

Chem. J. Chinese Universities ›› 2017, Vol. 38 ›› Issue (2): 217.doi: 10.7503/cjcu20160664
• Physical Chemistry • Previous Articles Next Articles
BULIN Chaoke1, GUO Ting2, ZHANG Bangwen1,*( ), DAI Zhian1, YU Huitao1, XING Ruiguang1, ZI Luxiong1
), DAI Zhian1, YU Huitao1, XING Ruiguang1, ZI Luxiong1
Received:2016-09-21
Online:2017-02-10
Published:2017-01-17
Contact:
ZHANG Bangwen
E-mail:bangwenz@126.com
Supported by:CLC Number:
TrendMD:
BULIN Chaoke, GUO Ting, ZHANG Bangwen, DAI Zhian, YU Huitao, XING Ruiguang, ZI Luxiong. Fast Removal of Aqueous Mn(Ⅱ) Using Partially Reduced Graphene Oxide-Fe3O4†[J]. Chem. J. Chinese Universities, 2017, 38(2): 217.
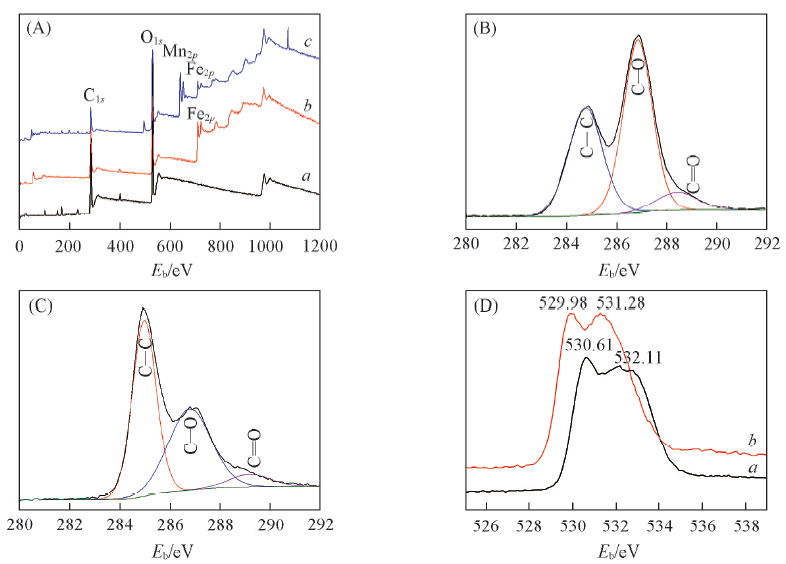
Fig.5 Survey XPS spectra of GO(a), PRGO-Fe3O4(b) and PRGO-Fe3O4-Mn(c)(A), C1s XPS spectra of GO(B) and PRGO-Fe3O4(C) and O1s XPS spectra of PRGO-Fe3O4(a) and PRGO-Fe3O4-Mn(b)(D)
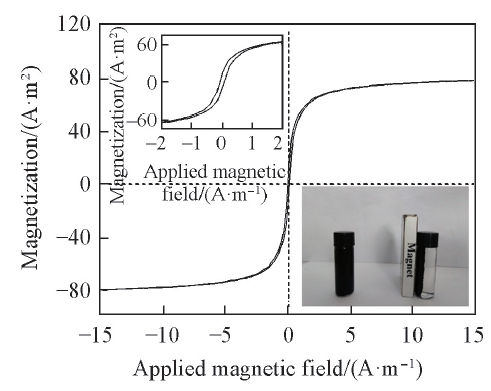
Fig.6 Magnetization curve of PRGO-Fe3O4Inset at the upper left corner shows the magnification of hysteresis loop; inset at the lower right corner shows the magnetic separation of PRGO-Fe3O4 from water under an external magnetic field.
| Model | Parameter | R2 | Model | Parameter | R2 |
|---|---|---|---|---|---|
| Langmuir | Qmax=943.3962 mg/g | 0.9439 | Pseudo first order | k1=1.8585 min-1 | 0.9647 |
| KL=0.2494 L/mg | Qe=98.0600 mg/g | ||||
| Freundlich | n=2.6687 | 0.9908 | Pseudo second order | k2=0.2034 g·mg-1·min-1 | 0.9999 |
| KL=541.6608 | Qe=404.8583 mg/g | ||||
| Temkin | AT=5.4466 J/mol | 0.9962 | Intra-particle diffusion | Kpi=13.4465 mg·g-1·min-0.5 | 0.9925 |
| BT=1.8775 L/g | c=98.0611 mg/g | ||||
| D-R | Qmax=1757.5042 mg/g | 0.8855 | Liquid film diffusion | 0.9648 | |
| E=39.3643 kJ/mol |
Table 1 Parameters of the adsorption models
| Model | Parameter | R2 | Model | Parameter | R2 |
|---|---|---|---|---|---|
| Langmuir | Qmax=943.3962 mg/g | 0.9439 | Pseudo first order | k1=1.8585 min-1 | 0.9647 |
| KL=0.2494 L/mg | Qe=98.0600 mg/g | ||||
| Freundlich | n=2.6687 | 0.9908 | Pseudo second order | k2=0.2034 g·mg-1·min-1 | 0.9999 |
| KL=541.6608 | Qe=404.8583 mg/g | ||||
| Temkin | AT=5.4466 J/mol | 0.9962 | Intra-particle diffusion | Kpi=13.4465 mg·g-1·min-0.5 | 0.9925 |
| BT=1.8775 L/g | c=98.0611 mg/g | ||||
| D-R | Qmax=1757.5042 mg/g | 0.8855 | Liquid film diffusion | 0.9648 | |
| E=39.3643 kJ/mol |
| Temperature/K | ΔG/(kJ/mol) | ΔH/(kJ/mol) | ΔS/(J·K-1·mo-1) |
|---|---|---|---|
| 293 | -10.32 | 16.70 | 93.24 |
| 298 | -10.79 | ||
| 303 | -11.26 | ||
| 313 | -11.72 |
Table 2 Thermodynamic data of liquid film diffusion at different temperature according to Van’t Hoff formula
| Temperature/K | ΔG/(kJ/mol) | ΔH/(kJ/mol) | ΔS/(J·K-1·mo-1) |
|---|---|---|---|
| 293 | -10.32 | 16.70 | 93.24 |
| 298 | -10.79 | ||
| 303 | -11.26 | ||
| 313 | -11.72 |
| [1] | Imran A., Chem. Rev., 2012, 112, 5073—5091 |
| [2] | Jerome C., Alexandra F., Youmin G., Benoit C., David F., Chem. Soc. Rev., 2014, 43, 5594—5617 |
| [3] | Yan C. Q., Liu B., Lu G. X., Li Y. X., Yang Q. B., Song Y., Chem. J. Chinese Universities,2016, 37(1), 189—194 |
| (闫春秋, 刘斌, 鲁冠秀, 李耀先, 杨清彪, 宋岩. 高等学校化学学报, 2016, 37(1), 189—194) | |
| [4] | Wang H. L., Zhao Y., Ma L. K., Fan P. H., Xu C. B., Jiao C. L., Lin A. J., Chem. J. Chinese Universities,2016, 37(2), 335—341 |
| (王会丽, 赵越, 马乐宽, 范鹏浩, 徐从斌, 焦春磊, 林爱军. 高等学校化学学报, 2016, 37(2), 335—341) | |
| [5] | Wang Z. T., Xiao C. F., Zhao J., Hu X., Xu N. K., Chem. J. Chinese Universities,2014, 35(11), 2410—2417 |
| (王子涛, 肖长发, 赵健, 胡霄, 徐乃库. 高等学校化学学报, 2014, 35(11), 2410—2417) | |
| [6] | Guo X. Y., Fan G., Di J. Z., Dong Y. R., Non-Metallic Mines,2015, 38(4), 67—70 |
| (郭旭颖, 范戈, 狄军贞, 董艳荣. 非金属矿, 2015, 38(4), 67—70) | |
| [7] | Cheng Y., Yang Y. H., Yu S. S., Zhang Y. Q., Ning P., Shi L., Zhang H. Y., Bulletin of the Chinese Ceremic Society. ,2015, 34(7), 1851—1856 |
| (程杨, 杨月红, 于珊珊, 张玉琴, 宁平, 石磊, 张怀予. 硅酸盐通报, 2015, 34(7), 1851—1856) | |
| [8] | Xiao L. P., Li Y., Guo Y., Liu Z., Non-Metallic Mine,2016, 39(3), 23—25 |
| (肖利萍, 李莹, 郭悦, 刘喆. 非金属矿, 2016, 39(3), 23—25) | |
| [9] | Xiao L. P., Li Y., Guo Y., Liu Z., Non-Metallic Mines,2016, 39(2), 28—30, 34 |
| (肖利萍, 李莹, 刘喆. 非金属矿, 2016, 39(2), 28—30, 34) | |
| [10] | Mariana T., Svetlana D. G., Velyana G. G., Lyubomir T. V., J. Mol. Liq., 2015, 211, 938—947 |
| [11] | Khaled Z. A. W., Habd E. M., Mostafa M. H. K., Journal of Environmental Chemical Engineering,2015, 3, 179—186 |
| [12] | Yan H., Li H. J., Tao X., Li K., Yang H., Li A. M., Xiao S. J., Cheng R. S., ACS Appl. Mater. Interfaces,2014, 6, 9871—9880 |
| [13] | Xu R., Zhou G. Y., Tang Y. H., Chu L., Liu C. B., Zeng Z. B., Luo S. L., Chem. Eng. J., 2015, 275, 179—188 |
| [14] | Yu H. T., Zhang B. W., Bulin C. K., Li R. H., Xing R. G., Sci Rep. ,2016, 6, 1—7 |
| [15] | Cui L. M., Wang Y. G., Gao L., Hu L. H., Yan L. G., Wei Q., Du. B., Chem. Eng. J., 2015, 281, 1—10 |
| [16] | Zhang W. J., Shi X. H., Zhang Y. X., Gu W., Li B. Y., Xian Y. Z., J. Mater. Chem. A,2013, 1, 1745—1753 |
| [17] | Bian Y., Bian Z. Y., Zhang J. X., Ding A. Z., Liu S. L., Wang H., Appl. Surf. Sci., 2015, 329, 269—275 |
| [18] | Peng L., Xu Z., Liu Z., Wei Y. Y., Sun H. Y., Li Z. Zhao X. L., Gao C., Nature Comm., 2015, 6, 5716 |
| [19] | Li J., Zhang S. W., Chen C. L., Zhao G. X., Yang X., Li J. X., Wang X. K., ACS Appl. Mater. Interfaces,2012, 4, 4991—5000 |
| [20] | Wuhan University. Analytical Chemistry, Higher Education Press, Beijing, 2001, 328 |
| (武汉大学. 分析化学, 北京:高等教育出版社, 2001, 328) | |
| [21] | Kondo S., Ishikawa T., Abe I., Translated by Li G. X., Adsorption Science, Chemical Industry Press, Beijing, 2006, 140 |
| (近藤精一, 石川达雄, 安部郁夫. 吸附科学. 李国希译. 北京:化学工业出版社, 2006, 140) | |
| [22] | Li X.L., Preparation of Novel Composite Adsorbents Based on Polymer Matrix and the Research on the Adsorption of Heavy Metals in Aqueous Solutions, Lanzhou University, Lanzhou, 2013 |
| (李晓丽, 聚合物基新型复合吸附材料的制备及对水体中重金属污染物的吸附性能研究, 兰州: 兰州大学, 2013) | |
| [23] | Zheng Y. M., Yu L., Chen., J. Colloid Interface Sci., 2012, 367, 362—369 |
| [1] | MA Jianxin, LIU Xiaodong, XU Na, LIU Guocheng, WANG Xiuli. A Multi-functional Zn(II) Coordination Polymer with Luminescence Sensing, Amperometric Sensing, and Dye Adsorption Performance [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210585. |
| [2] | LIU Shuaizhuo,ZHANG Qian,LIU Ning,XIAO Wenyan,FAN Leiyi,ZHOU Ying. One-step Synergistic Hydrophobic Modification of Melamine Sponge and Its Application † [J]. Chem. J. Chinese Universities, 2020, 41(3): 521. |
| [3] | WU Shanshan,WEI Chanling,ZHAO Lijuan,TIAN Yang,WANG Xia,GONG Bolin. Preparation and Enrichment Properties of Novel Magnetic Restricted Access Media-molecularly Imprinted Composites† [J]. Chem. J. Chinese Universities, 2019, 40(6): 1150. |
| [4] | JI Yuchun,MAO Wenhui,LIAO Hejie,WANG Jilin,LONG Fei,GU Yunle. Boron Nitride Nanotube-nanosheet Hierarchical Structures andIts Optical/adsorption Properties† [J]. Chem. J. Chinese Universities, 2019, 40(2): 216. |
| [5] | KANG Yuanyuan, GUO Zeqing, ZHOU Jianping. Hydrothermal Preparation and Adsorption Property of MoS2/Na2Fe2Ti6O16† [J]. Chem. J. Chinese Universities, 2018, 39(7): 1364. |
| [6] | GUO Ming, ZHANG Xinge, ZENG Chuchu, YIN Xinxin. Preparation and Properties Characterization of Intelligent Molecularly Imprinted Polymer Based on Diles-Alder Reaction† [J]. Chem. J. Chinese Universities, 2018, 39(3): 566. |
| [7] | WANG Huili, ZHAO Yue, MA Lekuan, FAN Penghao, XU Congbin, JIAO Chunlei, LIN Aijun. Preparation of Composite Modified Expanded Graphite and Its Adsorption on Acid Brilliant Blue Dye† [J]. Chem. J. Chinese Universities, 2016, 37(2): 335. |
| [8] | XU Junge, LI Yunqin, YUAN Baoling, CUI Haojie, FU Minglai. Synthesis and Characterization of 3D Flower-like α-FeOOH Nanostructures† [J]. Chem. J. Chinese Universities, 2015, 36(1): 48. |
| [9] | CHEN Youning, GAO Li, HE Maofang, WEI Yinmao. High-capacity Polyvinyltetrazole-grafted Chelating Resin for Adsorption of Heavy Metal Ions† [J]. Chem. J. Chinese Universities, 2014, 35(7): 1596. |
| [10] | WANG Zitao, XIAO Changfa, ZHAO Jian, HU Xiao, XU Naiku. Preparation of Reduced Graphene Oxide-based Melamine Sponge and Its Absorption Properties† [J]. Chem. J. Chinese Universities, 2014, 35(11): 2410. |
| [11] | MA Chao, XU Yan, GUO Xin-Yu, LUO Xiang-Ren, WU Xue-Min. Adsorption Properties of Comb-shaped Copolymer of Polycarboxylic Acid Superplasticizer on the Interface of Fipronil Particles [J]. Chem. J. Chinese Universities, 2013, 34(6): 1441. |
| [12] | DONG Jia-Bin, WU Jian-Bo, YANG Jing, SONG Wei, DAI Xiao-Jun, YE Zheng-De, GONG Bo-Lin. Preparation of High-capacity IDA Chelating Resin and Its Adsorption Properties [J]. Chem. J. Chinese Universities, 2013, 34(3): 714. |
| [13] | WANG Yan-Ling, LIU Jun-Bo, TANG Shan-Shan, CHANG Hai-Bo, LIANG Da-Dong. Preparation and Properties of Molecularly Imprinted Nanofiber Memberanes Towards Enrofloxacin [J]. Chem. J. Chinese Universities, 2013, 34(12): 2880. |
| [14] | LU Hong-Yan, YANG Li-Xin*, WU Sai-Xiang, ZHANG Ling-Jun, ZHOU Li, LIU Xiao-Li. Preparation of Three-dimensionally Ordered Macroporous Spinel Li1.6Mn1.6O4 and Adsorption Characteristics of Lithium Ion-sieve [J]. Chem. J. Chinese Universities, 2011, 32(10): 2268. |
| [15] |
YAN Ai-Guo, QIU Guan-Zhou, LIU Xiao-He*, SHI Rong-Rong, ZHANG Ning, YI Ran, LI Yong-Bo, GAO Guan-Hua.
Size-controlled Synthesis, Characterization and Microwave Absorption Efficiency of Fe3O4 Nanocrystallines [J]. Chem. J. Chinese Universities, 2008, 29(1): 23. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||