

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (11): 1947.doi: 10.7503/cjcu20160497
• Articles: Inorganic Chemistry • Previous Articles Next Articles
XING Dan, MA Yingxia*( ), RUAN Yongxin, DU Xueyan*(
), RUAN Yongxin, DU Xueyan*( )
)
Received:2016-07-13
Online:2016-11-10
Published:2016-10-19
Contact:
MA Yingxia,DU Xueyan
E-mail:mayx2011818@163.com;duxy@lut.cn
Supported by:CLC Number:
TrendMD:
XING Dan, MA Yingxia, RUAN Yongxin, DU Xueyan. Preparation and Characterization of Amino-functionalized Magnetic Graphite Nanosheet Nanocomposites†[J]. Chem. J. Chinese Universities, 2016, 37(11): 1947.
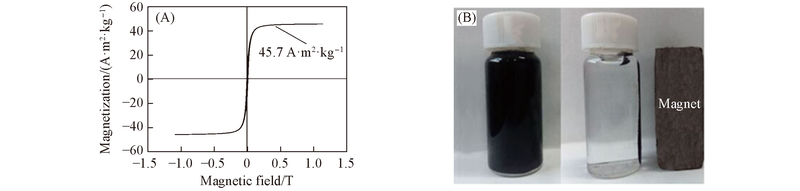
Fig.4 Magnetization curve for NH2-GNS/Fe3O4 nanocomposites(A) and digital photos of NH2-GNS/Fe3O4 nanocomposites before(left) and after(right) magnetic separation in aqueous solution at room temperature(B)
| Langmuir | Freundlich | ||||
|---|---|---|---|---|---|
| qm/(mg·g-1) | KL/(L·mg-1) | R2 | KF/(mg·g-1) | n/(mg·L-1) | R2 |
| 12.6103 | 0.8564 | 0.9997 | 5.9769 | 4.2571 | 0.7958 |
Table 1 Langmuir and Freundlich isotherm parameters for adsorption of Ag(Ⅰ) on NH2-GNS/Fe3O4 nanocomposites*
| Langmuir | Freundlich | ||||
|---|---|---|---|---|---|
| qm/(mg·g-1) | KL/(L·mg-1) | R2 | KF/(mg·g-1) | n/(mg·L-1) | R2 |
| 12.6103 | 0.8564 | 0.9997 | 5.9769 | 4.2571 | 0.7958 |
| [1] | Liu F., L. , Wang Z., M. , Li G., Y. , Desalination and Water Treatment, 2014, 52( 37-39), 7172- 7179 |
| [2] | 孟宪慧. 含银废水中银离子及银的配位离子的吸附与解吸研究, 沈阳: 东北大学, 2005) |
| Meng X., H. , Study on Adsorption and Desorption of Ag + and Coordination Compound of Silver in Silver-containing Wastewater, Northeas-tern University, Shenyang, 2005 ( | |
| [3] | 张帆, 李菁, 谭建华, 王波, 黄福. 化工进展, 2013, 32( 11), 2749- 2756 |
| Zhang, F. , Li, J. , Tan J., H. , Wang, B. , Huang, F. , Chemical Industry and Engineering Progress, 2013, 32( 11), 2749- 2756 ( | |
| [4] | 王旭恒, 王少芬, 闫新创, 宋燕. 广州化工, 2014, 42( 9), 93- 95 |
| Wang X., H. , Wang S., F. , Yan X., C. , Song, Y. , Guangzhou Chemical Industry, 2014, 42( 9), 93- 95 ( | |
| [5] | Hou H., B. , Yu D., M. , Hu G., H. , Langmuir, 2015, 31( 4), 1376- 1384 |
| [6] | 王子涛, 肖长发, 赵健, 胡霄, 徐乃库. 高等学校化学学报, 2014, 35( 11), 2410- 2417 |
| Wang Z., T. , Xiao C., F. , Zhao, J. , Hu, X. , Xu N., K. , Chem. J. Chinese Universities, 2014, 35( 11), 2410- 2417 ( | |
| [7] | 李国显. 石墨烯/纳米复合材料的制备及吸波性能, 南京: 南京航空航天大学, 2012) |
| Li G., X. , Synthesis and Microwave Absorbing Properties of Graphene/Magnetic-particle Nanocomposite Materials, Nanjing University of Aeronautics and Astronautics, Nanjing, 2012 ( | |
| [8] | He, F. , Lam, K. , Ma, D. , Fan J., T. , Chan L., H. , Zhang L., M. , Carbon, 2013, 58, 175- 184 |
| [9] | Ma Y., X. , Li Y., F. , Zhao G., H. , Yang L., Q. , Wang J., Z. , Shan, X. , Yan, X. , Carbon, 2012, 50( 8), 2976- 2986 |
| [10] | 任芳, 朱光明, 任鹏刚. 复合材料学报, 2014, 31( 2), 263- 272 |
| Ren, F. , Zhu G., M. , Ren P., G. , Acta Materiae Compositae Sinica, 2014, 31( 2), 263- 272 ( | |
| [11] | Xu K., L. , Chen G., M. , Qiu, D. , Journal of Materials Chemistry A, 2013, 1( 40), 12395- 12399 |
| [12] | Moussa, M. , El-Kady M., F. , Wang, H. , Michimore, A. , Zhou Q., Q. , Xu, J. , MajeswkiP., Ma, J. , Nanotechnology, 2015, 26( 7), 1- 11 |
| [13] | Rajaura R., S. , Srivastava, S. , Sharma, V. , Sharma P., K. , Lal, C. , Singh, M. , Palsania H., S. , Vijay Y., K. , International Journal of Hydrogen Energy, 2016, 41( 22), 9454- 9461 |
| [14] | Liu X., W. , Shen L., Y. , Hu Y., H. , Water Air Soil Pollut., 2016, 227( 5), 1- 12 |
| [15] | 杨梖, 白雪, 顾海鑫. 环境工程, 2015, 4, 25- 29 |
| Yang, B. , Bai, X. , Gu H., X. , Environmental Engineering, 2015, 4, 25- 29 ( | |
| [16] | Tan Y., Q. , Chen, M. , Hao Y., M. , Chemical Engineering Journal, 2012, 191( 2012011), 104- 111 |
| [17] | Cao, W. , Ma Y., R. , Zhou, W. , Guo, L. , Chem. Res. Chinese Universities, 2015, 31( 4), 508- 513 |
| [18] | Wu X., W. , Ma H., W. , Yang, J. , Wang F., J. , Li Z., H. , Applied Surface Science, 2012, 258( 14), 5516- 5521 |
| [19] | 路翠萍. 磁性聚苯乙烯基大孔树脂的制备及对水溶液中金属离子的吸附性能研究, 兰州: 兰州理工大学, 2014) |
| Lu C., P. , Study on the Synthesis of Magnetic Polystyrene Based Macroporous Resins and Their Adsorption Performance to Metal Ions in Aqueous Solution, Lanzhou University of Technology, Lanzhou, 2014 ( | |
| [20] | Zeng Y., B. , Zhou, Y. , Zhou T., S. , Shi G., Y. , Electrochimica Acta, 2014, 130( 0), 504- 511 |
| [21] | Shen X., P. , Wu J., L. , Bai, S. , Zhou, H. , Journal of Alloys and Compounds, 2010, 506( 1), 136- 140 |
| [22] | Guo H., L. , Wang X., F. , Qian Q., Y. , Wang F., B. , Xia X., H. , ACS Nano, 2009, 3( 9), 2653- 2659 |
| [1] | JIANG Hongbin, DAI Wenchen, ZHANG Rao, XU Xiaochen, CHEN Jie, YANG Guang, YANG Fenglin. Research on Co3O4/UiO-66@α-Al2O3 Ceramic Membrane Separation and Catalytic Spraying Industry VOCs Waste Gas [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220025. |
| [2] | HAO Honglei, MENG Fanyu, LI Ruoyu, LI Yingqiu, JIA Mingjun, ZHANG Wenxiang, YUAN Xiaoling. Biomass Derived Nitrogen Doped Porous Carbon Materials as Adsorbents for Removal of Methylene Blue in Water [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220055. |
| [3] | WANG Hongning, HUANG Li, QING Jiang, MA Tengzhou, JIANG Wei, HUANG Weiqiu, CHEN Ruoyu. Activation of Biochar from Cattail and the VOCs Adsorption Application [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210824. |
| [4] | CHEN Xiaolu, YUAN Zhenyan, ZHONG Yingchun, REN Hao. Preparation of Triphenylamine Based PAF-106s via Mechanical Ball Milling and C2 Hydrocarbons Adsorption Property [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210771. |
| [5] | MENG Xianglong, YANG Ge, GUO Hailing, LIU Chenguang, CHAI Yongming, WANG Chunzheng, GUO Yongmei. Synthesis of Nano-zeolite and Its Adsorption Performance for Hydrogen Sulfide [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210687. |
| [6] | TAN Lejian, ZHONG Xuanshu, WANG Jin, LIU Zongjian, ZHANG Aiying, YE Lin, FENG Zengguo. Low Critical Dissolution Temperature Behavior of β⁃Cyclodextrin and Its Application in the Preparation of β⁃Cyclodextrin Sheet Crystal with Ordered Nano⁃channel [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220405. |
| [7] | ZHENG Meiqi, MAO Fangqi, KONG Xianggui, DUAN Xue. Layered Double Hydroxides as Sorbent for Remediation of Radioactive Wastewater [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220456. |
| [8] | TIAN Xiaokang, ZHANG Qingsong, YANG Shulin, BAI Jie, CHEN Bingjie, PAN Jie, CHEN Li, WEI Yen. Porous Materials Inspired by Microbial Fermentation: Preparation Method and Application [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220216. |
| [9] | MA Jianxin, LIU Xiaodong, XU Na, LIU Guocheng, WANG Xiuli. A Multi-functional Zn(II) Coordination Polymer with Luminescence Sensing, Amperometric Sensing, and Dye Adsorption Performance [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210585. |
| [10] | ZHANG Chi, SUN Fuxing, ZHU Guangshan. Synthesis, N2 Adsorption and Mixed-matrix Membrane Performance of Bimetal Isostructural CAU-21 [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210578. |
| [11] | LIU Changhui, LIANG Guojun, LI Yanlu, CHENG Xiufeng, ZHAO Xian. Density Functional Theory Study of NH3 Adsorption on Boron Nanotubes [J]. Chem. J. Chinese Universities, 2021, 42(7): 2263. |
| [12] | WANG Hongning, HUANG Li, SONG Fujiao, ZHU Ting, HUANG Weiqiu, ZHONG Jing, CHEN Ruoyu. Synthesis and VOCs Adsorption Properties of Hollow Carbon Nanospheres [J]. Chem. J. Chinese Universities, 2021, 42(6): 1704. |
| [13] | WANG Longjie, FAN Hongchuan, QIN Yu, CAO Qiue, ZHENG Liyan. Research Progress of Metal-organic Frameworks in the Field of Chemical Separation and Analysis [J]. Chem. J. Chinese Universities, 2021, 42(4): 1167. |
| [14] | YAN Yanhong, WU Simin, YAN Yilun, TANG Xihao, CAI Songliang, ZHENG Shengrun, ZHANG Weiguang, GU Fenglong. Sulfonic Acid-functionalized Spherical Covalent Organic Framework with Ultrahigh Capacity for the Removal of Cationic Dyes [J]. Chem. J. Chinese Universities, 2021, 42(3): 956. |
| [15] | HU Xueyi, HAN Lulu, FANG Yun, XIA Yongmei. Admicelles and Adsolubilization of Extended Surfactants on Alumina [J]. Chem. J. Chinese Universities, 2021, 42(3): 843. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||