

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (9): 1716.doi: 10.7503/cjcu20160037
• Physical Chemistry • Previous Articles Next Articles
WEI Bing, CUI Yu, MA Shoutao, YANG Quanning, SUN Guoxin*( )
)
Received:2016-01-15
Online:2016-09-10
Published:2016-08-17
Contact:
SUN Guoxin
E-mail:chm_sungx@ujn.edu.cn
Supported by:CLC Number:
TrendMD:
WEI Bing, CUI Yu, MA Shoutao, YANG Quanning, SUN Guoxin. Extraction Interfacial Properties of La3+ from HNO3 System with TODGA†[J]. Chem. J. Chinese Universities, 2016, 37(9): 1716.
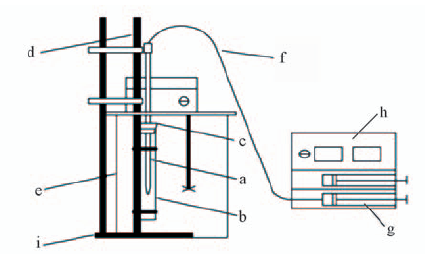
Fig.1 Schematic diagram of drop volume device a. Drop volume tube; b. sample tube; c. rubber plug with a groove; d. sample tube rack; e. glass thermostatic water bath; f. injection line; g. syringe; h. trace injection pump; i. retort stand
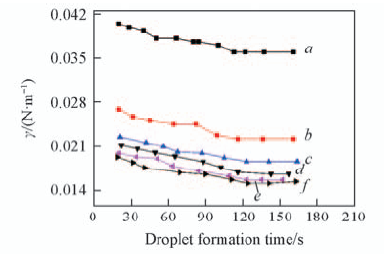
Fig.2 Effect of droplet formation time on the interfacial tension c L a 3 + =1×10-3 mol/L; c HN O 3 =1 mol/L; c NaN O 3 =1 mol/L; T=298 K. c(TODGA)/(mol·L-1): a. 0; b. 0.01; c. 0.05; d. 0.10; e. 0.15; f. 0.20.
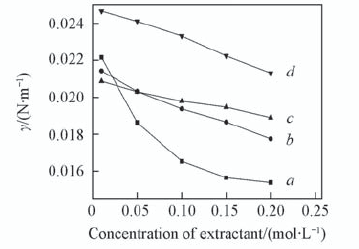
Fig.6 Effect of extractant concentration on the interfacial tension with different types of diluents c L a 3 + =1×10-3 mol/L; c HN O 3 =1 mol/L; c NaN O 3 =1 mol/L; T=298 K; t=150 s. a. TODGA/octane; b. TODGA/cyclohexane; c. TODGA/benzene;d. TODGA/toluene.
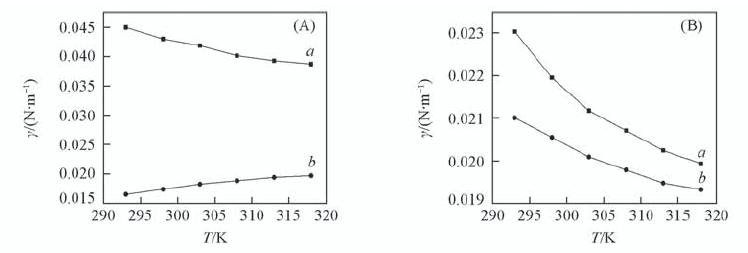
Fig.7 Effect of temperature on the interfacial tension c L a 3 + =1×10-3 mol/L; c HN O 3 =1 mol/L; c NaN O 3 =1 mol/L; t=150 s. (A) a. Octane; b. 0.01 mol/L TODGA/octane.(B) a. Benzene; b. 0.1 mol/L TODGA/benzene.
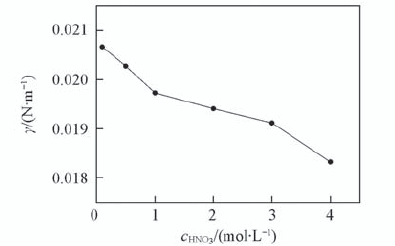
Fig.8 Effect of HNO3 concentration on the interfacial tension with 0.1 mol/L TODGA/octane c L a 3 + =1×10-3 mol/L; c NaN O 3 =1 mol/L; T=298 K; t=150 s.
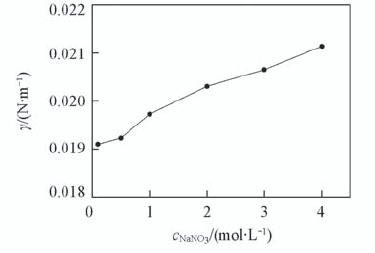
Fig.9 Effect of NaNO3 concentration on the interfacial tension with 0.1 mol/L TODGA/octane c L a 3 + =1×10-3 mol/L; c HN O 3 =1 mol/L; T=298 K; t=150 s.
| [1] | Chen L., Li Y., Wang Z. W., Peng Z. Y., Yang Z. M., Yuan L. H., Feng W., Chem. J. Chinese Universities,2015, 36(8), 1485—1490 |
| (陈龙, 李艳, 王真文, 彭智勇, 杨泽明, 袁立华, 冯文. 高等学校化学学报,2015, 36(8), 1485—1490) | |
| [2] | Dutta S., Mohapatra P. K., Manchanda V. K., Appl. Radiat. Isot., 2011, 69, 158—162 |
| [3] | Huang S., Ding S. D., Jin Y. D., Ma L. J., Xia C. Q., Li S. J., Wu Y. X., Huang H., Chem. J. Chinese Universities,2014, 35(7), 1369—1378 |
| (黄松, 丁松东, 金永东, 马利建, 夏传琴, 李首建, 吴宇轩, 黄璜. 高等学校化学学报,2014, 35(7), 1369—1378) | |
| [4] | Zhu W. B., Ye G. A., Li F. F., Li H. R., J. Radioanal. Nucl. Chem., 2013, 298, 1749—1755 |
| [5] | Zhu W. B., Ye G. A., Li F. F., Li H. R., Journal of Nuclear and Radiochemistry ,2013, 35(4), 229—235 |
| (朱文彬, 叶国安, 李峰峰, 李会蓉. 核化学与放射化学 ,2013, 35(4), 229—235) | |
| [6] | Sun G. X., Yang Y. H., Sun S. X., Shen J. R., Chem. J. Chinese Universities,1995, 16(11), 1677—1679 |
| (孙国新, 杨永会, 孙思修, 沈静兰. 高等学校化学学报,1995, 16(11), 1677—1679) | |
| [7] | Wu D. B., Xiong Y., Li D. Q., J. Colloid Interface Sci., 2005, 290, 235—240 |
| [8] | Zhang K., Du C. L., Li R. X., Wu D. C., Journal of Sichuan University,2004, 36(2), 58—61 |
| (张科, 杜宗良, 李瑞霞, 吴大诚. 四川大学学报,2004, 36(2), 58—61) | |
| [9] | Fang Y. W., Wang J. H., Chem. J. Chinese Universities,1996, 17(2), 298—299 |
| (方奕文, 王镜和. 高等学校化学学报,1996, 17(2), 298—299) | |
| [10] | Joseph D. B., Michael J. N., Raymond R. D., Derek Y. C. C., Rico F. T., J. Colloid Interface Sci., 2015, 454, 226—237 |
| [11] | M. Soledade C S. Santos., Ana Paula Paiva., J. Colloid Interface Sci., 2014, 413, 78—85 |
| [12] | Jiang R., Zhao J. X., You Y., Acta Chim. Sinica,2005, 63(2), 126—130 |
| (姜蓉, 赵剑曦, 游毅. 化学学报,2005, 63(2), 126—130) | |
| [13] | Guo L. S., Cao P. Y., Journal of Dalian University,1988, 3(6), 41—42 |
| (郭立山, 曹佩玉. 大连大学学报,1988, 3(6), 41—42) | |
| [14] | Xiong H. J., Li W., Zhang Y. Y., Journal of Wuhan Polytechnic University,2007, 26(3), 42—44 |
| (熊会军, 李炜, 张誉赢. 武汉工业学院学报,2007, 26(3), 42—44) | |
| [15] | Zhao J. M., Sun X. B., Li W., J. Colloid Interface Sci., 2006, 294, 429—435 |
| [16] | Sun G. X., Liu M., Cui Y., Yuan M. L., Yin S. H., Solvent Extr. Ion Exch., 2010, 28(4), 482—494 |
| [17] | Lando J. L., Oakley H. T., J. Colloid Interfce Sci., 1967, 25(4), 526—530 |
| [18] | Wilkinson M. C., Kidwell R. L., J. Colloid Interfce Sci., 1971, 35(1), 114—119 |
| [19] | Manori J., Thomas L. B., J. Phys. Chem. B., 2009, 113(34), 11662—11671 |
| [20] | Nave S., Molodo G., Madic C., Testard F., Solvent Extr. Ion Exch., 2004, 22(4), 527—551 |
| [21] | Zhang Y. J., Cui. Y., Hu Y. F., Sun G. X., Chin. J. Inorg. Chem., 2010, 26(4), 663—667 |
| (张艳菊, 崔玉, 胡玉芬, 孙国新. 无机化学学报, 2010, 26(4), 663—667) | |
| [22] | Yang B. H., Uranium ore Processing Technology, Beijing Research Institute of Chemical Engineering and Metallurgy, Beijing, 2002, 352 |
| (杨伯和. 铀矿加工工艺学, 北京: 核工业北京化工冶金研究院, 2002, 352) | |
| [23] | Liu J. J., Wang W. W., Li D. Q., Colloids Surf. A: Physicochem. Eng., 2007, 311, 124—130 |
| [24] | Sarikhani K., Jeddi K., Thompson R. B., Park C. B., Chen P., Thermochimica Acta,2015, 609, 1—6 |
| [25] | Zhang Y. J., Cui Y., Liu M., Yin S. H., Sun G. X., Chin. J. Inorg. Chem., 2008, 24(12) , 2047—2050 |
| (张艳菊, 崔玉, 刘敏, 尹少宏, 孙国新. 无机化学学报, 2008, 24(12) , 2047—2050) | |
| [26] | Gujar R. B., Ansari S. A., Murali M. S., Mohapatra P. K., Manchanda V. K., J. Radioanal. Nucl. Chem., 2010, 284, 377—385 |
| [27] | Zhu W. B., Ye G. A., Li F. F., Jiang D. X., Li H. R., J. Nucl. Radiochem., 2011, 33(6), 328—334 |
| (朱文彬, 叶国安, 李峰峰, 蒋德祥, 李会蓉. 核化学与放射化学, 2011, 33(6), 328—334) | |
| [28] | Liu X. L., Sun G. X., Cai X. C., Yang X. F., Li Y. X., Sun Z. M., Cui Y., J. Radioanal. Nucl. Chem., 2015, 306, 549—553 |
| [1] | TANG Quanjun, LIU Yingxin, MENG Rongwei, ZHANG Ruotian, LING Guowei, ZHANG Chen. Application of Single-atom Catalysis in Marine Energy [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220324. |
| [2] | BI Gening, XIAO Xiaohua, LI Gongke. Development and Validation of Multiple Physical Fields Coupling Model for Microwave-assisted Extraction [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210739. |
| [3] | ZHENG Haijiao, JIANG Liyan, JIA Qiong. Arginine Functionalized Magnetic Nanoparticles and Its Application in Phosphopeptides Enrichment [J]. Chem. J. Chinese Universities, 2021, 42(3): 717. |
| [4] | DENG Jiewei, YANG Yunyun, LIN Li, LUAN Tiangang. Rapid Classification of Daphniamagna and Daphnia pulex by Surface-coated Probe Nanoelectrospray Ionization Mass Spectrometry Lipidomics [J]. Chem. J. Chinese Universities, 2020, 41(9): 2011. |
| [5] | LI Xiaoqian, ZHANG Hua, LU Haijian, LIU Chang, LIU Qinglong, MA Xiayu, FANG Yuanping, LIANG Dapeng. Mechanism of Photocatalytic Degradation of Rhodamine B by TiO2 Nanowire Array with Internal Extraction Electrospray Ionization Mass Spectrometry [J]. Chem. J. Chinese Universities, 2020, 41(9): 2003. |
| [6] | WANG Xiaoru,ZHANG Na,XING Jun. Preparation and Application of Melamine Imprinted Material Using Itaconic Acid as Multidentate Functional Monomer [J]. Chem. J. Chinese Universities, 2020, 41(7): 1521. |
| [7] | ZHANG Xiaofei, WU Lie, LI Shanshan, ZHU Manyu, CHENG Xiaowei, JIANG Xiu’e. Effect of Phase Behavior of Phospholipids on Lipid Membrane Damage Induced by Graphene Oxide † [J]. Chem. J. Chinese Universities, 2020, 41(4): 661. |
| [8] | PIAO Huilan,MA Pinyi,QIN Zucheng,JIANG Yanxiao,SUN Ying,WANG Xinghua,SONG Daqian. Determination of Triazine Herbicides from Fruit Juice Samples Using Effervescence Assisted Microextraction Method Based on Acidic Ionic Liquid Packed Syringe [J]. Chem. J. Chinese Universities, 2020, 41(2): 228. |
| [9] | WANG Tianqi,YU Qiongwei,FENG Yuqi. Analysis of Imidazole Propionic Acid in Serum of Patients with Type 2 Diabetes Based on NiO@SiO2 Solid-phase Extraction Coupled with Liquid Chromatography-Mass Spectrometry † [J]. Chem. J. Chinese Universities, 2020, 41(2): 262. |
| [10] | CHENG Xiankun, HOU Xue, TIAN Huan, ZHANG Menglong, WEI Hao, ZHAO Zhuo. Synthesis of Macrocyclic Thiacrown Ethers and Their Selective Extraction for Ag(Ⅰ) and Tl(Ⅰ) † [J]. Chem. J. Chinese Universities, 2019, 40(9): 1881. |
| [11] | WU Rong,WANG Huimin,CAO Jun,CHEN Luyao,SU Erzheng. Simultaneous Extraction of Salidroside and Tyrosol from Rhodiolarosea L. Using Tailor-made Deep Eutectic Solvents† [J]. Chem. J. Chinese Universities, 2019, 40(5): 918. |
| [12] | ZHANG Lirong,WANG Zhipeng,LIU Ying,XU Chao,CHEN Jing,DING Songdong. Extraction of Trivalent Americium and Europiumfrom Nitric Acid Solution with Di(2-ethylhexyl)dithiophosphinic Acid† [J]. Chem. J. Chinese Universities, 2019, 40(1): 1. |
| [13] | MENG Zihui,WANG Yifei,XIE Tengsheng,CHEN Wei,QIU Lili,XUE Min. Molecularly Imprinted Hollow Spheres for the Solid Phase Extraction of Protein† [J]. Chem. J. Chinese Universities, 2019, 40(1): 62. |
| [14] | SU Rui,WANG Yihan,CHEN Changbao,SUN Xiuli,LIU Shuying,YANG Hongmei. Determination of Brominated Flame Retardants in Environmental Water by Microwave-assisted Ionic Liquid/ionic Liquid Dispersive Liquid-liquid Microextraction Coupled with DART-Orbitrap Mass Spectrometry† [J]. Chem. J. Chinese Universities, 2018, 39(9): 1934. |
| [15] | XUE Yun, CAO Meng, YANG Xue, XU Yanlu, YAN Yongde, JI Debin, MA Fuqiu. Electrochemical Extraction of La and Zn-La Alloy Codeposition in Chloride System† [J]. Chem. J. Chinese Universities, 2018, 39(6): 1145. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||