

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (9): 1596.doi: 10.7503/cjcu20160253
• Articles: Inorganic Chemistry • Previous Articles Next Articles
GONG Wenpeng, TIAN Chaoqiang, DU Xiaogang, YANG Shuijin*( )
)
Received:2016-04-18
Online:2016-09-10
Published:2016-08-26
Contact:
YANG Shuijin
E-mail:yangshuijin@163.com
Supported by:CLC Number:
TrendMD:
GONG Wenpeng, TIAN Chaoqiang, DU Xiaogang, YANG Shuijin. Synthesis and Characterization of Composite H6P2Mo18O62/Zn(BDC)(Bipy)0.5 and Its Adsorption Activity for Methylene Blue†[J]. Chem. J. Chinese Universities, 2016, 37(9): 1596.
| Sample | Element | w(%) | n(Mo):n(P) |
|---|---|---|---|
| H6P2Mo18O62 | P | 2.13 | 9.1 |
| Mo | 60.02 | ||
| Zn | 0 | ||
| H6P2Mo18O62/Zn(BDC)(Bipy)0.5 | P | 1.74 | 9.0 |
| Mo | 48.59 | ||
| Zn | 20.61 |
Table 1 Elemental analysis of Zn(BDC)(Bipy)0.5 and H6P2Mo18O62/Zn(BDC)(Bipy)0.5
| Sample | Element | w(%) | n(Mo):n(P) |
|---|---|---|---|
| H6P2Mo18O62 | P | 2.13 | 9.1 |
| Mo | 60.02 | ||
| Zn | 0 | ||
| H6P2Mo18O62/Zn(BDC)(Bipy)0.5 | P | 1.74 | 9.0 |
| Mo | 48.59 | ||
| Zn | 20.61 |
| Sample | SBET/(m2·g-1) | Vtotal/(cm3·g-1) | D/nm |
|---|---|---|---|
| Zn(BDC)(Bipy)0.5 | 710.6 | 0.32 | 1.79 |
| H6P2Mo18O62/Zn(BDC)(Bipy)0.5 | 249.9 | 0.12 | 1.92 |
Table 2 Structural properties of Zn(BDC)(Bipy)0.5 and H6P2Mo18O62/Zn(BDC)(Bipy)0.5
| Sample | SBET/(m2·g-1) | Vtotal/(cm3·g-1) | D/nm |
|---|---|---|---|
| Zn(BDC)(Bipy)0.5 | 710.6 | 0.32 | 1.79 |
| H6P2Mo18O62/Zn(BDC)(Bipy)0.5 | 249.9 | 0.12 | 1.92 |
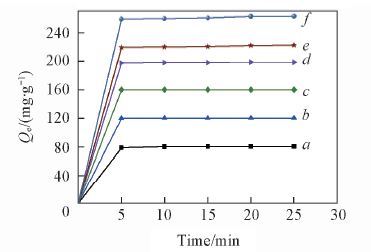
Fig.7 Effect of initial mass concentration on adsorption capacities for MB Initial mass concentration of MB/(mg·mL-1): a. 40;b. 60; c. 80; d. 100; e. 120; f. 140.
| Kinetics model | Initial concentration of MB/ (mg·L-1) | Qe,exp/ (mg·g-1) | Qe,cal/ (mg·g-1) | K1/min-1 or K2/ (g·mg-1·min-1) | R2 |
|---|---|---|---|---|---|
| Pseudo-first-order model | 90 | 179.43 | 1.1693 | 0.09343 | 0.9661 |
| 100 | 198.64 | 1.7124 | 0.10918 | 0.9669 | |
| Pseudo-second-order model | 90 | 179.43 | 179.53 | 0.000021 | 1 |
| 100 | 198.64 | 198.81 | 0.0000157 | 1 |
Table 3 Parameters of pseudo-first-order and pseudo-second-order adsorption kinetics model in different initial concentrations
| Kinetics model | Initial concentration of MB/ (mg·L-1) | Qe,exp/ (mg·g-1) | Qe,cal/ (mg·g-1) | K1/min-1 or K2/ (g·mg-1·min-1) | R2 |
|---|---|---|---|---|---|
| Pseudo-first-order model | 90 | 179.43 | 1.1693 | 0.09343 | 0.9661 |
| 100 | 198.64 | 1.7124 | 0.10918 | 0.9669 | |
| Pseudo-second-order model | 90 | 179.43 | 179.53 | 0.000021 | 1 |
| 100 | 198.64 | 198.81 | 0.0000157 | 1 |
| Temperature/K | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| Qm/(mg·g-1) | KL/(L·mg-1) | RL | R2 | KF/(mg·g-1) | n | R2 | |
| 293 | 277.78 | 0.00319 | 0.7337 | 0.9986 | 244.84 | 13.18 | 0.9606 |
| 303 | 275.48 | 1.9621 | 0.00459 | 0.9893 | 194.54 | 7.76 | 0.9137 |
| 313 | 273.22 | 0.7205 | 0.01242 | 0.9695 | 157.66 | 5.76 | 0.7916 |
Table 4 Isotherm parameters of the adsorption of methylene blue by H6P2Mo18O62/Zn(BDC)(Bipy)0.5 at different temperature
| Temperature/K | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| Qm/(mg·g-1) | KL/(L·mg-1) | RL | R2 | KF/(mg·g-1) | n | R2 | |
| 293 | 277.78 | 0.00319 | 0.7337 | 0.9986 | 244.84 | 13.18 | 0.9606 |
| 303 | 275.48 | 1.9621 | 0.00459 | 0.9893 | 194.54 | 7.76 | 0.9137 |
| 313 | 273.22 | 0.7205 | 0.01242 | 0.9695 | 157.66 | 5.76 | 0.7916 |
| ΔGo/(kJ·mol-1) | ΔHo/(kJ·mol-1) | ΔSo/(J·mol-1·K-1) | ||
|---|---|---|---|---|
| 293 K | 303 K | 313 K | ||
| -14.87 | -11.33 | -9.5 | -93.81 | -270.31 |
Table 5 Thermodynamic parameters of adsorption methylene blue onto H6P2Mo18O62/Zn(BDC)(Bipy)0.5
| ΔGo/(kJ·mol-1) | ΔHo/(kJ·mol-1) | ΔSo/(J·mol-1·K-1) | ||
|---|---|---|---|---|
| 293 K | 303 K | 313 K | ||
| -14.87 | -11.33 | -9.5 | -93.81 | -270.31 |
| [1] | Rebane R., Leito I., Yurchenko S., J. Chromatogr. A,2010, 1217, 747—757 |
| [2] | Lin H., Yang S., Ni Y., J. Wood. Chem. Technol., 2010, 30, 118—128 |
| [3] | Gupta V. K., Shilpi A., Saleh T. A., Instrum. Sci. Technol., 2007, 202(4—7), 915—919 |
| [4] | Carneiro P. A., Umbuzeiro G. A., Oliveira D. P., J. Hazard. Mater., 2010, 174, 694—699 |
| [5] | Marin M. O., Prete V. D., Moruno E. G., Food Control., 2009, 20, 298—303 |
| [6] | Farahani M., Rozaimah S. A. S., Soraya H., Procedia. Environ. Sci., 2011, 10, 203—208 |
| [7] | Crini G., Bioresour. Technol., 2006, 97, 1061—1085 |
| [8] | Gupta V. K., Saleh T. A., Environ. Sci. Pollut. Res., 2013, 20(5), 2828—2843 |
| [9] | Mittal A., Kaur D., Malviya A., Mittal J., Gupta V. K., J. Colloid Interface Sci., 2009, 337, 345—354 |
| [10] | Gupta V. K., Agarwal S., Saleh T. A., J. Hazard. Mater., 2011, 185, 17—23 |
| [11] | Zhang W. J., Zhou C. J., Zhou W. C., Bull. Environ. Contam. Toxicol., 2011, 87, 86—90 |
| [12] | Wang H. L., Zhao Y., Ma L. K., Fan P. H., Xu C. B., Jiao C. L., Lin A. J., Chem. J. Chinese Universities,2016, 37(2), 335—341 |
| (王会丽, 赵越, 马乐宽, 范鹏浩, 徐从斌, 焦春磊, 林爱军. 高等学校化学学报, 2016, 37(2), 335—341) | |
| [13] | Hasan Z., Jhung S. H., J. Hazard Mater., 2015, 283, 329—339 |
| [14] | Tong M., Liu D., Yang Q., Devautour V. S., Maurin G., Zhong C., J. Mater. Chem. A,2013, 1, 8534—8537 |
| [15] | Haque E., Lo V., Minett A. I., J. Mater. Chem. A,2014, 2, 193—203 |
| [16] | Dai M., Su X. R., Wang X., Wu B., Ren Z. G., Zhou X., Lang J. P., Cryst. Growth & Des., 2014, 14, 240—248 |
| [17] | Chen B. L., Liang C. D., Yang J., Contreras D. S., Clancy Y. L., Lobkousky E. B., Yaghi O. M., Dai S., Angew. Chem. Int. Ed., 2006, 45, 1390—1393 |
| [18] | Liang D. D., Liu S. X., Ma F. J., Adv. Synth. Catal., 2011, 353, 733—742 |
| [19] | Ma F. J., Liu S. X., Liang D. D., Eur. J. Inorg. Chem., 2010, 3756—3761 |
| [20] | Sha J. Q., Peng J., Lan Y. Q., Inorg. Chem., 2008, 47, 5145—5153 |
| [21] | Yan A., Yao X. S., Wang E. B., Chem. Eur. J., 2014, 20, 6927—6933 |
| [22] | Yi F. Y., Zhu W., Sun Z. M., Chem. Commun., 2015, 51, 3336—3339 |
| [23] | Fu H., Li Y. G., Lu Y., Chen W. L., Wu Q., Meng J. X., Wang X. L., Zhang Z. M., Wang E. B., Crystal. Growth & Des., 2011, 11, 458—465 |
| [24] | Sun C. Y., Liu S. X., Liang D. D., J. Am. Chem. Soc., 2009, 131, 1883—1888 |
| [25] | Park D. R., Song J. H., Lee S. H., Applied Catalysis A: General,2008, 349, 222—228 |
| [26] | Wang E. B., Gao L.H., Liu J. F., Acta Chim. Sinica,1988, 46, 757—762 |
| (王恩波, 高丽华, 刘景福. 化学学报, 1988, 46, 757—762) | |
| [27] | Liu Y.W., Liu S. M., Liu S. X., J. Am. Chem. Soc., 2015, 137, 12697—12703 |
| [28] | Liu H., Zhao Y. G., Zhang Z. J., Nijem N., Chabal Y. J., Adv. Funct. Mater., 2011, 21, 4754—4762 |
| [29] | Canioni R., Marchal R. C., Secheresse F., Horcajada P., Serre C., J. Mater. Chem., 2011, 21, 1226—1233 |
| [30] | Lin S., Song Z., Che G., Microporous Mesoporous Mater., 2014, 193(2), 27—34 |
| [31] | Luo H. M., Zhao X., Chen N. L., Ion Exchange And Adsorption,2011, 27(2), 152—159 |
| [32] | Eftekhari S., Habibi Y. A., Sohrabnezhand S., Hazard. Mater., 2010, 78, 349—355 |
| [33] | Li R., Ren X. Q., Zhao J. S., J. Mater. Chem. A, 2014, 2, 2168—2173 |
| [34] | Liu X. X., Gong W. P., Luo J., Appl. Surf. Sci., 2016, 362, 517—524 |
| [35] | Chen S. H., Zhang J., Zhang C. L., Desalination,2010, 252, 149—156 |
| [36] | Langmuir I., J. Am. Chem. Soc., 1918, 40, 1361—1403 |
| [37] | Hameed B. H., J. Hazard. Mater., 2008, 154, 204—212 |
| [1] | JIANG Hongbin, DAI Wenchen, ZHANG Rao, XU Xiaochen, CHEN Jie, YANG Guang, YANG Fenglin. Research on Co3O4/UiO-66@α-Al2O3 Ceramic Membrane Separation and Catalytic Spraying Industry VOCs Waste Gas [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220025. |
| [2] | HAO Honglei, MENG Fanyu, LI Ruoyu, LI Yingqiu, JIA Mingjun, ZHANG Wenxiang, YUAN Xiaoling. Biomass Derived Nitrogen Doped Porous Carbon Materials as Adsorbents for Removal of Methylene Blue in Water [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220055. |
| [3] | WANG Hongning, HUANG Li, QING Jiang, MA Tengzhou, JIANG Wei, HUANG Weiqiu, CHEN Ruoyu. Activation of Biochar from Cattail and the VOCs Adsorption Application [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210824. |
| [4] | CHEN Xiaolu, YUAN Zhenyan, ZHONG Yingchun, REN Hao. Preparation of Triphenylamine Based PAF-106s via Mechanical Ball Milling and C2 Hydrocarbons Adsorption Property [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210771. |
| [5] | MENG Xianglong, YANG Ge, GUO Hailing, LIU Chenguang, CHAI Yongming, WANG Chunzheng, GUO Yongmei. Synthesis of Nano-zeolite and Its Adsorption Performance for Hydrogen Sulfide [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210687. |
| [6] | TAN Lejian, ZHONG Xuanshu, WANG Jin, LIU Zongjian, ZHANG Aiying, YE Lin, FENG Zengguo. Low Critical Dissolution Temperature Behavior of β⁃Cyclodextrin and Its Application in the Preparation of β⁃Cyclodextrin Sheet Crystal with Ordered Nano⁃channel [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220405. |
| [7] | WANG Shoubai, WU Xiuming, SHU Chen, ZHONG Min, HUANG Wei, YAN Deyue. Gas Separation Performance of Polyimide Homogeneous MembranesContaining tert-Butyl Groups [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220357. |
| [8] | TIAN Xiaokang, ZHANG Qingsong, YANG Shulin, BAI Jie, CHEN Bingjie, PAN Jie, CHEN Li, WEI Yen. Porous Materials Inspired by Microbial Fermentation: Preparation Method and Application [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220216. |
| [9] | ZHENG Meiqi, MAO Fangqi, KONG Xianggui, DUAN Xue. Layered Double Hydroxides as Sorbent for Remediation of Radioactive Wastewater [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220456. |
| [10] | TANG Yuanhui, LI Chunyu, LIN Yakai, ZHANG Chunhui, LIU Ze, YU Lixin, WANG Haihui, WANG Xiaolin. Dissipative Particle Dynamics Simulation of the Effect of Polymer Chain Rigidity on Membranes Formation by Nonsolvent Induced Phase Separation Process [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220169. |
| [11] | LIU Xueguang, YANG Xiaoshan, MA Jingjing, LIU Weisheng. Separating Methyl Blue Selectively from the Mixture of Dyes by Europium Metal-organic Frameworks [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210715. |
| [12] | MA Jianxin, LIU Xiaodong, XU Na, LIU Guocheng, WANG Xiuli. A Multi-functional Zn(II) Coordination Polymer with Luminescence Sensing, Amperometric Sensing, and Dye Adsorption Performance [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210585. |
| [13] | ZHANG Chi, SUN Fuxing, ZHU Guangshan. Synthesis, N2 Adsorption and Mixed-matrix Membrane Performance of Bimetal Isostructural CAU-21 [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210578. |
| [14] | CHANG Shuqing, XIN Xu, HUANG Yaqi, ZHANG Xincong, FU Yanghe, ZHU Weidong, ZHANG Fumin, LI Xiaona. Pyroelectrically-induced Catalytic Performance of Zr-based MOF Under Cold-hot Alternation [J]. Chem. J. Chinese Universities, 2021, 42(8): 2558. |
| [15] | SU Yingli, REN Haisheng, LI Xiangyuan. Application of New Nonequilibrium Solvation Theory in Electronic Spectra of Organic Dyes [J]. Chem. J. Chinese Universities, 2021, 42(7): 2254. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||