

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (2): 335.doi: 10.7503/cjcu20150544
• Physical Chemistry • Previous Articles Next Articles
WANG Huili1, ZHAO Yue2, MA Lekuan2, FAN Penghao1, XU Congbin1, JIAO Chunlei1, LIN Aijun1,*( )
)
Received:2015-07-14
Online:2016-02-10
Published:2016-01-06
Contact:
LIN Aijun
E-mail:ajlin@126.com
Supported by:CLC Number:
TrendMD:
WANG Huili, ZHAO Yue, MA Lekuan, FAN Penghao, XU Congbin, JIAO Chunlei, LIN Aijun. Preparation of Composite Modified Expanded Graphite and Its Adsorption on Acid Brilliant Blue Dye†[J]. Chem. J. Chinese Universities, 2016, 37(2): 335.
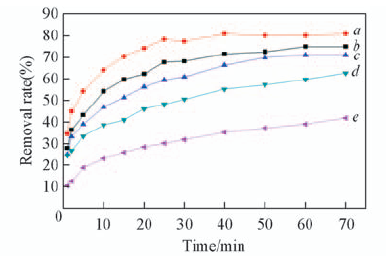
Fig.1 Adsorption activities of M-EG&EG in the reduction of acid brilliant blue a. 11.662 mg/g CTAB; b. 17.493 mg/g CTAB; c. 5.831 mg/g CTAB; d. 11.662 mg/g without KBr; e. EG.
| NaCl | NaOH | Pb(NO3)2 | |||
|---|---|---|---|---|---|
| Molar fraction(%) | Removal rate(%) | Molar fraction(%) | Removal rate(%) | c/(mg·L-1) | Removal rate(%) |
| 0 | 85.06 | 0 | 85.06 | 0 | 85.06 |
| 1 | 88.39 | 1 | 85.81 | 33.3 | 91.79 |
| 2 | 88.52 | 1.5 | 87.92 | 66.7 | 93.41 |
| 3 | 87.71 | 2 | 86.8 | 100 | 94.16 |
| 4 | 87.43 | 2.5 | 87.55 | 133.3 | 94.28 |
| 5 | 86.48 | 3 | 88.42 | 166.7 | 94.66 |
Table 1 Effects of metal compounds on reduction rate of acid brilliant blue
| NaCl | NaOH | Pb(NO3)2 | |||
|---|---|---|---|---|---|
| Molar fraction(%) | Removal rate(%) | Molar fraction(%) | Removal rate(%) | c/(mg·L-1) | Removal rate(%) |
| 0 | 85.06 | 0 | 85.06 | 0 | 85.06 |
| 1 | 88.39 | 1 | 85.81 | 33.3 | 91.79 |
| 2 | 88.52 | 1.5 | 87.92 | 66.7 | 93.41 |
| 3 | 87.71 | 2 | 86.8 | 100 | 94.16 |
| 4 | 87.43 | 2.5 | 87.55 | 133.3 | 94.28 |
| 5 | 86.48 | 3 | 88.42 | 166.7 | 94.66 |
| Adsorbent | Pseudo-first-order | Pseudo-second order | ||||
|---|---|---|---|---|---|---|
| Qe/(mg·g-1) | k1/h-1 | R2 | Qe/(mg·g-1) | k2/h-1 | R2 | |
| M-EG | 67.69 | 0.4026 | 0.7049 | 71.43 | 0.3267 | 0.9976 |
| EG | 62.18 | 0.0805 | 0.4626 | 66.22 | 0.1086 | 0.983 |
Table 2 Fitting parameters of kinetic equations for acid brilliant blue on M-EG
| Adsorbent | Pseudo-first-order | Pseudo-second order | ||||
|---|---|---|---|---|---|---|
| Qe/(mg·g-1) | k1/h-1 | R2 | Qe/(mg·g-1) | k2/h-1 | R2 | |
| M-EG | 67.69 | 0.4026 | 0.7049 | 71.43 | 0.3267 | 0.9976 |
| EG | 62.18 | 0.0805 | 0.4626 | 66.22 | 0.1086 | 0.983 |
| Adsorbent | Langmuir parameter | Freundlich parameter | ||||
|---|---|---|---|---|---|---|
| Qm/(mg·g-1) | b/(L·mg-1) | R2 | n | KF | R2 | |
| M-EG | 526.32 | 0.0322 | 0.9785 | 1.9066 | 15.929 | 0.8311 |
| EG | 100.00 | 0.0805 | 0.8142 | 2.9011 | 12.627 | 0.7708 |
Table 3 Isotherms constants for acid brilliant blue on M-EG
| Adsorbent | Langmuir parameter | Freundlich parameter | ||||
|---|---|---|---|---|---|---|
| Qm/(mg·g-1) | b/(L·mg-1) | R2 | n | KF | R2 | |
| M-EG | 526.32 | 0.0322 | 0.9785 | 1.9066 | 15.929 | 0.8311 |
| EG | 100.00 | 0.0805 | 0.8142 | 2.9011 | 12.627 | 0.7708 |
| [1] | Li F., Wang G. Y., Zhang Y., Ling H. R., Chem. J. Chinese Universities, 2015, 36(7), 1351—1357 |
| (李锋, 王桂燕, 张岩, 李洪仁. 高等学校化学学报, 2015, 36(7), 1351—1357) | |
| [2] | Li K. B., Zhao F., Wei H., Zhang T., Wang Q. Q., Chem. J. Chinese Universities, 2011, 32(8), 1812—1818 |
| (李克斌, 赵锋, 魏红, 张涛, 王勤勤. 高等学校化学学报, 2011, 32(8), 1812—1818 ) | |
| [3] | Ren N. Q., Zhou X. J., Guo W. Q., Yang S. S., CIESC Journal, 2013, 64(1), 84—94 |
| (任南琪, 周显娇, 郭婉茜, 杨珊珊. 化工学报, 2013, 64(1), 84—94) | |
| [4] | McMullan G., Meehan C., Conneely A., Kirby N., Robinson T., Nigam P., Banat I. M., Marchant R., Smyth W. F., Applied Microbiology and Biotechnology, 2001, 56, 81—87 |
| [5] | Wang Y. Z., Chen M. X., Hu C., Acta Scientiae Circumstantiae, 2000, 20(6), 772—775 |
| (王怡中, 陈梅雪, 胡春. 环境科学学报, 2000, 20(6), 772—775) | |
| [6] | Liu Y., Degradation of Dye Wastewater by Ccatalytic Wet Oxidation Under Room Temperature and Atmospheric Pressure, Harbin Institute of Technology, Harbin, 2006 |
| (刘琰. 常温常压催化湿式氧化工艺处理染料废水的研究, 哈尔滨: 哈尔滨工业大学, 2006) | |
| [7] | Huo H. L., Lü L. Y., Wang H., Chem. J. Chinese Universities, 2015, 36(5), 939—944 |
| (霍海玲, 吕丽云, 王虹. 高等学校化学学报, 2015, 36(5), 939—944) | |
| [8] | Teng M., Qiao J., Li F., Bera P. K., Carbon, 2012, 50, 2877—2886 |
| [9] | Bae J. H., Song D. I., Jeon Y. W., Separation Science and Technology, 2000, 35(3), 353—365 |
| [10] | Meng F. P., Yi H. C ., Materials Review, 2009, 23(7), 69—73 |
| (孟范平, 易怀昌. 材料导报, 2009, 23(7), 69—73) | |
| [11] | Liao P., Ismael Z. M., Zhang W., Yuan S., Tong M., Wang K., Bao J., Chem. Eng. J., 2012, 195, 339—346 |
| [12] | Chen X. L., Deng L. C., Wang X. F., Guan S., Chem. J. Chinese Universities, 2014, 35(12), 2510—2515 |
| (陈晓磊, 邓李川, 王晓峰, 关爽. 高等学校化学学报, 2014, 35(12), 2510—2515) | |
| [13] | Huang C., Chang K., Ou H., Chiang Y., Wang C., Micropor. Mesopor. Mater., 2011, 141, 102—109 |
| [14] | Zhou X., Ge X., Tang R. Z., Chen T., Wang G. Y., Chin. J. Catal., 2014, 35(4), 481—489 |
| (周喜, 葛鑫, 唐荣芝, 陈彤, 王公应. 催化学报, 2014, 35(4), 481—489) | |
| [15] | Yang M. X., Ma J., Sun Y. R., Chem. J. Chinese Universities, 2014, 35(3), 570—575 |
| (杨明轩, 马杰, 孙怡然. 高等学校化学学报, 2014, 35(3), 570—575) | |
| [16] | Liu P., Zhang L., Sep. Purif. Technol., 2007, 58, 32—39 |
| [17] | Chen X. W., Zheng X., Wang X. F., Wang J. H., Chem. J. Chinese Universities, 2015, 36(8), 1641—1647 |
| (陈旭伟, 郑旭, 王晓峰, 王建华. 高等学校化学学报, 2015, 36(8), 1641—1647) | |
| [18] | Yang M. Q., Zhang N., Pagliaro M., Xu Y. J., Chem. Soc. Rev., 2014, 43, 8240—8254 |
| [19] | Zhang N., Zhang Y. H., Xu Y. J., Nanoscale, 2012, 4, 5792—5813 |
| [20] | Li W., Han C., Liu W., Zhang M. H., Tao K., Catal. Today, 2007, 125, 278—281 |
| [21] | Wei Y. X., Tu W. X., Chem. J. Chinese Universities, 2014, 35(11), 2397—2402 |
| (魏英祥, 涂伟霞. 高等学校化学学报, 2014, 35(11), 2397—2402) | |
| [22] | Ding M. J., Huang H., Yang P., Chem. J. Chinese Universities, 2015, 36(5), 989—995 |
| (丁敏娟, 黄徽, 杨平. 高等学校化学学报, 2015, 36(5), 989—995) | |
| [23] | Yue X. Q., Duan W. Y., Lu Y., Zhang F. C., Zhang R. J., Bull. Mater. Sci., 2011, 34, 1569—1573 |
| [24] | Li J. T., Li M., Li J. H., Sun H. W., Ultrasonics Sonochemistry, 2007, 14, 62—66 |
| [25] | Cao N. Z., Shen W. C., Wen S. Z., Liu Y. J., Material Science & Technology, 1997, 5(2), 121—123 |
| (曹乃珍, 沈万慈, 温诗铸, 刘英杰. 材料科学与工艺, 1997, 5(2), 121—123) | |
| [26] | Lu Y., Zhong J. B., Li J. Z., Zeng J., He J. J., Chemical Research and Application, 2013, 25(6), 866—870 |
| (卢燕, 钟俊波, 李建章, 曾俊, 贺进进. 化学研究与应用, 2013, 25(6), 866—870) | |
| [27] | Fu M., Wang R. F., Zhao X. B., Ding J. N., Chen Z., Functional Materials, 2009, 40(8), 1322—1325 |
| (付猛, 王荣飞, 赵晓兵, 丁建宁, 陈志刚. 功能材料, 2009, 40(8), 1322—1325) | |
| [28] | Xiao X., Zhao Z.G., Surfactant Application Principle, Chemical Industry Press, Beijing, 2003, 78—83 |
| (肖进新, 赵振国. 表面活性剂应用原理, 北京: 化学工业出版社, 2003, 78—83) | |
| [29] | Cao N. Z., Shen W. C., Wen S. Z., Acta Physico-Chimica, 1996, 12(8), 766—768 |
| (曹乃珍,沈万慈, 温诗铸. 物理化学学报, 1996, 12(8), 766—768) | |
| [30] | Zhang W. H., Yin D., Lu N., Li Z. Y., Yang J. L., Chem. J. Chinese Universities, 2015, 36(11), 2081—2086 |
| (张文华, 殷迪, 卢宁, 李振宇, 杨金龙. 高等学校化学学报, 2015, 36(11), 2081—2086) | |
| [31] | Steurer P., Wissert R., Thomann R., Mülhaup R., Rapid Commun., 2009, 30, 316—327 |
| [32] | Han Z. D., Wang J. Q., Chinese Journal of Inorganic Chemistry, 2003, 12, 1366—1370 |
| (韩志东, 王建祺. 无机化学学报, 2003, 12, 1366—1370) | |
| [33] | Stankovich S., Piner R. D., Chen X. Q., Wu N. Q., Nguyen S. T., Ruoff R. S., J. Mater. Chem., 2006, 16, 155—158 |
| [34] | Weng S.F., Fourier Transform Infrared Spectroscopy, Chemical Industry Press, Beijing, 2010, 377—388 |
| (翁诗甫. 傅里叶变换红外光谱分析, 北京: 化学工业出版社, 2010, 377—388) | |
| [35] | Jiang B. J., Tian C. G., Wang L., Xu Y. X., Wang R. H., Qiao Y. J., Ma Y. G., Fu H. G., Chem. Commun., 2010, 46, 4920—4922 |
| [36] | Wang J., Guo B. D., Zhang X. D., Zhang Z. H., Ultrasonics Sonochemistry, 2005, 12(5), 331—337 |
| [37] | Yu G. F., Qing C. L., Mou S. S., Acta Scientiae Circumstantiae, 2001, 21(5), 601—606 |
| (余贵芬, 青长乐, 牟树森. 环境科学学报, 2001, 21(5), 601—606) | |
| [38] | Sun X., Li L. Q., Pan G. X., Journal of Agro-Environment Science, 2014, 33(8), 1637—1643 |
| (孙璇, 李恋卿, 潘根兴. 农业环境科学学报, 2014, 33(8), 1637—1643) | |
| [39] | Li R. Y., Chen D., Li L. Q., Journal of Agro-Environment Science, 2015, 34(5), 1001—1008 |
| (李瑞月, 陈德, 李恋卿. 农业环境科学学报, 2015, 34(5), 1001—1008) | |
| [40] | Sun Y. L., Ni J. R., Environmental Chemistry, 2002, 21(1), 37—44 |
| (孙玉玲, 倪晋仁. 环境化学, 2002, 21(1), 37—44) |
| [1] | MA Jianxin, LIU Xiaodong, XU Na, LIU Guocheng, WANG Xiuli. A Multi-functional Zn(II) Coordination Polymer with Luminescence Sensing, Amperometric Sensing, and Dye Adsorption Performance [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210585. |
| [2] | LIU Shuaizhuo,ZHANG Qian,LIU Ning,XIAO Wenyan,FAN Leiyi,ZHOU Ying. One-step Synergistic Hydrophobic Modification of Melamine Sponge and Its Application † [J]. Chem. J. Chinese Universities, 2020, 41(3): 521. |
| [3] | WU Shanshan,WEI Chanling,ZHAO Lijuan,TIAN Yang,WANG Xia,GONG Bolin. Preparation and Enrichment Properties of Novel Magnetic Restricted Access Media-molecularly Imprinted Composites† [J]. Chem. J. Chinese Universities, 2019, 40(6): 1150. |
| [4] | JI Yuchun,MAO Wenhui,LIAO Hejie,WANG Jilin,LONG Fei,GU Yunle. Boron Nitride Nanotube-nanosheet Hierarchical Structures andIts Optical/adsorption Properties† [J]. Chem. J. Chinese Universities, 2019, 40(2): 216. |
| [5] | KANG Yuanyuan, GUO Zeqing, ZHOU Jianping. Hydrothermal Preparation and Adsorption Property of MoS2/Na2Fe2Ti6O16† [J]. Chem. J. Chinese Universities, 2018, 39(7): 1364. |
| [6] | GUO Ming, ZHANG Xinge, ZENG Chuchu, YIN Xinxin. Preparation and Properties Characterization of Intelligent Molecularly Imprinted Polymer Based on Diles-Alder Reaction† [J]. Chem. J. Chinese Universities, 2018, 39(3): 566. |
| [7] | BULIN Chaoke, GUO Ting, ZHANG Bangwen, DAI Zhian, YU Huitao, XING Ruiguang, ZI Luxiong. Fast Removal of Aqueous Mn(Ⅱ) Using Partially Reduced Graphene Oxide-Fe3O4† [J]. Chem. J. Chinese Universities, 2017, 38(2): 217. |
| [8] | XU Junge, LI Yunqin, YUAN Baoling, CUI Haojie, FU Minglai. Synthesis and Characterization of 3D Flower-like α-FeOOH Nanostructures† [J]. Chem. J. Chinese Universities, 2015, 36(1): 48. |
| [9] | CHEN Youning, GAO Li, HE Maofang, WEI Yinmao. High-capacity Polyvinyltetrazole-grafted Chelating Resin for Adsorption of Heavy Metal Ions† [J]. Chem. J. Chinese Universities, 2014, 35(7): 1596. |
| [10] | WANG Zitao, XIAO Changfa, ZHAO Jian, HU Xiao, XU Naiku. Preparation of Reduced Graphene Oxide-based Melamine Sponge and Its Absorption Properties† [J]. Chem. J. Chinese Universities, 2014, 35(11): 2410. |
| [11] | MA Chao, XU Yan, GUO Xin-Yu, LUO Xiang-Ren, WU Xue-Min. Adsorption Properties of Comb-shaped Copolymer of Polycarboxylic Acid Superplasticizer on the Interface of Fipronil Particles [J]. Chem. J. Chinese Universities, 2013, 34(6): 1441. |
| [12] | DONG Jia-Bin, WU Jian-Bo, YANG Jing, SONG Wei, DAI Xiao-Jun, YE Zheng-De, GONG Bo-Lin. Preparation of High-capacity IDA Chelating Resin and Its Adsorption Properties [J]. Chem. J. Chinese Universities, 2013, 34(3): 714. |
| [13] | WANG Yan-Ling, LIU Jun-Bo, TANG Shan-Shan, CHANG Hai-Bo, LIANG Da-Dong. Preparation and Properties of Molecularly Imprinted Nanofiber Memberanes Towards Enrofloxacin [J]. Chem. J. Chinese Universities, 2013, 34(12): 2880. |
| [14] | LU Hong-Yan, YANG Li-Xin*, WU Sai-Xiang, ZHANG Ling-Jun, ZHOU Li, LIU Xiao-Li. Preparation of Three-dimensionally Ordered Macroporous Spinel Li1.6Mn1.6O4 and Adsorption Characteristics of Lithium Ion-sieve [J]. Chem. J. Chinese Universities, 2011, 32(10): 2268. |
| [15] |
YAN Ai-Guo, QIU Guan-Zhou, LIU Xiao-He*, SHI Rong-Rong, ZHANG Ning, YI Ran, LI Yong-Bo, GAO Guan-Hua.
Size-controlled Synthesis, Characterization and Microwave Absorption Efficiency of Fe3O4 Nanocrystallines [J]. Chem. J. Chinese Universities, 2008, 29(1): 23. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||