiqing, WU Zhichao, GU Wenling, ZHU Chengzhou. Recent Advances in Single-atom Materials for Enzyme-like Catalysis and Biomedical Applications [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220077. |
| [3] | ZHU Haotian, JIN Meixiu, TANG Wensi, SU Fang, LI Yangguang. Properties of Transition Metal-biimidazole-Dawson-type Tungstophosphate Hybrid Compounds as Supports for Enzyme Immobilization [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220328. |
| [4] | SHU Xin, WANG Di, YUAN Chunling, QIN Xiu, WANG Yilin. Colorimetric Determination of Sarcosine with Carbon Quantum Dots as Mimetic Peroxidase [J]. Chem. J. Chinese Universities, 2021, 42(6): 1761. |
| [5] | LI Liu, SUN Shiyong, LYU Rui, GOLUBEV Yevgeny Aleksandrovich, WANG Ke, DONG Faqin, DUAN Tao, KOTOVA Olga Borisovna, KOTOVA Elena Leonidovna. Construction of Fe-aminoclay-glucose Oxidase Nanocomposite Catalyst and Its Multi-enzyme Cascade Analysis [J]. Chem. J. Chinese Universities, 2021, 42(3): 803. |
| [6] | LI Mei, XIA Xiaojuan, CHEN Zhixiong, YANG Meng, LI Ziying, YANG Tong, MENG Shuang, YANG Yunhui, HU Rong. Construction of a Label-free Electrochemical Ochratoxin Aptasensor Based on Pt Nanoparticles@ metal-organic Framework Nanomimetic Enzyme [J]. Chem. J. Chinese Universities, 2021, 42(12): 3615. |
| [7] | SHUAI Die, ZHAO Meijuan, CHEN Bingnian, WANG Li. Inhibitory Effect of Four Kinds of Keegin-type Phosphomolybdate on Tyrosinase and Melanin Formation and Its Antioxidant Activities [J]. Chem. J. Chinese Universities, 2021, 42(12): 3579. |
| [8] | KUANG Xiaojun, YI Jingwei, FANG Xiaoxia, LAI Dongmei, XU Hong. Preparation of Water-soluble Coumarin Fluorescent Substrate and Its Application in Droplet Based Digital Detection [J]. Chem. J. Chinese Universities, 2021, 42(11): 3537. |
| [9] | ZHANG Jiayi, DING Zhenyao, WANG Dandan, CHEN Liping, FENG Xinjian. Fabrication of Triphase Enzyme Electrode Based on Porous Gold Substrate for High-performance Electrochemical Biosensor [J]. Chem. J. Chinese Universities, 2021, 42(10): 3167. |
| [10] | WANG Huan, SUO Jinquan, WANG Chunyan, WANG Runwei. Glucose Oxidase Immobilization with Amino Dendritic Mesoporous Silica Nanoparticles and Its Application in Glucose Detection [J]. Chem. J. Chinese Universities, 2020, 41(8): 1731. |
| [11] | XIE Xingyu, ZHAO Yaxiang, ZHAO Lizhi, LI Rishun, WU Dihao, YE Hui, XIN Qingping, LI Hong, ZHANG Yuzhong. Colorimetric Detection Method for H2O2 Based on Two-dimensional Metal-organic Frameworks of Metalloporphyrin [J]. Chem. J. Chinese Universities, 2020, 41(8): 1776. |
| [12] | GAO Xia,PAN Huibin,QIAO Chengfang,CHEN Fengying,ZHOU Yuan,YANG Wenhua. Construction of HRP Immobilized Enzyme Reactor Based on Hierarchically Porous Metal-organic Framework and Its Dye Degradation Application† [J]. Chem. J. Chinese Universities, 2020, 41(7): 1591. |
| [13] | XIA Yun, Lü Wangyang, CHEN Wenxing, LI Nan. Fiber Supported Carbon Black Metal Phthalocyanine Axial Complex to Mimic Enzyme for Highly Efficient Catalytic Degradation of Organic Pollutant in Water† [J]. Chem. J. Chinese Universities, 2020, 41(7): 1582. |
| [14] | ZHU Ling,WANG Yuchen,ZHAO Jiangyuan,WEN Mengliang,LI Minggang,HAN Xiulin. Transformation of Ginsenoside Rb3 and C-Mx by Recombinant β-Xylosidase † [J]. Chem. J. Chinese Universities, 2020, 41(5): 1010. |
| [15] | TENG Yu,YANG Shaoming,BAI Chaopeng,ZHANG Jian. Preparation of HRP Molecularly Imprinted Electrochemical Sensor with Multi-walled Carbon Nanotubes as Sensitizing Materials and the Detection of H2O2 † [J]. Chem. J. Chinese Universities, 2020, 41(1): 78. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||