

Chem. J. Chinese Universities ›› 2017, Vol. 38 ›› Issue (6): 1059.doi: 10.7503/cjcu20160797
• Organic Chemistry • Previous Articles Next Articles
CHEN Dongxing1, SHI Jinyu2, CHEN Qiufang2, ZHANG Rui2, GONG Guoqing2, XU Yungen1,2,*( ), ZHU Qihua1,2,*(
), ZHU Qihua1,2,*( )
)
Received:2016-11-16
Online:2017-06-10
Published:2017-05-23
Contact:
XU Yungen,ZHU Qihua
E-mail:xyg@cpu.edu.cn;zhuqihua@cpu.edu.cn
Supported by:CLC Number:
TrendMD:
CHEN Dongxing, SHI Jinyu, CHEN Qiufang, ZHANG Rui, GONG Guoqing, XU Yungen, ZHU Qihua. Design, Synthesis and Biological Evaluation of Thrombin Inhibitors with 1,2,3,4-Tetrahydrobenzo[4,5]imidazo[1,2-a]pyrazine Nucleus†[J]. Chem. J. Chinese Universities, 2017, 38(6): 1059.
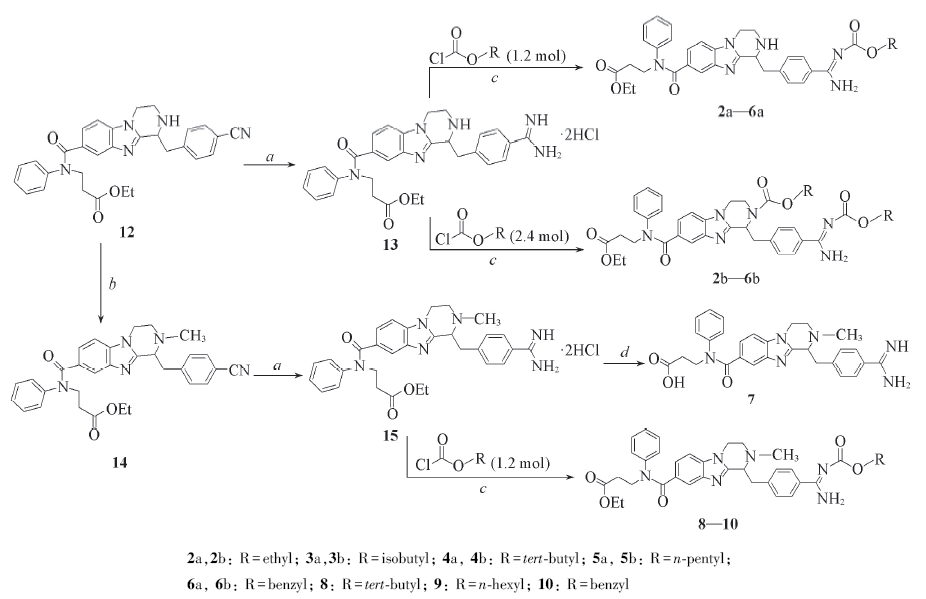
Scheme 3 Synthetic routes of target compounds 2a—6a, 2b—6b, 7—10Reagents and conditions: a. i: HCl, EtOH, -5—0 ℃, 6 h; ii: (NH4)2CO3, EtOH, 25 ℃, 12 h, 38.7%; b. CH3I, K2CO3, DMF, r. t., 88.6%; c. K2CO3, THF/H2O, 0—25 ℃, 1 h, 40.3%—66.0%; d. i: LiOH, EtOH, H2O, 2 h; ii: CH3CO2H, pH=6.0, 29.9%.
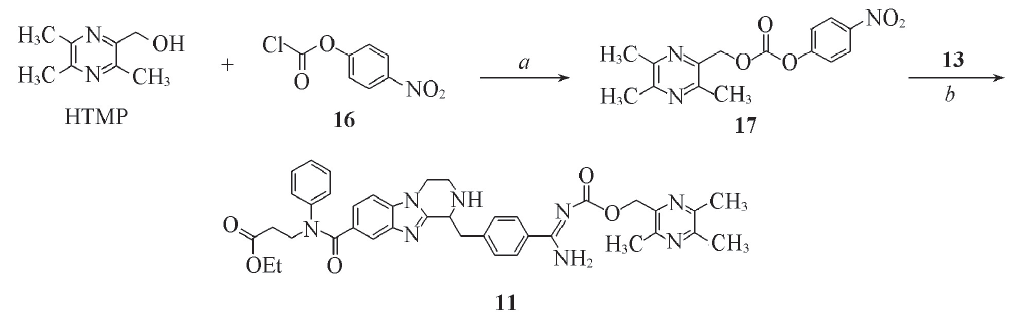
Scheme 4 Synthetic routes of target compound 11Reagents and conditions: a. Pyridine, CH2Cl2, 0 ℃ to r. t., 12 h, 82.1%; b. K2CO3, THF/H2O, 0 ℃ to r. t., 12 h, 35.7%.
| Compd. | Appearance | Yield(%) | m. p./℃ | HRMS(calcd. ), m/z[M+H]+ |
|---|---|---|---|---|
| 2a | White solid | 44.2 | 114—116 | 597.2823(597.2820) |
| 2b | White solid | 48.3 | 106—108 | 669.3044(669.3031) |
| 3a | White solid | 40.3 | 108—110 | 625.3148(625.3133) |
| 3b | White solid | 44.6 | 94—96 | 725.3673(725.3657) |
| 4a | White solid | 48.0 | 160—162 | 625.3141(625.3133) |
| 4b | White solid | 66.0 | 148—150 | 725.3662(725.3657) |
| 5a | White solid | 42.6 | 108—110 | 639.3287(639.3289) |
| 5b | White solid | 47.4 | 88—90 | 753.3978(753.3970) |
| 6a | White solid | 43.7 | 130—132 | 659.2979(659.2976) |
| 6b | White solid | 54.3 | 120—122 | 793.3354(793.3344) |
| 7 | White solid | 29.9 | > 250 | 511.2453(511.2452) |
| 8 | White solid | 57.4 | 112—114 | 639.3295(639.3289) |
| 9 | White solid | 59.6 | 82—84 | 667.3603(667.3602) |
| 10 | White solid | 54.5 | 94—96 | 673.3145(673.3133) |
| 11 | Yellow solid | 35.7 | 104—106 | 703.3356(703.3351) |
Table 1 Appearance, yields, melting points and HRMS data of target compounds 2a—6a, 2b—6b and 7—11
| Compd. | Appearance | Yield(%) | m. p./℃ | HRMS(calcd. ), m/z[M+H]+ |
|---|---|---|---|---|
| 2a | White solid | 44.2 | 114—116 | 597.2823(597.2820) |
| 2b | White solid | 48.3 | 106—108 | 669.3044(669.3031) |
| 3a | White solid | 40.3 | 108—110 | 625.3148(625.3133) |
| 3b | White solid | 44.6 | 94—96 | 725.3673(725.3657) |
| 4a | White solid | 48.0 | 160—162 | 625.3141(625.3133) |
| 4b | White solid | 66.0 | 148—150 | 725.3662(725.3657) |
| 5a | White solid | 42.6 | 108—110 | 639.3287(639.3289) |
| 5b | White solid | 47.4 | 88—90 | 753.3978(753.3970) |
| 6a | White solid | 43.7 | 130—132 | 659.2979(659.2976) |
| 6b | White solid | 54.3 | 120—122 | 793.3354(793.3344) |
| 7 | White solid | 29.9 | > 250 | 511.2453(511.2452) |
| 8 | White solid | 57.4 | 112—114 | 639.3295(639.3289) |
| 9 | White solid | 59.6 | 82—84 | 667.3603(667.3602) |
| 10 | White solid | 54.5 | 94—96 | 673.3145(673.3133) |
| 11 | Yellow solid | 35.7 | 104—106 | 703.3356(703.3351) |
| Compd. | 1H NMR(300 MHz), δa | 13C NMR(75 MHz), δb |
|---|---|---|
| 2a | 9.14(brs, 2H), 7.87(d, J=8.3 Hz, 2H), 7.43(s, 1H), 7.38(d, J=8.3 Hz, 2H), 7.32—7.21(m, 2H), 7.21—7.06(m, 5H), 4.34—4.24(m, 1H), 4.15—3.99(m, 5H), 3.96(q, J=7.2 Hz, 2H), 3.91—3.81(m, 1H), 3.57—3.45(m, 1H), 3.29—3.20(m, 1H), 3.07—2.84(m, 2H), 2.58(t, J=7.1 Hz, 2H), 1.18(t, J=6.9 Hz, 3H), 1.11(t, J=7.1 Hz, 3H) | 171.08, 170.63, 167.19, 163.87, 152.30, 142.83, 141.49, 140.86, 134.73, 132.51, 129.67, 129.05(2C), 128.78(2C), 127.33(2C), 127.28(2C), 126.19, 122.90, 119.95, 107.88, 60.78, 60.07, 55.29, 46.34, 42.50, 40.98, 38.95, 32.10, 13.93, 13.61 |
| 2b | 9.59(brs, 2H), 7.85—7.59(m, 3H), 7.26—7.02(m, 9H), 5.89—5.63(m, 1H), 4.65—4.44(m, 1H), 4.41—3.80(m, 11H), 3.62—3.27(m, 2H), 2.76(t, J=7.2 Hz, 2H), 1.36(t, J=6.8 Hz, 3H), 1.32—1.18(m, 6H) | 171.12, 170.38, 166.89, 164.02, 154.40, 150.11, 142.91, 141.21, 141.00, 134.20, 132.49, 130.10, 129.33(2C), 128.79(2C), 127.36(2C), 126.94(2C), 126.21, 123.18, 120.30, 107.95, 61.68, 60.89, 60.08, 53.49, 46.34, 41.36, 38.73, 36.87, 32.12, 13.91(2C), 13.62 |
| 3a | 9.07(brs, 2H), 7.89(d, J=8.0 Hz, 2H), 7.46(s, 1H), 7.40(d, J=8.2 Hz, 2H), 7.34—7.21(m, 3H), 7.21—7.18(m, 2H), 7.18—7.15(m, 1H), 7.15—7.10(m, 1H), 4.38—4.19(m, 1H), 4.11(t, J=7.0 Hz, 2H), 4.07—4.03(m, 1H), 3.99(q, J=7.1 Hz, 2H), 3.94—3.85(m, 1H), 3.81(d, J=6.6 Hz, 2H), 3.60—3.44(m, 1H), 3.31—3.20(m, 1H), 3.08—2.87(m, 2H), 2.61(t, J=7.1 Hz, 2H), 1.99—1.81(m, 1H), 1.14(t, J=7.1 Hz, 3H), 0.91(d, J=6.7 Hz, 6H) | 171.12, 170.64, 167.26, 164.34, 152.41, 142.93, 141.48, 140.98, 134.80, 132.90, 129.69, 129.11(2C), 128.78(2C), 127.35(2C), 127.23(2C), 126.16, 122.92, 120.06, 107.83, 71.25, 60.07, 55.42, 46.36, 42.65, 41.11, 39.04, 32.13, 27.33, 18.77(2C), 13.62 |
| 3b | 9.07(brs, 2H), 7.88(s, 2H), 7.51(s, 1H), 7.35(d, J=8.6 Hz, 1H), 7.31—7.27(m, 1H), 7.27—7.21(m, 3H), 7.21—7.18(m, 2H), 7.18—7.04(m, 2H), 5.69—5.38(m, 1H), 4.45—4.27(m, 1H), 4.24—4.15(m, 1H), 4.11(t, J=7.2 Hz, 2H), 3.99(q, J=7.1 Hz, 3H), 3.81(d, J=6.6 Hz, 2H), 3.76—3.66(m, 1H), 3.60—3.51(m, 1H), 3.49—3.39(m, 1H), 3.33—3.24(m, 2H), 2.61(t, J=7.1 Hz, 2H), 1.97—1.81(m, 1H), 1.63—1.45(m, 1H), 1.14(t, J=7.1 Hz, 3H), 0.91(d, J=6.6 Hz, 6H), 0.83(s, 3H), 0.68(s, 3H) | 171.10, 170.36, 166.91, 164.15, 154.56, 150.06, 142.91, 141.22, 140.77, 134.20, 132.77, 130.10, 129.32(2C), 128.79(2C), 127.36(2C), 126.98(2C), 126.21, 123.16, 120.30, 107.96, 71.85, 71.30, 60.06, 53.58, 46.33, 41.31, 39.21, 37.24, 32.11, 27.30(2C), 18.75(2C), 18.50(2C), 13.62 |
| 4a | 9.03(brs, 2H), 7.83(d, J=8.2 Hz, 2H), 7.43(s, 1H), 7.36(d, J=8.2 Hz, 2H), 7.31—7.19(m, 3H), 7.18—7.15(m, 2H), 7.15—7.08(m, 2H), 4.32—4.20(m, 1H), 4.08(t, J=7.1 Hz, 2H), 4.04—4.00(m, 1H), 3.96(q, J=7.1 Hz, 2H), 3.91—3.81(m, 1H), 3.56—3.45(m, 1H), 3.28—3.18(m, 1H), 3.04—2.86(m, 2H), 2.58(t, J=7.1 Hz, 2H), 1.42(s, 9H), 1.11(t, J=7.1 Hz, 3H) | 171.20, 170.63, 166.66, 162.89, 152.33, 142.95, 141.35, 140.93, 134.78, 132.98, 129.68, 129.16(2C), 128.80(2C), 127.36(2C), 127.19(2C), 126.16, 123.00, 120.11, 107.85, 79.37, 60.10, 55.42, 46.37, 42.63, 41.12, 39.01, 32.09, 27.69(3C), 13.64 |
| 4b | 9.05(brs, 2H), 7.94(d,J=5.6 Hz, 2H), 7.50(s, 1H), 7.39—7.10(m, 9H), 5.60—5.43(m, 1H), 4.44—4.28(m, 1H), 4.26—4.15(m, 1H), 4.11(t, J=6.7 Hz, 2H), 3.99(q, J=7.1 Hz, 2H), 3.94—3.83(m, 1H), 3.57—3.39(m, 1H), 3.30—3.20(m, 2H), 2.61(t, J=7.2 Hz, 2H), 1.45(s, 9H), 1.34—1.19(m, 3H), 1.14(t, J=7.1 Hz, 3H), 1.12—0.99(m, 6H) | 171.13, 170.40, 166.19, 162.93, 153.23, 150.49, 142.94, 141.26, 140.97, 134.26, 132.62, 130.03, 129.38(2C), 128.81(2C), 127.37(2C), 126.96(2C), 126.21, 123.15, 120.26, 107.98, 80.74, 79.50, 60.07, 53.66, 46.35, 41.43, 39.11, 36.29, 32.12, 27.69(6C), 13.63 |
| 5a | 9.18(brs, 2H), 7.89(d, J=8.3 Hz, 2H), 7.46(s, 1H), 7.40(d, J=8.3 Hz, 2H), 7.35—7.21(m, 3H), 7.21—7.15(m, 3H), 7.15—7.10(m, 1H), 4.32—4.21(m, 1H), 4.10(t, J=6.9 Hz, 2H), 4.07—3.94(m, 5H), 3.94—3.82(m, 1H), 3.59—3.47(m, 1H), 3.31—3.20(m, 1H), 3.06—2.88(m, 2H), 2.61(t, J=6.9 Hz, 2H), 1.67—1.54(m, 2H), 1.37—1.27(m, 4H), 1.14(t, J=7.1 Hz, 3H), 0.88(t, J=7.0 Hz, 3H) | 171.18, 170.65, 167.21, 164.06, 152.19, 142.85, 141.48, 140.88, 134.74, 132.63, 129.69, 129.18(2C), 128.81(2C), 127.35(2C), 127.28(2C), 126.21, 122.99, 120.05, 107.90, 65.19, 60.13, 55.38, 46.36, 42.49, 41.09, 38.96, 32.07, 28.03, 27.57, 21.91, 13.64, 13.50 |
| 5b | 9.13(brs, 2H), 7.90(s, 2H), 7.50(s, 1H), 7.39—7.28(m, 2H), 7.28—7.22(m, 3H), 7.22—7.07(m, 4H), 5.65—5.41(m, 1H), 4.46—4.29(m, 1H), 4.28—4.16(m, 1H), 4.11(t, J=6.7 Hz, 2H), 4.05—3.86(m, 5H), 3.76—3.61(m, 1H), 3.61—3.41(m, 2H), | 171.16, 170.39, 166.71, 164.36, 154.57, 150.08, 142.97, 141.28, 140.90, 134.24, 132.77, 130.15, 129.37(2C), 128.82(2C), 127.39(2C), 126.93(2C), 126.22, 123.22, 120.37, 107.95, 65.94, |
| Compd. | 1H NMR(300 MHz), δa | 13C NMR(75 MHz), δb |
| 5b | 3.32—3.22(m, 2H), 2.61(t, J=7.0 Hz, 2H), 1.68—1.52(m, 2H), 1.39—1.17(m, 8H), 1.14(t, J=7.1 Hz, 3H), 1.11—0.95(m, 2H), 0.95—0.72(m, 6H) | 65.18, 60.10, 53.61, 46.37, 41.37, 39.24, 37.12, 32.16, 28.05, 27.97, 27.60, 27.49, 21.91, 21.79, 13.66, 13.48, 13.45 |
| 6a | 9.06(brs, 2H), 7.90(d,J=7.9 Hz, 2H), 7.46(s, 1H), 7.44—7.36(m, 5H), 7.36—7.32(m, 1H), 7.33—7.27(m, 2H), 7.27—7.21(m, 2H), 7.21—7.10(m, 4H), 5.10(s, 2H), 4.33—4.22(m, 1H), 4.10(t, J=7.0 Hz, 2H), 4.06—4.03(m, 1H), 3.99(q, J=7.0 Hz, 2H), 3.94—3.80(m, 1H), 3.59—3.47(m, 1H), 3.31—3.20(m, 1H), 3.09—2.82(m, 2H), 2.61(t, J=7.0 Hz, 2H), 1.14(t, J=7.1 Hz, 3H) | 171.13, 170.64, 167.43, 163.97, 152.34, 142.95, 141.67, 140.96, 136.18, 134.78, 132.67, 129.74, 129.17(2C), 128.79(2C), 127.88(2C), 127.67(2C), 127.42, 127.36(2C), 127.23(2C), 126.16, 122.96, 120.08, 107.84, 66.66, 60.08, 55.38, 46.38, 42.61, 41.08, 39.05, 32.13, 13.64 |
| 6b | 9.15(brs, 2H), 7.87(d, J=8.3 Hz, 2H), 7.50(s, 1H), 7.44—6.97(m, 19H), 5.69—5.47(m, 1H), 5.12(s, 2H), 4.97(s, 2H), 4.70—4.56(m, 1H), 4.42—4.24(m, 1H), 4.23—4.15(m, 1H), 4.11(t, J=6.8 Hz, 2H), 3.99(q, J=7.1 Hz, 2H), 3.68—3.37(m, 2H), 3.29—3.11(m, 1H), 2.61(t, J=7.2 Hz, 2H), 1.14(t, J=7.1 Hz, 3H) | 171.14, 170.36, 166.19, 162.93, 153.23, 149.93, 142.92, 141.22, 140.83, 136.15, 135.79, 134.18, 132.52, 130.16, 129.30(2C), 128.80(2C), 128.03(2C), 127.89(2C), 127.69(2C), 127.63(2C), 127.59, 127.44, 127.37(2C), 126.96(2C), 126.22, 123.22, 120.33, 107.97, 66.70, 66.37, 60.09, 53.73, 46.35, 41.33, 39.19, 37.16, 32.14, 13.64 |
| 7 | 9.40(s, 2H), 9.20(s, 2H), 7.81(d,J=7.7 Hz, 2H), 7.62(d, J=7.9 Hz, 2H), 7.58—7.43(m, 2H), 7.32—7.24(m, 2H), 7.24—7.19(m, 2H), 7.19—7.15(m, 1H), 7.15—7.10(m, 1H), 5.11(brs, 1H), 4.42(brs, 2H), 4.10—3.99(m, 2H), 3.66(brs, 2H), 3.49(brs, 2H), 2.77(s, 3H), 2.60—2.52(m, 2H) | 172.53, 169.36, 165.24, 148.40, 143.25, 143.03, 140.55, 133.80, 131.25, 129.87(2C), 129.23(2C), 128.10(2C), 127.99(2C), 126.74, 126.22, 123.82, 118.56, 110.43, 60.70, 49.68, 46.27, 38.45, 37.94, 37.90, 32.10 |
| 8 | 9.05(brs, 2H), 7.70(d, J=8.2 Hz, 2H), 7.47(s, 1H), 7.33—7.14(m, 9H), 4.16—4.12(m, 1H), 4.10(t, J=7.1 Hz, 2H), 4.06—4.03(m, 1H), 3.99(q, J=7.1 Hz, 2H), 3.92—3.77(m, 1H), 3.39—3.37(m, 1H),3.28—3.15(m, 2H), 2.92—2.77(m, 1H), 2.61(t, J=6.9 Hz, 2H), 2.40(s, 3H), 1.44(d, J=4.8 Hz, 9H), 1.14(t, J=7.1 Hz, 3H) | 171.18, 170.63, 166.73, 160.39, 152.10, 144.01, 142.89, 141.10, 134.40, 131.26, 129.62, 129.39(2C), 128.79(2C), 127.36(2C), 126.47(2C), 126.19, 122.80, 119.94, 107.84, 79.78, 62.11, 60.09, 48.88, 46.35, 41.98, 39.94, 36.84, 32.09, 27.64(3C), 13.64 |
| 9 | 8.94(brs, 2H), 7.74(d, J=8.3 Hz, 2H), 7.47(s, 1H), 7.37—7.00(m, 9H), 4.17—4.05(m, 3H), 4.06—3.92(m, 5H), 3.91—3.75(m, 1H), 3.55(d, J=27.6 Hz, 1H), 3.27—3.13(m, 2H), 2.94—2.75(m, 1H), 2.61(t, J=6.8 Hz, 2H), 2.40(s, 3H),1.68—1.49(m, 2H), 1.36—1.25(m, 6H), 1.14(t, J=7.1 Hz, 3H), 0.86(t, J=6.6 Hz, 3H) | 171.18, 170.58, 167.28, 161.05, 152.07, 142.97, 142.29, 141.20, 134.47, 131.79, 129.62, 129.38(2C), 128.78(2C), 127.35(2C), 126.43(2C), 126.13, 122.81, 120.06, 107.80, 65.14, 62.15, 60.08, 48.88, 46.35, 41.95, 39.84, 36.91, 32.09, 31.04, 28.30, 25.13, 22.07, 13.65, 13.55 |
| 10 | 9.15(brs, 2H), 7.89(d, J=8.2 Hz, 2H), 7.53—7.35(m, 4H), 7.35—7.28(m, 1H), 7.29—7.22(m, 3H), 7.22—7.17(m, 3H), 7.17—7.14(m, 2H), 7.15—7.05(m, 2H), 5.13(s, 2H), 4.34—4.23(m, 1H), 4.12(t, J=6.7 Hz, 2H), 4.08—4.04(m, 1H), 4.01(t, J=6.7 Hz, 2H), 3.94—3.85(m, 1H), 3.57—3.49(m, 2H), 3.06—2.90(m, 2H), 2.69(t, J=7.2 Hz, 2H), 2.42(s, 3H), 0.87(t, J=6.5 Hz, 3H) | 171.18, 170.81, 167.63, 164.43, 152.41, 142.94, 141.60, 141.19, 137.74, 134.89, 132.95, 129.62, 129.43(2C), 128.83(2C), 127.87(2C), 127.67(2C), 127.37(2C), 127.23, 126.46(2C), 126.19, 123.10, 120.06, 107.88, 64.69, 61.82, 60.11, 48.82, 46.30, 41.93, 40.76, 37.08, 31.05, 13.56 |
| 11 | 9.55(brs, 2H), 7.81(d,J=7.9 Hz, 2H), 7.72(s, 1H), 7.34(d, J=8.4 Hz, 2H), 7.31(d, J=1.3 Hz, 1H), 7.25—7.18(m, 2H), 7.16—7.05(m, 4H), 5.30(s, 2H), 4.47—4.35(m, 1H), 4.34—4.22(m, 2H), 4.09(q, J=7.3 Hz, 2H), 4.05—3.96(m, 2H), 3.80—3.71(m, 1H), 3.47—3.37(m, 1H), 3.22—3.05(m, 2H), 2.80—2.71(m, 2H), 2.60(s, 3H), 2.51(s, 3H), 2.49(s, 3H), 1.23(t, J=7.1 Hz, 3H) | 171.09, 170.65, 167.46, 163.62, 152.37, 150.41, 148.61, 148.19, 144.86, 142.75, 141.48, 140.82, 134.72, 132.45, 129.59, 128.96(2C), 128.79(2C), 127.36(2C), 127.32(2C), 126.22, 122.87, 119.89, 107.92, 65.43, 60.09, 55.30, 46.32, 42.49, 40.99, 38.93, 32.06, 21.09, 20.81, 20.08, 13.61 |
Table 2 1H NMR and 13C NMR data of target compounds 2a—6a, 2b—6b and 7—11
| Compd. | 1H NMR(300 MHz), δa | 13C NMR(75 MHz), δb |
|---|---|---|
| 2a | 9.14(brs, 2H), 7.87(d, J=8.3 Hz, 2H), 7.43(s, 1H), 7.38(d, J=8.3 Hz, 2H), 7.32—7.21(m, 2H), 7.21—7.06(m, 5H), 4.34—4.24(m, 1H), 4.15—3.99(m, 5H), 3.96(q, J=7.2 Hz, 2H), 3.91—3.81(m, 1H), 3.57—3.45(m, 1H), 3.29—3.20(m, 1H), 3.07—2.84(m, 2H), 2.58(t, J=7.1 Hz, 2H), 1.18(t, J=6.9 Hz, 3H), 1.11(t, J=7.1 Hz, 3H) | 171.08, 170.63, 167.19, 163.87, 152.30, 142.83, 141.49, 140.86, 134.73, 132.51, 129.67, 129.05(2C), 128.78(2C), 127.33(2C), 127.28(2C), 126.19, 122.90, 119.95, 107.88, 60.78, 60.07, 55.29, 46.34, 42.50, 40.98, 38.95, 32.10, 13.93, 13.61 |
| 2b | 9.59(brs, 2H), 7.85—7.59(m, 3H), 7.26—7.02(m, 9H), 5.89—5.63(m, 1H), 4.65—4.44(m, 1H), 4.41—3.80(m, 11H), 3.62—3.27(m, 2H), 2.76(t, J=7.2 Hz, 2H), 1.36(t, J=6.8 Hz, 3H), 1.32—1.18(m, 6H) | 171.12, 170.38, 166.89, 164.02, 154.40, 150.11, 142.91, 141.21, 141.00, 134.20, 132.49, 130.10, 129.33(2C), 128.79(2C), 127.36(2C), 126.94(2C), 126.21, 123.18, 120.30, 107.95, 61.68, 60.89, 60.08, 53.49, 46.34, 41.36, 38.73, 36.87, 32.12, 13.91(2C), 13.62 |
| 3a | 9.07(brs, 2H), 7.89(d, J=8.0 Hz, 2H), 7.46(s, 1H), 7.40(d, J=8.2 Hz, 2H), 7.34—7.21(m, 3H), 7.21—7.18(m, 2H), 7.18—7.15(m, 1H), 7.15—7.10(m, 1H), 4.38—4.19(m, 1H), 4.11(t, J=7.0 Hz, 2H), 4.07—4.03(m, 1H), 3.99(q, J=7.1 Hz, 2H), 3.94—3.85(m, 1H), 3.81(d, J=6.6 Hz, 2H), 3.60—3.44(m, 1H), 3.31—3.20(m, 1H), 3.08—2.87(m, 2H), 2.61(t, J=7.1 Hz, 2H), 1.99—1.81(m, 1H), 1.14(t, J=7.1 Hz, 3H), 0.91(d, J=6.7 Hz, 6H) | 171.12, 170.64, 167.26, 164.34, 152.41, 142.93, 141.48, 140.98, 134.80, 132.90, 129.69, 129.11(2C), 128.78(2C), 127.35(2C), 127.23(2C), 126.16, 122.92, 120.06, 107.83, 71.25, 60.07, 55.42, 46.36, 42.65, 41.11, 39.04, 32.13, 27.33, 18.77(2C), 13.62 |
| 3b | 9.07(brs, 2H), 7.88(s, 2H), 7.51(s, 1H), 7.35(d, J=8.6 Hz, 1H), 7.31—7.27(m, 1H), 7.27—7.21(m, 3H), 7.21—7.18(m, 2H), 7.18—7.04(m, 2H), 5.69—5.38(m, 1H), 4.45—4.27(m, 1H), 4.24—4.15(m, 1H), 4.11(t, J=7.2 Hz, 2H), 3.99(q, J=7.1 Hz, 3H), 3.81(d, J=6.6 Hz, 2H), 3.76—3.66(m, 1H), 3.60—3.51(m, 1H), 3.49—3.39(m, 1H), 3.33—3.24(m, 2H), 2.61(t, J=7.1 Hz, 2H), 1.97—1.81(m, 1H), 1.63—1.45(m, 1H), 1.14(t, J=7.1 Hz, 3H), 0.91(d, J=6.6 Hz, 6H), 0.83(s, 3H), 0.68(s, 3H) | 171.10, 170.36, 166.91, 164.15, 154.56, 150.06, 142.91, 141.22, 140.77, 134.20, 132.77, 130.10, 129.32(2C), 128.79(2C), 127.36(2C), 126.98(2C), 126.21, 123.16, 120.30, 107.96, 71.85, 71.30, 60.06, 53.58, 46.33, 41.31, 39.21, 37.24, 32.11, 27.30(2C), 18.75(2C), 18.50(2C), 13.62 |
| 4a | 9.03(brs, 2H), 7.83(d, J=8.2 Hz, 2H), 7.43(s, 1H), 7.36(d, J=8.2 Hz, 2H), 7.31—7.19(m, 3H), 7.18—7.15(m, 2H), 7.15—7.08(m, 2H), 4.32—4.20(m, 1H), 4.08(t, J=7.1 Hz, 2H), 4.04—4.00(m, 1H), 3.96(q, J=7.1 Hz, 2H), 3.91—3.81(m, 1H), 3.56—3.45(m, 1H), 3.28—3.18(m, 1H), 3.04—2.86(m, 2H), 2.58(t, J=7.1 Hz, 2H), 1.42(s, 9H), 1.11(t, J=7.1 Hz, 3H) | 171.20, 170.63, 166.66, 162.89, 152.33, 142.95, 141.35, 140.93, 134.78, 132.98, 129.68, 129.16(2C), 128.80(2C), 127.36(2C), 127.19(2C), 126.16, 123.00, 120.11, 107.85, 79.37, 60.10, 55.42, 46.37, 42.63, 41.12, 39.01, 32.09, 27.69(3C), 13.64 |
| 4b | 9.05(brs, 2H), 7.94(d,J=5.6 Hz, 2H), 7.50(s, 1H), 7.39—7.10(m, 9H), 5.60—5.43(m, 1H), 4.44—4.28(m, 1H), 4.26—4.15(m, 1H), 4.11(t, J=6.7 Hz, 2H), 3.99(q, J=7.1 Hz, 2H), 3.94—3.83(m, 1H), 3.57—3.39(m, 1H), 3.30—3.20(m, 2H), 2.61(t, J=7.2 Hz, 2H), 1.45(s, 9H), 1.34—1.19(m, 3H), 1.14(t, J=7.1 Hz, 3H), 1.12—0.99(m, 6H) | 171.13, 170.40, 166.19, 162.93, 153.23, 150.49, 142.94, 141.26, 140.97, 134.26, 132.62, 130.03, 129.38(2C), 128.81(2C), 127.37(2C), 126.96(2C), 126.21, 123.15, 120.26, 107.98, 80.74, 79.50, 60.07, 53.66, 46.35, 41.43, 39.11, 36.29, 32.12, 27.69(6C), 13.63 |
| 5a | 9.18(brs, 2H), 7.89(d, J=8.3 Hz, 2H), 7.46(s, 1H), 7.40(d, J=8.3 Hz, 2H), 7.35—7.21(m, 3H), 7.21—7.15(m, 3H), 7.15—7.10(m, 1H), 4.32—4.21(m, 1H), 4.10(t, J=6.9 Hz, 2H), 4.07—3.94(m, 5H), 3.94—3.82(m, 1H), 3.59—3.47(m, 1H), 3.31—3.20(m, 1H), 3.06—2.88(m, 2H), 2.61(t, J=6.9 Hz, 2H), 1.67—1.54(m, 2H), 1.37—1.27(m, 4H), 1.14(t, J=7.1 Hz, 3H), 0.88(t, J=7.0 Hz, 3H) | 171.18, 170.65, 167.21, 164.06, 152.19, 142.85, 141.48, 140.88, 134.74, 132.63, 129.69, 129.18(2C), 128.81(2C), 127.35(2C), 127.28(2C), 126.21, 122.99, 120.05, 107.90, 65.19, 60.13, 55.38, 46.36, 42.49, 41.09, 38.96, 32.07, 28.03, 27.57, 21.91, 13.64, 13.50 |
| 5b | 9.13(brs, 2H), 7.90(s, 2H), 7.50(s, 1H), 7.39—7.28(m, 2H), 7.28—7.22(m, 3H), 7.22—7.07(m, 4H), 5.65—5.41(m, 1H), 4.46—4.29(m, 1H), 4.28—4.16(m, 1H), 4.11(t, J=6.7 Hz, 2H), 4.05—3.86(m, 5H), 3.76—3.61(m, 1H), 3.61—3.41(m, 2H), | 171.16, 170.39, 166.71, 164.36, 154.57, 150.08, 142.97, 141.28, 140.90, 134.24, 132.77, 130.15, 129.37(2C), 128.82(2C), 127.39(2C), 126.93(2C), 126.22, 123.22, 120.37, 107.95, 65.94, |
| Compd. | 1H NMR(300 MHz), δa | 13C NMR(75 MHz), δb |
| 5b | 3.32—3.22(m, 2H), 2.61(t, J=7.0 Hz, 2H), 1.68—1.52(m, 2H), 1.39—1.17(m, 8H), 1.14(t, J=7.1 Hz, 3H), 1.11—0.95(m, 2H), 0.95—0.72(m, 6H) | 65.18, 60.10, 53.61, 46.37, 41.37, 39.24, 37.12, 32.16, 28.05, 27.97, 27.60, 27.49, 21.91, 21.79, 13.66, 13.48, 13.45 |
| 6a | 9.06(brs, 2H), 7.90(d,J=7.9 Hz, 2H), 7.46(s, 1H), 7.44—7.36(m, 5H), 7.36—7.32(m, 1H), 7.33—7.27(m, 2H), 7.27—7.21(m, 2H), 7.21—7.10(m, 4H), 5.10(s, 2H), 4.33—4.22(m, 1H), 4.10(t, J=7.0 Hz, 2H), 4.06—4.03(m, 1H), 3.99(q, J=7.0 Hz, 2H), 3.94—3.80(m, 1H), 3.59—3.47(m, 1H), 3.31—3.20(m, 1H), 3.09—2.82(m, 2H), 2.61(t, J=7.0 Hz, 2H), 1.14(t, J=7.1 Hz, 3H) | 171.13, 170.64, 167.43, 163.97, 152.34, 142.95, 141.67, 140.96, 136.18, 134.78, 132.67, 129.74, 129.17(2C), 128.79(2C), 127.88(2C), 127.67(2C), 127.42, 127.36(2C), 127.23(2C), 126.16, 122.96, 120.08, 107.84, 66.66, 60.08, 55.38, 46.38, 42.61, 41.08, 39.05, 32.13, 13.64 |
| 6b | 9.15(brs, 2H), 7.87(d, J=8.3 Hz, 2H), 7.50(s, 1H), 7.44—6.97(m, 19H), 5.69—5.47(m, 1H), 5.12(s, 2H), 4.97(s, 2H), 4.70—4.56(m, 1H), 4.42—4.24(m, 1H), 4.23—4.15(m, 1H), 4.11(t, J=6.8 Hz, 2H), 3.99(q, J=7.1 Hz, 2H), 3.68—3.37(m, 2H), 3.29—3.11(m, 1H), 2.61(t, J=7.2 Hz, 2H), 1.14(t, J=7.1 Hz, 3H) | 171.14, 170.36, 166.19, 162.93, 153.23, 149.93, 142.92, 141.22, 140.83, 136.15, 135.79, 134.18, 132.52, 130.16, 129.30(2C), 128.80(2C), 128.03(2C), 127.89(2C), 127.69(2C), 127.63(2C), 127.59, 127.44, 127.37(2C), 126.96(2C), 126.22, 123.22, 120.33, 107.97, 66.70, 66.37, 60.09, 53.73, 46.35, 41.33, 39.19, 37.16, 32.14, 13.64 |
| 7 | 9.40(s, 2H), 9.20(s, 2H), 7.81(d,J=7.7 Hz, 2H), 7.62(d, J=7.9 Hz, 2H), 7.58—7.43(m, 2H), 7.32—7.24(m, 2H), 7.24—7.19(m, 2H), 7.19—7.15(m, 1H), 7.15—7.10(m, 1H), 5.11(brs, 1H), 4.42(brs, 2H), 4.10—3.99(m, 2H), 3.66(brs, 2H), 3.49(brs, 2H), 2.77(s, 3H), 2.60—2.52(m, 2H) | 172.53, 169.36, 165.24, 148.40, 143.25, 143.03, 140.55, 133.80, 131.25, 129.87(2C), 129.23(2C), 128.10(2C), 127.99(2C), 126.74, 126.22, 123.82, 118.56, 110.43, 60.70, 49.68, 46.27, 38.45, 37.94, 37.90, 32.10 |
| 8 | 9.05(brs, 2H), 7.70(d, J=8.2 Hz, 2H), 7.47(s, 1H), 7.33—7.14(m, 9H), 4.16—4.12(m, 1H), 4.10(t, J=7.1 Hz, 2H), 4.06—4.03(m, 1H), 3.99(q, J=7.1 Hz, 2H), 3.92—3.77(m, 1H), 3.39—3.37(m, 1H),3.28—3.15(m, 2H), 2.92—2.77(m, 1H), 2.61(t, J=6.9 Hz, 2H), 2.40(s, 3H), 1.44(d, J=4.8 Hz, 9H), 1.14(t, J=7.1 Hz, 3H) | 171.18, 170.63, 166.73, 160.39, 152.10, 144.01, 142.89, 141.10, 134.40, 131.26, 129.62, 129.39(2C), 128.79(2C), 127.36(2C), 126.47(2C), 126.19, 122.80, 119.94, 107.84, 79.78, 62.11, 60.09, 48.88, 46.35, 41.98, 39.94, 36.84, 32.09, 27.64(3C), 13.64 |
| 9 | 8.94(brs, 2H), 7.74(d, J=8.3 Hz, 2H), 7.47(s, 1H), 7.37—7.00(m, 9H), 4.17—4.05(m, 3H), 4.06—3.92(m, 5H), 3.91—3.75(m, 1H), 3.55(d, J=27.6 Hz, 1H), 3.27—3.13(m, 2H), 2.94—2.75(m, 1H), 2.61(t, J=6.8 Hz, 2H), 2.40(s, 3H),1.68—1.49(m, 2H), 1.36—1.25(m, 6H), 1.14(t, J=7.1 Hz, 3H), 0.86(t, J=6.6 Hz, 3H) | 171.18, 170.58, 167.28, 161.05, 152.07, 142.97, 142.29, 141.20, 134.47, 131.79, 129.62, 129.38(2C), 128.78(2C), 127.35(2C), 126.43(2C), 126.13, 122.81, 120.06, 107.80, 65.14, 62.15, 60.08, 48.88, 46.35, 41.95, 39.84, 36.91, 32.09, 31.04, 28.30, 25.13, 22.07, 13.65, 13.55 |
| 10 | 9.15(brs, 2H), 7.89(d, J=8.2 Hz, 2H), 7.53—7.35(m, 4H), 7.35—7.28(m, 1H), 7.29—7.22(m, 3H), 7.22—7.17(m, 3H), 7.17—7.14(m, 2H), 7.15—7.05(m, 2H), 5.13(s, 2H), 4.34—4.23(m, 1H), 4.12(t, J=6.7 Hz, 2H), 4.08—4.04(m, 1H), 4.01(t, J=6.7 Hz, 2H), 3.94—3.85(m, 1H), 3.57—3.49(m, 2H), 3.06—2.90(m, 2H), 2.69(t, J=7.2 Hz, 2H), 2.42(s, 3H), 0.87(t, J=6.5 Hz, 3H) | 171.18, 170.81, 167.63, 164.43, 152.41, 142.94, 141.60, 141.19, 137.74, 134.89, 132.95, 129.62, 129.43(2C), 128.83(2C), 127.87(2C), 127.67(2C), 127.37(2C), 127.23, 126.46(2C), 126.19, 123.10, 120.06, 107.88, 64.69, 61.82, 60.11, 48.82, 46.30, 41.93, 40.76, 37.08, 31.05, 13.56 |
| 11 | 9.55(brs, 2H), 7.81(d,J=7.9 Hz, 2H), 7.72(s, 1H), 7.34(d, J=8.4 Hz, 2H), 7.31(d, J=1.3 Hz, 1H), 7.25—7.18(m, 2H), 7.16—7.05(m, 4H), 5.30(s, 2H), 4.47—4.35(m, 1H), 4.34—4.22(m, 2H), 4.09(q, J=7.3 Hz, 2H), 4.05—3.96(m, 2H), 3.80—3.71(m, 1H), 3.47—3.37(m, 1H), 3.22—3.05(m, 2H), 2.80—2.71(m, 2H), 2.60(s, 3H), 2.51(s, 3H), 2.49(s, 3H), 1.23(t, J=7.1 Hz, 3H) | 171.09, 170.65, 167.46, 163.62, 152.37, 150.41, 148.61, 148.19, 144.86, 142.75, 141.48, 140.82, 134.72, 132.45, 129.59, 128.96(2C), 128.79(2C), 127.36(2C), 127.32(2C), 126.22, 122.87, 119.89, 107.92, 65.43, 60.09, 55.30, 46.32, 42.49, 40.99, 38.93, 32.06, 21.09, 20.81, 20.08, 13.61 |
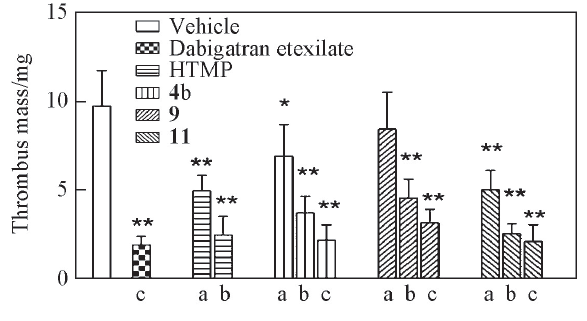
Fig.1 Antithrombotic effect of tested compounds, dabigatran etexilate and HTMP in vivoAverage thrombus mass is the mean±SD from eight independent experiments. Statistical significance compared with the vehicle group is *P<0.05, **P<0.01. Dose/(mg·kg-1): a. 1; b. 5; c. 20.
| [1] | Fu Y., Wang D.W., Chinese J. Clin., 2006, 34(8), 51—53 |
| (付研, 王大为. 中国临床医生, 2006, 34(8), 51—53) | |
| [2] | Mackman N., Becker R.C., Arterioscler. Thromb. Vasc. Biol., 2010, 30(3), 369—371 |
| [3] | Rupin A., Marx I., Vallez M.O., Mennecier P., Gloanec P., de Nanteuil G., Verbeuren T. J., J. Thromb. Haemost., 2011, 9(7), 1375—1382 |
| [4] | Xiao Z., Theroux P., Circulation, 1998, 97(3), 251—256 |
| [5] | Ishibashi H., Koide M., Obara S., Kumasaka Y., Tamura K., J. Stroke. Cerebrovasc. Dis., 2013, 22(5), 656—660 |
| [6] | Di Nisio M., Middeldorp S., Buller H.R., N. Engl. J. Med., 2005, 353(10), 1028—1040 |
| [7] | Yang H.R., Ren Y. J., Gao X. D., Gao Y. H., Chem. Res. Chinese Universities, 2016, 32(6), 973—978 |
| [8] | Connolly S.J., Ezekowitz M. D., Yusuf S., Eikelboom J., Oldgren J., Parekh A., Pogue J., Reilly P. A., Themeles E., Varrone J., Wang S., Alings M., Xavier D., Zhu J., Diaz R., Lewis B. S., Darius H., Diener H. C., Joyner C. D., Wallentin L., Engl. J. Med., 2009, 361(12), 1139—1151 |
| [9] | Grave S., Aust. Nurs. J., 2011, 19(6), 30—33 |
| [10] | Martins H.S., Scalabrini-Neto A., Velasco I. T., Lancet, 2007, 370(9604), 2002—2003 |
| [11] | Hauel N.H., Nar H., Priepke H., Ries U., Stassen J. M., Wienen W., J. Med. Chem., 2002, 45(9), 1757—1766 |
| [12] | Halton J.M., Lehr T., Cronin L., Lobmeyer M. T., Haertter S., Belletrutti M., Mitchell L. G., Thromb. Haemost., 2016, 116(3), 461—471 |
| [13] | Imberti D., Pomero F., Benedetti R., Fenoglio L., Intern. Emerg. Med., 2016, 11(7), 895—900 |
| [14] | Yang X.Z., Yang W. H., Xu Y. G., Diao X. J., He G. W., Gong G. Q., Eur. J. Med. Chem., 2012, 57, 21—28 |
| [15] | Yang X.Z., Diao X. J., Yang W. H., Li F., He G. W., Gong G. Q., Xu Y. G., Bioorg. Med. Chem. Lett., 2013, 23(7), 2089—2092 |
| [16] | Wang S.C., Dai P., Xu Y. G., Chen Q. F., Zhu Q. H., Gong G. Q., Arch. Pharm., 2015, 348(8), 595—605 |
| [17] | Chen D.X., Wang S. C., Diao X. J., Zhu Q. H., Shen H. L., Han X. Q., Wang Y. W., Gong G. Q., Xu Y. G., Bioorg. Med. Chem., 2015, 23(23), 7405—7416 |
| [18] | Li M., Handa S., Ikeda Y., Goto S., Thromb. Res., 2001, 104(1), 15—28 |
| [19] | Liu J.B., Li Y. X., Chen Y. W., Wu C. C., Wan Y. Y., Wei W., Xiong L. X., Zhang X., Yu S. J., Li Z. M., Chem. Res. Chinese Universities, 2016, 32(1), 41—48 |
| [20] | Jia C.Q., Yang D. Y., Che C. L., Ma Y. Q., Rui C. H., Yan X. J., Qin Z. H., Chem. J. Chinese Universities, 2016, 37(5), 892—901 |
| (贾长青, 杨冬燕, 车传亮, 马永强, 芮昌辉, 闫晓静, 覃兆海. 高等学校化学学报, 2016, 37(5), 892—901) |
| [1] | CAO Kaiyue, PENG JinWu, LI Hongbin, SHI Chengying, WANG Peng, LIU Baijun. High-temperature Proton Exchange Membranes Based on Cross-linked Polybenzimidazole/hyperbranched-polymer Blends [J]. Chem. J. Chinese Universities, 2021, 42(6): 2049. |
| [2] | LIANG Minhui, WANG Peng, LI Hongbin, LI Tianyang, CAO Kaiyue, PENG Jinwu, LIU Zhenchao, LIU Baijun. Preparation of High-temperature Proton Exchange Membranes Based on Semi-interpenetrating Polymer Networks [J]. Chem. J. Chinese Universities, 2020, 41(12): 2845. |
| [3] | SONG Xipeng, LIU Jinyu, WANG Lihua, HAN Xutong, HUANG Qinglin. Preparation of Polybenzimidazole/Polyvinylpyrrolidone Proton Exchange Membranes for Vanadium Redox Flow Battery† [J]. Chem. J. Chinese Universities, 2019, 40(7): 1543. |
| [4] | ZHAO Caixiu, YANG Yi, LIU Yiting, JIANG Ying, YUAN Fang, WANG Rui, CHEN Dongju. High Flux Polybenzimidazole Solvent Resistant Nanofiltration Membranes: Morphology Control and Performance† [J]. Chem. J. Chinese Universities, 2018, 39(4): 785. |
| [5] | LIU Zhiqing, XUE Fei, LEI Zhenkai, LIU Chenjiang. Synthesis of ILs 1-Alkyl-3-carboxymethyl Benzimidazole Double Trifluoromethanesulfonimide and Application in Desulfurization of Fuels† [J]. Chem. J. Chinese Universities, 2016, 37(5): 886. |
| [6] | XU Yan-Yan, YU Shu-Ping, HAN Ke-Fei, YU Jing-Hua, ZHU Hong, WANG Zhong-Ming. Microwave-Assisted Synthesis of Novel Polybenzimidazole Resin and Performance of Its Proton Exchange Membranes [J]. Chem. J. Chinese Universities, 2013, 34(6): 1547. |
| [7] | ZHOU Yu, WANG Da-Ming, ZHAO Xiao-Gang, ZHOU Hong-Wei, CHEN Chun-Hai, DANG Guo-Dong. Synthesis and Properties of Polyimides Containing Benzimidazole Group [J]. Chem. J. Chinese Universities, 2013, 34(10): 2427. |
| [8] | YI Ping-Gui, YANG Xi-Chun, YU Xian-Yong, LIU Zheng-Jun, LIU Jin, WANG Zhao-Xu, LI Xiao-Fang. Proton Transfer of 2-(2-Amino-3-pyridyl)-benzimidazole Under the Inclusion Interaction with Cucurbit[8]uril [J]. Chem. J. Chinese Universities, 2012, 33(12): 2657. |
| [9] | ZHAO Jing, SHENG Li, XU Hong-Jie, FANG Jian-Hua, YIN Jie. Synthesis and Characterization of Novel Sulfonated Polybenzimidazoles [J]. Chem. J. Chinese Universities, 2012, 33(03): 645. |
| [10] | WANG Bin, LIU Chen-Jiang, WANG Ji-De, LEI Zhen-Kai, HU Dong-Lin. Synthesis of Functionalized Ionic Liquids Based on Benzimidazolium and Their Properties [J]. Chem. J. Chinese Universities, 2012, 33(01): 76. |
| [11] | HU Song-Qing*, MI Si-Qi, JIA Xiao-Lin, GUO Ai-Ling, CHEN Sheng-Hui, ZHANG Jun, LIU Xin-Yong. 3D-QSAR Study and Molecular Design of Benzimidazole Derivatives as Corrosion Inhibitors [J]. Chem. J. Chinese Universities, 2011, 32(10): 2402. |
| [12] | SHENG Li, XU Hong-Jie*, FANG Jian-Hua, YIN Jie. Synthesis and Characterization of Soluble Copolybenzimidazoles [J]. Chem. J. Chinese Universities, 2010, 31(7): 1461. |
| [13] | WANG Xiao-Ke, TIAN Mi*. Synthesis of Benzimidazole Derivatives in Water Under Imidazolium Salt Grafted Iodobenzene Diacetate Assisted [J]. Chem. J. Chinese Universities, 2010, 31(2): 296. |
| [14] | HUANG Xia-Yun, SHI Zi-Xing*, YIN Jie. Growth of Hydroxyapatite on Sulfonated Polybenzimidazole Membranes [J]. Chem. J. Chinese Universities, 2010, 31(1): 1. |
| [15] | SHI Da-Qing1,2*, LI Zheng-Yi2, DOU Guo-Lan2, SHI Chun-Ling2, WANG Xiang-Shan2, JI Shun-Jun1. Synthesis of Benzoimidazo[1,2-c]quinazoline Derivatives Promoted by Low-valent Titanium [J]. Chem. J. Chinese Universities, 2007, 28(10): 1889. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||