

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (6): 1145.doi: 10.7503/cjcu20170605
• Articles: Inorganic Chemistry • Previous Articles Next Articles
XUE Yun1,2,*( ), CAO Meng1, YANG Xue2, XU Yanlu2, YAN Yongde2, JI Debin2, MA Fuqiu1
), CAO Meng1, YANG Xue2, XU Yanlu2, YAN Yongde2, JI Debin2, MA Fuqiu1
Received:2017-09-06
Online:2018-06-10
Published:2018-05-23
Contact:
XUE Yun
E-mail:E-mail: xueyunhrbeu@163.com
Supported by:CLC Number:
TrendMD:
XUE Yun, CAO Meng, YANG Xue, XU Yanlu, YAN Yongde, JI Debin, MA Fuqiu. Electrochemical Extraction of La and Zn-La Alloy Codeposition in Chloride System†[J]. Chem. J. Chinese Universities, 2018, 39(6): 1145.
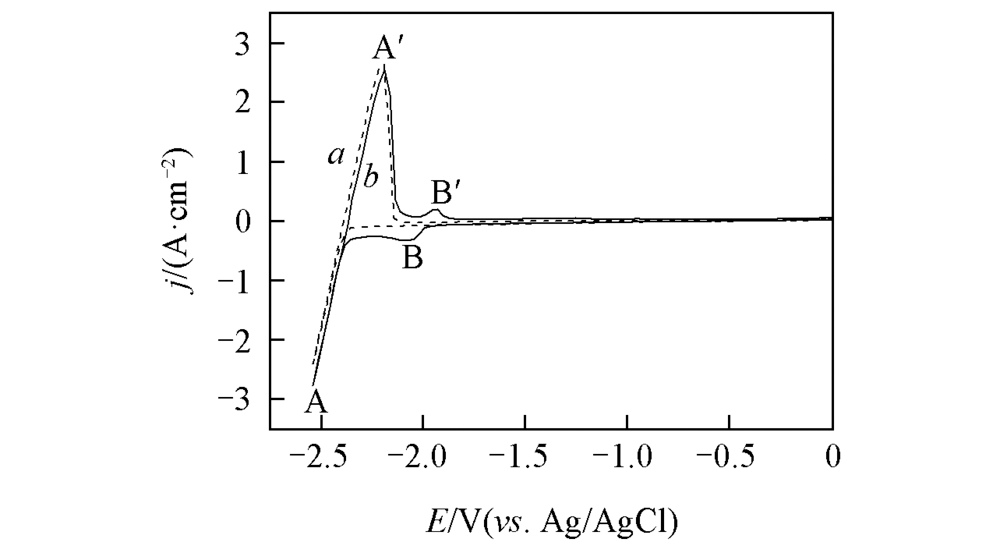
Fig.1 Cyclic voltammograms curves on a Mo electrode(S=0.322 cm2) in the LiCl-KCl melt salt sytem before(a) and after(b) the addition of 1.3×10-4 mol/mL LaCl3 at 723 K(scan rate: 0.2 V/s)
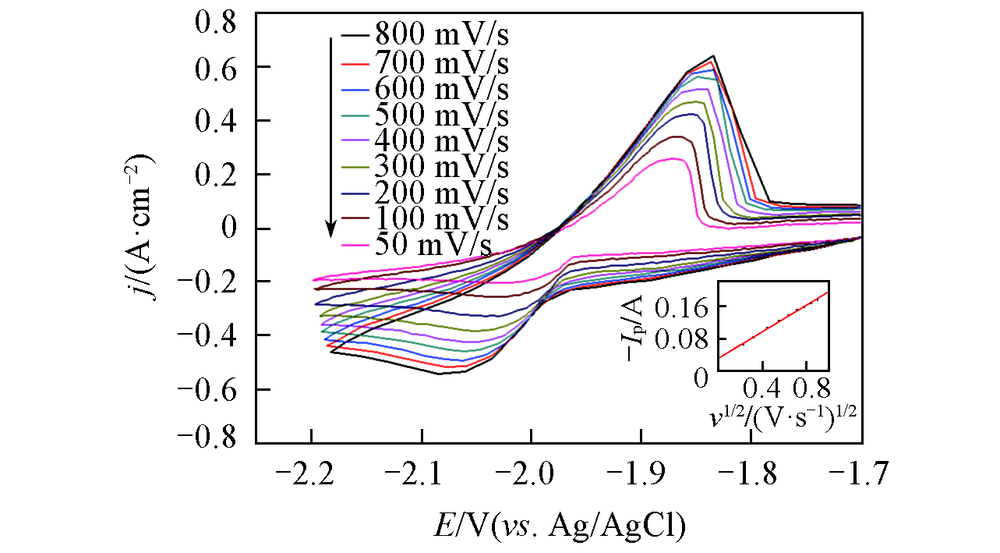
Fig.2 Cyclic voltammograms curves of LiCl-KCl-LaCl3(1.3×10-4 mol/mL) melt salt sytem on a Mo electrode(S=0.322 cm2) at different scan rates at 723 KInset: relationship of the cathodic peak current with the square root of the sweep rate.
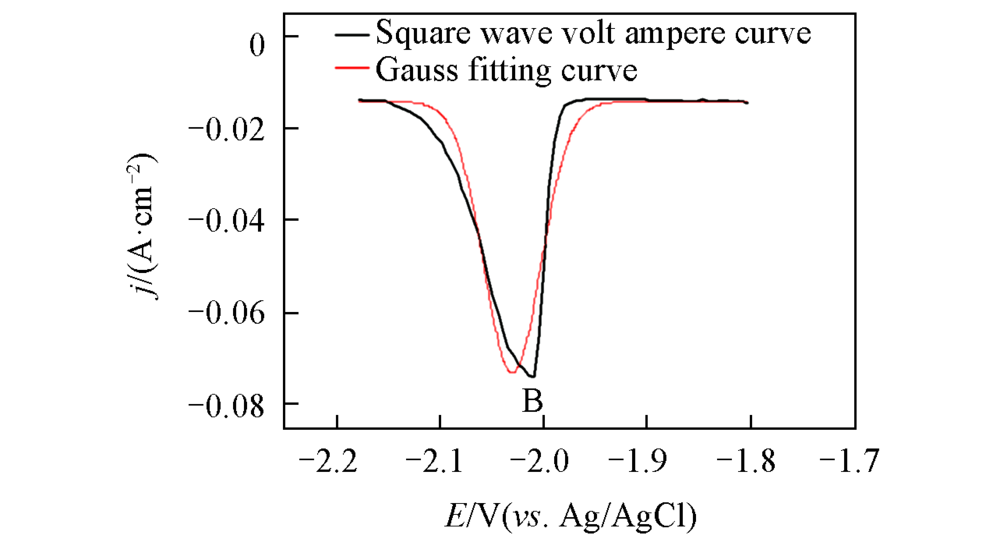
Fig.3 Square wave voltammogram obtained on a Mo electrode(S=0.322 cm2) in the LiCl-KCl-LaCl3(1.3×10-4 mol/mL) melt salt system at 723 KPulse height: 25 mV; potential step: 1 mV; frequency: 10 Hz.
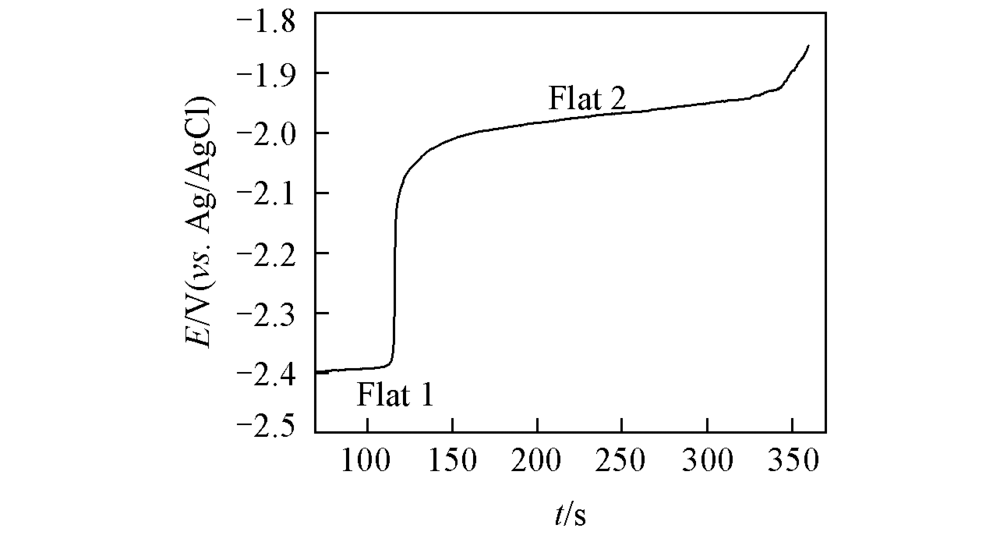
Fig.4 Open-circuit chronopotentiogram for a Mo electrode(S=0.322 cm2) in the LiCl-KCl-LaCl3(1.3×10-4 mol/mL) melt salt system at 723 K[deposition potential: -2.4 V(vs. Ag/AgCl); deposition time: 70 s]
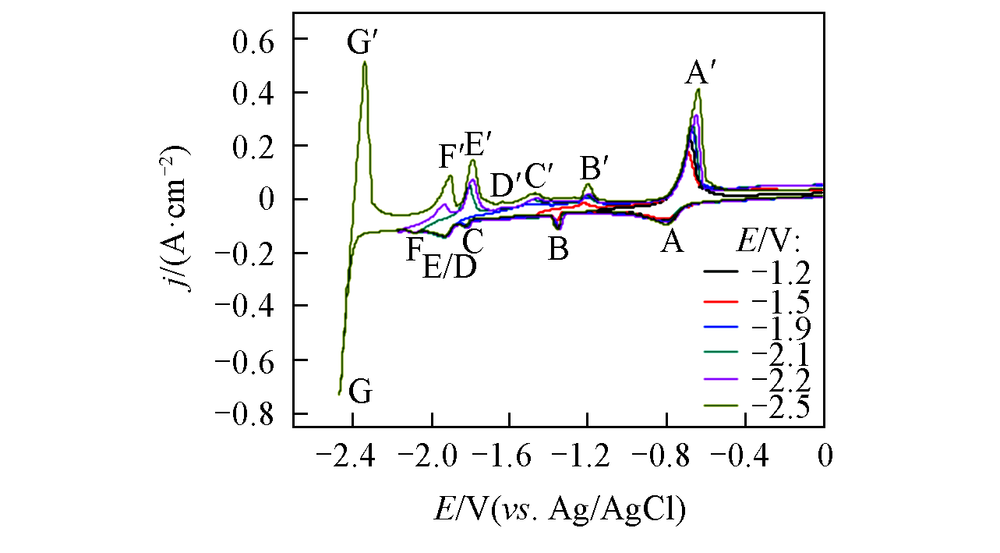
Fig.5 Cyclic voltammograms curves on a Mo electrode(S=0.322 cm2) in LiCl-KCl-ZnCl2(1.2×10-4 mol/mL)-LaCl3(1.3×10-4 mol/mL) melt salt system at different cathodic limits at 723 K(scan rate: 0.1 V/s)
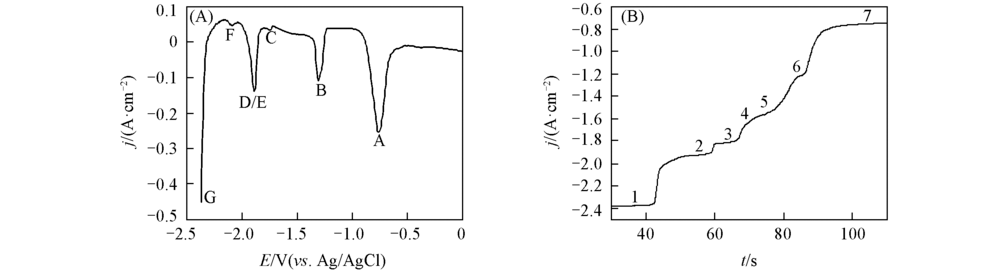
Fig.6 Square wave voltammogram(A) and open-circuit chronopotentiogram(B) on a Mo electrode in the LiCl-KCl-ZnCl2(1.2×10-4 mol/mL)-LaCl3(1.3×10-4 mol/mL) melt salt system at 723 K(A) Pulse height: 25 mV; potential step: 1 mV; frequency: 10 Hz; (B) deposition potential: -2.4 V(vs Ag/AgCl); deposition time: 30 s.
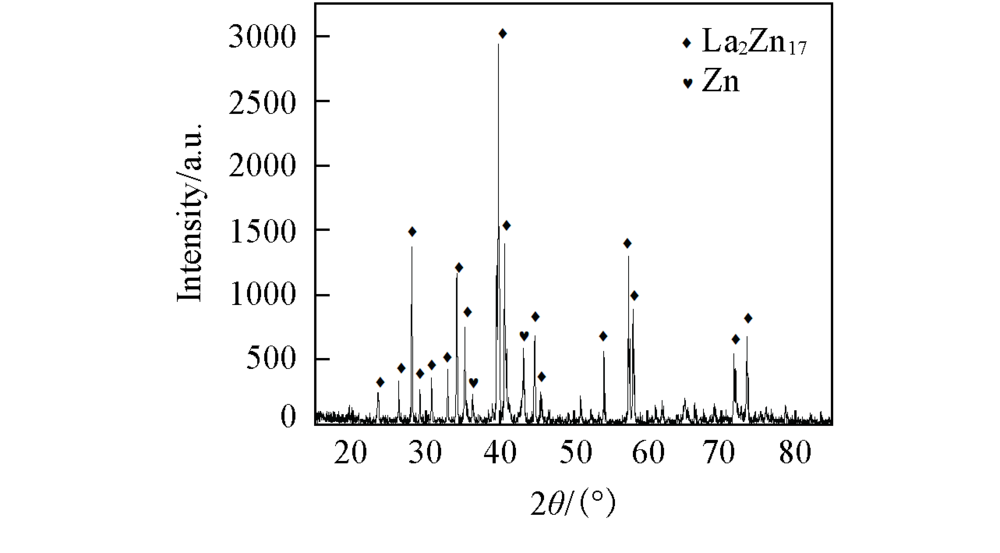
Fig.7 XRD patterns of the deposits obtained under galvanostatic electrolysis at a Mo electrode in LiCl-KCl-ZnCl2(8.8×10-4 mol/mL)-LaCl3(1.8×10-4 mol/mL) melt salt system at 923 K at 2 A for 2.5 h
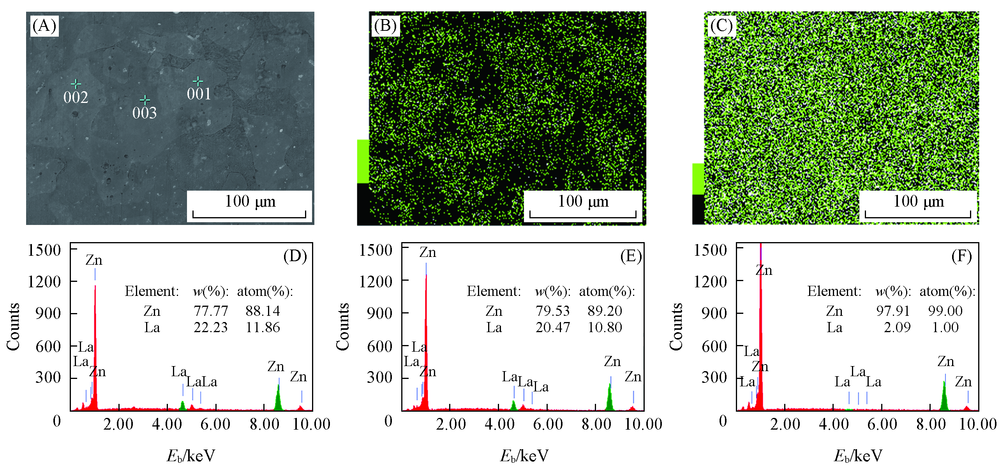
Fig.8 Cross-sectional SEM images(A—C) and EDS analysis(D—F) of the deposit obtained by galvanostatic electrolysis at -6.25 A/cm2 for 2.5 h on a Mo electrode in LiCl-KCl-ZnCl2(8.8×10-4 mol/mL)-LaCl3(1.8×10-4 mol/mL) melt salt system at 923 K(A) SEM image; (B) mapping of La; (C) mapping of Zn; (D)—(F) represent points 001, 002, 003 in (A), respec-tively.
| [1] | Salvatores M., Palmiotti G .S., Prog. Part. Nucl. Phys., 2011, 66, 144—166 |
| [2] | Cometto M., Wydler P., Chawla R., Ann. Nucl. Energy., 2008, 35, 1447—1460 |
| [3] | Castrillejo Y., Bermejo M. R., Arocas P. D., Martinez A. M., J. Electroanal. Chem., 2005, 579(2), 343—358 |
| [4] | Bermejo M. R., Barrado E., Martínez A. M., Castrillejo Y., J. Electroanal. Chem., 2008, 617(1), 85—100 |
| [5] | Massot L, Chamelot P, Taxil P., Electrochim. Acta, 2005, 50(28), 5510—5517 |
| [6] | Gibilaro M., Massot L., Chamelot P., Cassayre L., Taxil P., Electrochim. Acta, 2009, 55(1), 281—287 |
| [7] | Liu K., Liu Y. L., Yuan L. Y., Li Y., He H., Yang Z. Y., Zhao X. L., Chai Z. P., Shi W. Q., Electrochim. Acta., 2014, 129, 401—409 |
| [8] | Liu Y. L., Yan Y. D., Han W., Zhang M. L., Yuan L. Y., Lin R. S., Ye G. A., He H., Chai Z. F., Shi W. Q., Electrochim. Acta, 2013, 114, 180—188 |
| [9] | Kurata M., Sakamura Y., Hijikata T., Kinnoshita K., J. Nucl. Mater., 1995, 227(1), 110—121 |
| [10] | Moriyama H., Yamana H., Nishikawa S., Wakayama Y., Moritani K., Mitsugashira T., Yamana H., J. Alloy. Compd., 1998, 271, 587—591 |
| [11] | Serp J., Allibert M., Terrier A. L., J. Electrochem. Soc., 2005, 152(3), C167—C172 |
| [12] | Aspinall H.C., Chemistry of the f-Block Elements, CRC Press, Boca Raton, 2001 |
| [13] | Vandarkuzhali S., Gogoi N., Ghosh S., Prabhakara R., Nagarajan K., Electrochim. Acta, 2012, 59, 245—255 |
| [14] | Tang H., Pesic B., Electrochim. Acta, 2014, 119, 120—130 |
| [15] | Chen L. J., Zhang M. L., Han W., Yan Y. D., Cao P., Chem. J. Chinese Universities, 2012, 33(2), 327—330 |
| (陈丽军, 张密林, 韩伟, 颜永得, 曹鹏. 高等学校化学学报, 2012, 33(2), 327—330) | |
| [16] | Li M., Li W., Han W., Zhang M. L., Yan Y. D., Chem. J. Chinese Universities, 2014, 35(12), 2662—2667 |
| (李梅, 李炜, 韩伟, 张密林, 颜永得. 高等学校化学学报, 2014, 35(12), 2662—2667) | |
| [17] | Sang W. K., Do H. A., Eung H. K., Ho G. A., J. Ind. Eng. Chem., 2009, 1(15), 86—91 |
| [18] | Sylvie D., Sébastien J., Davide R., Electrochim. Acta, 2014, 144, 383—390 |
| [19] | Tang H., Yan Y. D., Zhang M. L., Xue Y., Zhang Z. J., He W. C., He H., Acta Phys.-Chim. Sin., 2013, 29(8), 1698—1704 |
| [20] | Ramaley L., Krasue M. S., J. Anal. Chem., 1969, 41(11), 1362—1365 |
| [21] | Chamelot P., Lafage B., Taxil P., Electrochim. Acta, 1997, 43(5/6), 607—616 |
| [22] | Hamel C., Chamelot P., Laplace A., Walle E., Dugne O., Taxil P., Electrochim. Acta, 2007, 52(12), 3995—4003 |
| [23] | Kuznetsov S. A., Hayashi H., Minato K., Gaune-Escard M., J. Nucl. Med., 2005, 344(1—3), 169—172 |
| [24] | Berche A., Benigni P., Rogez J., Record M.C., Intermetallics, 2014, 45, 46—52 |
| [25] | Berche A., Benigni P., Rogez J., Record M. C., Thermochim. Acta, 2011, 523(1/2), 70—78 |
| [26] | Liu Y. L., Ye G. A., Liu K., Yuan L. Y., Chai Z. F., Shi W. Q., Electrochim. Acta, 2015, 168, 206—215 |
| [27] | Berche A., Marinellib F., Mikaeliana G., Rogez J., Record M. C., J. Alloy. Compd., 2009, 475, 79—85 |
| [28] | Berche A., Benigni P., Rogez J., Record M .C., Calphad., 2012, 36, 65—70 |
| [1] | ZHANG Qingbin, KONG Xianggui, WANG Xin, CHENG Cheng. NaYF4∶Yb3+,Er3+ Upconverting Nanoparticles Surface Ligand Exchange in Ternary Mixture Solvent and Optical Properties† [J]. Chem. J. Chinese Universities, 2014, 35(2): 224. |
| [2] | LEI Bing-Fu, LIU Ying-Liang, TANG Gong-Ben, YE Ze-Ren, SHI Chun-Shan . Unusual Afterglow Properties of Tm3+Doped Yttrium Oxysulfide [J]. Chem. J. Chinese Universities, 2003, 24(5): 782. |
| [3] | LEI Bing-Fu, LIU Ying-Liang, TANG Gong-Ben, YE Ze-Ren, SHI Chun-Shan. A New Orange-red Long-lasting Phosphor Material Y2O2S∶ Sm3+ [J]. Chem. J. Chinese Universities, 2003, 24(2): 208. |
| [4] | JIA Zhi-Hong, LI Hong, YE Ze-Ren, SHI Chun-Shan . Gd3+→Eu2+ Energy Transfer in BaLiF3:Eu,Gd [J]. Chem. J. Chinese Universities, 2002, 23(3): 349. |
| [5] | SU Hai-Quan, JIA Zhi-Hong, SHI Chun-Shan . Vibronic Transitions and Site Substitution of Eu2+ and Codoped Ions in KMgF3:Eu-X (X=Ce, Cr, Gd, Cu) System [J]. Chem. J. Chinese Universities, 2001, 22(10): 1620. |
| [6] | SU Hai-Quan, JIA Zhi-Hong, SHI Chun-Shan . Site Substitution and Energy Transfer of Eu2+ and Ce3+ in KMgF3:Eu and KMgF3:Eu-Ce Single Crystals [J]. Chem. J. Chinese Universities, 2001, 22(7): 1081. |
| [7] | ZHANG Xian-Ming, SU Hai-Quan, YE Ze-Ren, JIA Zhi-Hong, ZANG Chun-Yu, SHI Chun-Shan . Energy Transfer from Ce3+ to Eu2+ and Electron Transfer from Ce3+ to Eu3+ in BaY2F8:Ce, Eu Systems [J]. Chem. J. Chinese Universities, 2001, 22(3): 358. |
| [8] | LI Hong, JIA Zhi-Hong, FENG Shou-Hua, SHI Chun-Shan. Hydrothermal Synthesis and Spectral Properties of the Eu Doped KZnF3 [J]. Chem. J. Chinese Universities, 2000, 21(12): 1805. |
| [9] | ZHANG Xian-Ming, SU Hai-Quan, ZANG Chun-Yu, WANG Fu-Shan, SHI Chun-Shan. Energy Transfer from Ce3+to Eu2+in KZnF3 [J]. Chem. J. Chinese Universities, 1999, 20(9): 1334. |
| [10] | LIU Xing-Zhi, XUE Hong, ZHAO Jin, SONG Yu-Lin, ZANG Shu-Liang . Synthesis and Crystal Structure of In[(C2H5O)2PS2]3 [J]. Chem. J. Chinese Universities, 1999, 20(2): 196. |
| [11] | LI Xiao-Jing, ZHANG Shan-Rong, ZHANG Shu-Gong, PEI Feng-Kui . Studies on the Interaction of Rare Earth Ions with BSA [J]. Chem. J. Chinese Universities, 1999, 20(1): 127. |
| [12] | SONG Fu-Quan, MU Ping, BI Lan-Rong, WANG Zhao-Yu . Synthesis and Structural Studies of Tetramethyldisiloxanyl Dicyclopentadienyl Lanthanide Chlorides [J]. Chem. J. Chinese Universities, 1998, 19(9): 1436. |
| [13] | LING Da-Ren, LIU Yu-Cheng, ZHENG Zu-Ying . Bidisperse Pore Diffusivity of Eu3+ Ion in Porous Ion Exchange Resins [J]. Chem. J. Chinese Universities, 1998, 19(2): 169. |
| [14] | JING Hai-Qiang, WU Guo-Qing, DU Bao-Shi . Preparation of Phosphors MBPO5: Eu2+ and MBPO5: Yb2+ in Air [J]. Chem. J. Chinese Universities, 1997, 18(9): 1425. |
| [15] | AN Yong-Lin, SHI Chun-Shan, NI Jia-Zhuan. Single Crystal Growth and Spectroscopic Properties of KZnF3: Eu3+ [J]. Chem. J. Chinese Universities, 1997, 18(9): 1429. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||