

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (8): 1476.doi: 10.7503/cjcu20160264
• Physical Chemistry • Previous Articles Next Articles
XU Kai, LI Yi, ZHAO Nan, DU Wenxiu, ZENG Weiwei, GAO Shuai, CHENG Xiaonong*( ), YANG Juan*(
), YANG Juan*( )
)
Received:2016-04-20
Online:2016-07-19
Published:2016-07-19
Contact:
CHENG Xiaonong,YANG Juan
E-mail:xncheng@mail.ujs.edu.cn;yangjuan6347@mail.ujs.edu.cn
Supported by:CLC Number:
TrendMD:
XU Kai,LI Yi,ZHAO Nan,DU Wenxiu,ZENG Weiwei,GAO Shuai,CHENG Xiaonong,YANG Juan. Synthesis of Hollow PtNi/Graphene Cellular Monolith Catalysts and Their Electrochemical Performance†[J]. Chem. J. Chinese Universities, 2016, 37(8): 1476.
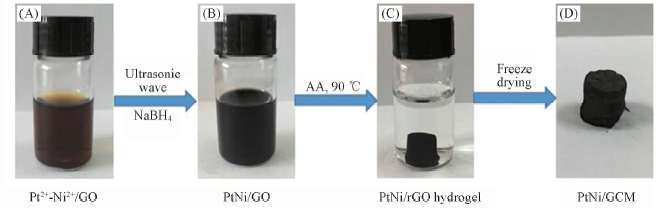
Fig.1 Schematic showing the synthesis mechanism of alloyed PtNi nanoparticles supported by GCM using sonochemical-assisted synthesis and gelatinization method
| Catalyst | Onset potential/V | Half-wave potential/V | Mass activity/(A·mg-1 Pt) | Specific activity/(mA·cm-2) | ||||
|---|---|---|---|---|---|---|---|---|
| Initial | Final | Initial | Final | Initial | Final | Initial | Final | |
| PtNi/GCM | 0.606 | 0.588 | 0.551 | 0.490 | 0.2311 | 0.1014 | 0.2164 | 0.0950 |
| Pt/C | 0.574 | 0.537 | 0.494 | 0.354 | 0.1999 | 0.0347 | 0.2854 | 0.0495 |
Table 1 Comparison of durability(after 30000 cycles) of PtNi/GCM and commercial Pt/C catalysts
| Catalyst | Onset potential/V | Half-wave potential/V | Mass activity/(A·mg-1 Pt) | Specific activity/(mA·cm-2) | ||||
|---|---|---|---|---|---|---|---|---|
| Initial | Final | Initial | Final | Initial | Final | Initial | Final | |
| PtNi/GCM | 0.606 | 0.588 | 0.551 | 0.490 | 0.2311 | 0.1014 | 0.2164 | 0.0950 |
| Pt/C | 0.574 | 0.537 | 0.494 | 0.354 | 0.1999 | 0.0347 | 0.2854 | 0.0495 |
| [1] |
Fang, Q. , Shen, Y. , Chen, B. , Chem. Eng. J., 2015, 264, 753- 771
doi: 10.1063/1.4932381 URL |
| [2] | Wang, X. , Zhuang, J. , Peng, Q , Xun, W. , Li Y., D. , Inorg. Chem., 2007, 45( 17), 6661- 6665 |
| [3] |
Hu C., G. , Cheng H., H. , Zhao, Y. , Hu, Y. , Liu, Y. , Dai L., M. , Qu L., T. , Adv. Mater., 2012, 24( 40), 5493- 5498
doi: 10.1002/adma.201200498 URL pmid: 22886893 |
| [4] |
Xia, W. , Mahmood, A. , Liang Z., B. , Zuo R., Q. , Guo S., J. , Angew. Chem. Int. Ed., 2016, 128( 8), 2698- 2726
doi: 10.1007/978-3-319-25907-9_7 URL |
| [5] |
何卫, 邹亮亮, 周毅, 卢向军, 李媛, 张校刚, 杨辉. 高等学校化学学报, 2012, 33( 1), 133- 138
doi: 10.3969/j.issn.0251-0790.2012.01.022 |
|
He, W. , Zou L., L. , Zhou, Y. , Lu X., J. , Li, Y. , Zhang X., G. , Yang, H. , Chem. J. Chinese Universities, 2012, 33( 1), 133- 138
doi: 10.3969/j.issn.0251-0790.2012.01.022 |
|
| [6] | Singh, V. , Joung, D. , Zhai, L. , Das, S. , Khondaker S., I. , Seal, S. , Prog. Mater. Sci., 2011, 56( 8), 1178- 1271 |
| [7] | Hu C., G. , Song, L. , Zhang Z., P. , Chen, N. , Feng Z., H. , Qu L., T. , Energy Environ. Sci., 2015, 8( 1), 31- 54 |
| [8] |
Kakati, N. , Maiti, J. , Lee S., H. , Jee S., H. , Viswanathan B., Y. , Young, S. , Chem. Rev., 2014, 114( 24), 12397- 12429
doi: 10.1021/cr400389f URL pmid: 25537109 |
| [9] | Fu S., F , Zhu C., Z. , Du, D. , Lin Y., H. , ACS Appl. Mater. Interfaces, 2015, 7( 25), 13842- 13848 |
| [10] | Bao, X. , National Sci. Rev., 2015, 2( 2), 137- 204 |
| [11] | Zhou Y., Z. , Yen C., H. , Fu, S. , Yang, G. , Zhu, C. , Du, D. , Wo P., C. , Cheng X., N. , Yang, J. , Wai C., M. , Lin Y., H. , Green Chem. Adv., 2015, 17, 3552- 3560 |
| [12] | Han B., H. , Carlton C., E. , Kongkanand, A. , Kulreja R., S. , Theobald B., R. , Gan, L. , Rachel, O. , Strasser, P. , Wagner F., T. , Yang S., H. , Energy Environ. Sci., 2015, 8( 1), 258- 266 |
| [13] | Cheng H., H. , Ye M., H. , Zhao, F. , Hu C., G. , Zhao, Y. , Liang, Y. , Chen, N. , Chen S., L. , Jiang, L. , Qu L., T. , Adv. Mater., 2016, 28( 17), 3305- 3312 |
| [14] |
Li Y., G. , Wu Y., Y. , J. Am. Chem. Soc., 2009, 131( 6), 5851- 5857
doi: 10.1021/ja907262c URL pmid: 19831351 |
| [15] |
Antonietti, M. , Fechler, N. , Fellinger, T. , Chem. Mater., 2014, 26( 1), 196- 210
doi: 10.1002/chin.201409239 URL |
| [16] | Zhao, F. , Zhao, Y. , Cheng H., H. , Qu L., T. , Angew. Chem. Int. Ed., 2015, 54( 49), 14951- 14955 |
| [17] |
Flannigan D., J. , Suslick K., S. , Nature, 2005, 434( 7029), 52- 55
doi: 10.1038/nature03361 URL pmid: 15744295 |
| [18] | Zhou Y., Z. , Yang G., H. , Pan H., B. , Zhu C., Z. , Fu S., F. , Shi Q., R. , Du, D. , Cheng X., N. , Yang, J. , Wai C., M. , Lin Y., H. , J. Mater.Chem. A, 2015, 3( 16), 8459- 8465 |
| [19] |
El-Shafei A., A. , J. Electroanal. Chem., 1999, 471( 2), 89- 95
doi: 10.1016/S0022-0728(99)00235-1 URL |
| [20] | 李志伟, 陶小军, 程亚敏. 无机化学学报, 2005, 21( 8), 1261- 1264 |
| Li Z., W. , Tao X., J. , Cheng Y., M. , Chinese J. Inorg. Chem., 2005, 21( 8), 1261- 1264 | |
| [21] |
黄建业, 王峰会, 侯绍行, 赵翔. 高等学校化学学报, 2014, 35( 9), 1968- 1974
doi: 10.7503/cjcu20140205 |
|
Huang J., Y. , Wang F., H. , Hou S., H. , Zhao, X. , Chem. J. Chinese Universities, 2014, 35( 9), 1968- 1974
doi: 10.7503/cjcu20140205 |
|
| [22] |
Bang J., H. , Suslick K., S. , J. Am. Chem. Soc., 2007, 129( 8), 2242- 2243
doi: 10.1021/ja0676657 URL pmid: 17269775 |
| [23] |
Sun, X. , Jiang K, Z. , Zhang, N. , Guo S., J. , Huang X., Q. , ACS Nano, 2015, 9( 7), 7634- 7640
doi: 10.1021/acsnano.5b02986 URL pmid: 26172056 |
| [24] |
Ding, L. , Li, Q. , Zhou D., D. , Cui, H. , An, H. , Zhai J., P. , J. Electroanal. Chem., 2011, 668( 668), 44- 50
doi: 10.1016/j.jelechem.2011.12.018 URL |
| [25] |
Guo S., J. , Sun S., H. , J. Am. Chem. Soc., 2012, 134( 5), 2492- 2495
doi: 10.1021/ja2104334 URL pmid: 22279956 |
| [26] |
Qin, Y. , Zhang, X. , Dai, X. , Small, 2016, 12( 4), 524- 533
doi: 10.1007/s12274-016-0986-0 URL |
| [27] | Mukerjee, S. , Srinivasan, S. , Soriaga M., P. , J. Electrochem. Soc., 1995, 142( 5), 1409- 1422 |
| [28] |
Stephens I., E. , Bondarenko A., S. , Grø, nbjerg U. , Rossmeisl, J. , Chorkendorff, I. , Energy Environ. Sci., 2012, 5( 5), 6744- 6762
doi: 10.1039/C2EE03590A URL |
| [29] | Shao Y., Y. , Yin G., P. , Wang J., J. , Shi P., F. , J. Electrochem. Soc., 2006, 153( 7), A1261- A1265 |
| [30] | Gonzá, lez E. , Arbiol, J. , Puntes V., F. , Science, 2011, 334( 6061), 1377- 1378 |
| [1] | FAN Jianling, TANG Hao, QIN Fengjuan, XU Wenjing, GU Hongfei, PEI Jiajing, CEHN Wenxing. Nitrogen Doped Ultra-thin Carbon Nanosheet Composited Platinum-ruthenium Single Atom Alloy Catalyst for Promoting Electrochemical Hydrogen Evolution Process [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220366. |
| [2] | CHENG Qian, YANG Bolong, WU Wenyi, XIANG Zhonghua. S-doped Fe-N-C as Catalysts for Highly Reactive Oxygen Reduction Reactions [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220341. |
| [3] | CHU Yuyi, LAN Chang, LUO Ergui, LIU Changpeng, GE Junjie, XING Wei. Single-atom Cerium Sites Designed for Durable Oxygen Reduction Reaction Catalyst with Weak Fenton Effect [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220294. |
| [4] | GU Yu, XI Baojuan, LI Jiangxiao, XIONG Shenglin. Structure Regulation of Single-atom Catalysts in Oxygen Reduction Reactions [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220036. |
| [5] | JIN Xiangyuan, ZHANG Libing, SUN Xiaofu, HAN Buxing. Electrocatalytic CO2 Reduction over Single-atom Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220035. |
| [6] | WONG Honho, LU Qiuyang, SUN Mingzi, HUANG Bolong. Rational Design of Graphdiyne-based Atomic Electrocatalysts: DFT and Self-validated Machine Learning [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220042. |
| [7] | ZHANG Xiaoyu, XUE Dongping, DU Yu, JIANG Su, WEI Yifan, YAN Wenfu, XIA Huicong, ZHANG Jianan. MOF-derived Carbon-based Electrocatalysts Confinement Catalyst on O2 Reduction and CO2 Reduction Reactions [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210689. |
| [8] | HE Yujing, LI Jiale, WANG Dongyang, WANG Fuling, XIAO Zuoxu, CHEN Yanli. Zinc-based Activated Fe/Co/N Doped Biomass Carbon Electrocatalysts with High Oxygen Reduction Activity [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220475. |
| [9] | WU Yaqiang, LIU Siming, JIN Shunjin, YAN Yongqing, WANG Zhao, CHEN Lihua, SU Baolian. Synthesis of Zn-Doped NiCoP Catalyst with Porous Double-layer Nanoarray Structure and Its Electrocatalytic Properties for Hydrogen Evolution [J]. Chem. J. Chinese Universities, 2021, 42(8): 2483. |
| [10] | YANG Tao, YAO Huiying, LI Qing, HAO Wei, CHI Lifeng, ZHU Jia. Density Functional Theoretical Studies on the Promising Electrocatalyst of M-BHT(M=Co or Cu) for CO2 Reduction to CH4 [J]. Chem. J. Chinese Universities, 2021, 42(4): 1268. |
| [11] | MA Jun, ZHONG Yang, ZHANG Shanshan, HUANG Yijun, ZHANG Lipeng, LI Yaping, SUN Xiaoming, XIA Zhenhai. Design and Theoretical Calculation of Heteroatoms Doped Graphdiyne Towards Efficiently Catalyzing Oxygen Reduction and Evolution Reactions [J]. Chem. J. Chinese Universities, 2021, 42(2): 624. |
| [12] | WANG Yuemin, MENG Qinglei, WANG Xian, GE Junjie, LIU Changpeng, XING Wei. Enhancement of Performance of Fe-N-C Catalysts by Copper and Sulfur Doping for the Oxygen Reduction Reaction [J]. Chem. J. Chinese Universities, 2020, 41(8): 1843. |
| [13] | ZHAO Guoqing, YUAN Zhao, WANG Lian, GUO Zhuo. Preparation of Ni2P/N, S co-Doped Reduced Graphene Oxide Composites and Their Electrocatalytic Properties for Hydrogen Evolution† [J]. Chem. J. Chinese Universities, 2020, 41(7): 1575. |
| [14] | JIANG Yuanyuan, LI Boyu, LU Yizhong, WU Tongshun, HAN Dongxue. Oxygen Evolution Reaction Electrocatalytic Performance Analysis of Electroless Plated Ni-Bx [J]. Chem. J. Chinese Universities, 2020, 41(12): 2774. |
| [15] | YIN Wenjing, LIU Xiao, QIAN Huidong, ZOU Zhiqing. Preparation and Oxygen Reduction Performance of Fe, N co-Doped arbon Nanoplate with High Density of Active Sites† [J]. Chem. J. Chinese Universities, 2019, 40(7): 1480. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||