

Chem. J. Chinese Universities ›› 2015, Vol. 36 ›› Issue (12): 2409.doi: 10.7503/cjcu20150549
• Organic Chemistry • Previous Articles Next Articles
KONG Lushi1, TIAN Guofeng1, LI Feng1, QI Shengli1,2,*( ), WU Dezhen1,2
), WU Dezhen1,2
Received:2015-07-14
Online:2015-12-10
Published:2015-11-17
Contact:
QI Shengli
E-mail:qisl@mail.buct.edu.cn
Supported by:CLC Number:
TrendMD:
KONG Lushi, TIAN Guofeng, LI Feng, QI Shengli, WU Dezhen. Synthesis and Properties of Triphenylamine-containing Asymmetrical Perylene Diimides†[J]. Chem. J. Chinese Universities, 2015, 36(12): 2409.
| Compd. | Elemental analysis(%, calcd.) | UV-Vis(CHCl3), λmax /nm | IR(KBr), | |||||
|---|---|---|---|---|---|---|---|---|
| C | H | N | ||||||
| PDI-ATPA | 80.62(80.52) | 5.41(5.27) | 5.63(5.73) | 304, 460, 491, 527 | 2958, 2925, 2856, 1700, 1660, 1591, 1497, 1340, 750 | |||
| PDI-TATPA | 78.12(78.38) | 5.15(5.02) | 5.64(5.74) | 310, 464, 493, 530 | 2954, 2925, 2858, 1697, 1656, 1589, 1502, 1340, 744 | |||
Table 1 Elemental analysis, UV-Vis, and IR data of PDI-ATPA and PDI-TATPA
| Compd. | Elemental analysis(%, calcd.) | UV-Vis(CHCl3), λmax /nm | IR(KBr), | |||||
|---|---|---|---|---|---|---|---|---|
| C | H | N | ||||||
| PDI-ATPA | 80.62(80.52) | 5.41(5.27) | 5.63(5.73) | 304, 460, 491, 527 | 2958, 2925, 2856, 1700, 1660, 1591, 1497, 1340, 750 | |||
| PDI-TATPA | 78.12(78.38) | 5.15(5.02) | 5.64(5.74) | 310, 464, 493, 530 | 2954, 2925, 2858, 1697, 1656, 1589, 1502, 1340, 744 | |||
| Compd. | 1H NMR(400 MHz, CDCl3), δ |
|---|---|
| PDI-ATPA | 8.71—8.59(m, 8H, PhH), 7.30(t, 4H, J=7.8 Hz, PhH), 7.21(d, 8H, J=7.4 Hz, PhH), 7.07(t, 2H, J=7.2 Hz, PhH), 4.16(t, 2H, J=8.0 Hz, CH2), 1.97(s, 1H, CH), 1.42—1.32(m, 8H, CH2), 0.89(t, 6H, J=7.4 Hz, CH3) |
| PDI-TATPA | 8.41—8.13(m, 24H, PhH), 7.41—7.34(m, 12H, PhH), 4.15(t, 6H,J=8.6 Hz, CH2), 1.92(s, 3H, CH), 1.42—1.33(m, 24H, CH2), 0.91(t, 18H, J=7.2 Hz, CH3) |
Table 2 1H NMR data of PDI-ATPA and PDI-TATPA
| Compd. | 1H NMR(400 MHz, CDCl3), δ |
|---|---|
| PDI-ATPA | 8.71—8.59(m, 8H, PhH), 7.30(t, 4H, J=7.8 Hz, PhH), 7.21(d, 8H, J=7.4 Hz, PhH), 7.07(t, 2H, J=7.2 Hz, PhH), 4.16(t, 2H, J=8.0 Hz, CH2), 1.97(s, 1H, CH), 1.42—1.32(m, 8H, CH2), 0.89(t, 6H, J=7.4 Hz, CH3) |
| PDI-TATPA | 8.41—8.13(m, 24H, PhH), 7.41—7.34(m, 12H, PhH), 4.15(t, 6H,J=8.6 Hz, CH2), 1.92(s, 3H, CH), 1.42—1.33(m, 24H, CH2), 0.91(t, 18H, J=7.2 Hz, CH3) |
| Compd. | λabs. max/nm | λem. max/nm | εmax/(cm-1·L·mol-1) | λedge/nm | △λ/nm | φb | |
|---|---|---|---|---|---|---|---|
| AsPDA-Ⅰ | 522 | 535 | 164200 | 555 | 2.23 | 13 | 0.99 |
| PDI-ATPA | 527 | 537 | 109000 | 587 | 2.11 | 10 | 0.02 |
| PDI-TATPA | 530 | 539 | 122000 | 612 | 2.03 | 9 | 0.01 |
Table 3 Optical data of AsPDA-Ⅰ, PDI-ATPA and PDI-TATPA
| Compd. | λabs. max/nm | λem. max/nm | εmax/(cm-1·L·mol-1) | λedge/nm | △λ/nm | φb | |
|---|---|---|---|---|---|---|---|
| AsPDA-Ⅰ | 522 | 535 | 164200 | 555 | 2.23 | 13 | 0.99 |
| PDI-ATPA | 527 | 537 | 109000 | 587 | 2.11 | 10 | 0.02 |
| PDI-TATPA | 530 | 539 | 122000 | 612 | 2.03 | 9 | 0.01 |
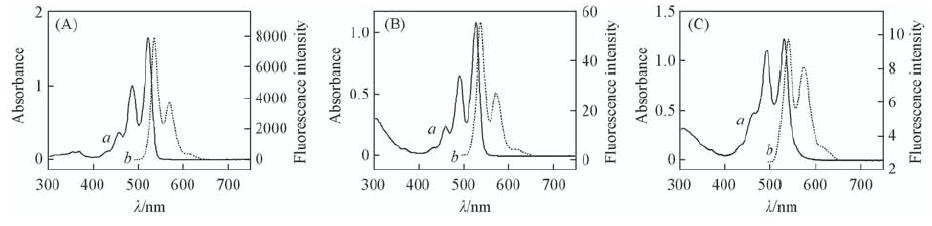
Fig.2 Normalized UV-Vis absorption(a) and flourescence emission spectra(b) of AsPDA-Ⅰ(A), PDI-ATPA(B) and PDI-TATPA(C) in CHCl3 c(CHCl3)=1×10-5 mol/L, λexcit.=485 nm.
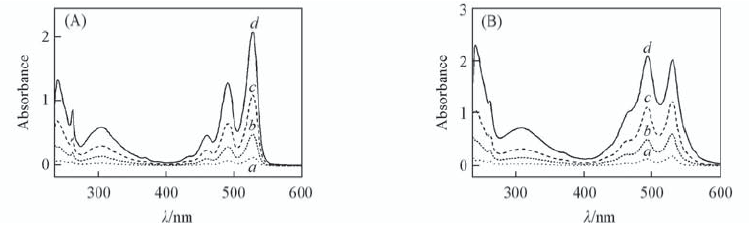
Fig.3 UV-Vis absorption spectra of PDI-ATPA(A) and PDI-TATPA(B) in CHCl3 with different concentrations c/(mol·L-1): a. 1×10-6; b. 5×10-6; c. 1×10-5; d. 2×10-5.
| Compd. | ELUMO/eV | EHOMO/eV | Energy gap/eV | Diploe moment |
|---|---|---|---|---|
| AsPDA-Ⅰ |  |  | 2.79 | 4.1594 |
| PDI-ATPA |  |  | 1.92 | 0.7271 |
| PDI-TATPA |  |  | 2.06 | 0.4360 |
Table 4 Molecular simulation results of AsPDA- I, PDI-ATPA and PDI-TATPA
| Compd. | ELUMO/eV | EHOMO/eV | Energy gap/eV | Diploe moment |
|---|---|---|---|---|
| AsPDA-Ⅰ |  |  | 2.79 | 4.1594 |
| PDI-ATPA |  |  | 1.92 | 0.7271 |
| PDI-TATPA |  |  | 2.06 | 0.4360 |
| [1] | Huang C., Barlow S., Marder S. R., J. Org. Chem., 2011, 76(8), 2386—2407 |
| [2] | Doossel L. F., Kamm V., Howard I. A., Laquai F., Pisula W., Feng X., Muullen K., J. Am. Chem. Soc., 2012, 134(13), 5876—5886 |
| [3] | Wurthner F., Chem. Commun., 2004, 5(14), 1564—1579 |
| [4] | Ramanan C., Smeigh A. L., Anthony J. E., Marks T. J., Wasielewski M. R., J. Am. Chem. Soc., 2011, 134(1), 386—397 |
| [5] | Huang J. C., Li H. Y., Mo X., Shi M. M., Wang M., Chen H. Z., Chem. Res. Chinese Universities,2014, 30(1), 63—67 |
| [6] | Kozma E., Catellani M., Dyes Pigments,2013, 98(1), 160—179 |
| [7] | Wang C. L., Dong H. L., Hu W. P., Liu Y. Q., Zhu D. B., Chem. Rev., 2012, 112(4), 2208—2267 |
| [8] | Belfield K. D., Bondar M. V., Hernandez F. E., Przhonska O. V., J. Phys. Chem. C,2008, 112(14), 5618—5622 |
| [9] | Wang T. Y., Hu X. X., Sun H. Y., Guo J. F., Liu D. Z., Li W., Wang L. C., Zhou X. Q., Chem. J. Chinese Universities,2014, 35(8), 1753—1760 |
| (汪天洋, 胡晓霞, 孙海亚, 郭建峰, 刘东志, 李巍, 王丽昌, 周雪琴. 高等学校化学学报,2014, 35(8), 1753—1760) | |
| [10] | Ding L. W., Yang X. G., Zhong W. B., Liu C., Liu Z. H., Zhang F. J., Chem. J. Chinese Universities,2013, 34(5), 1277—1283 |
| (丁立伟, 杨新国, 钟文斌, 刘存, 刘振辉, 张凤菊. 高等学校化学学报,2013, 34(5), 1277—1283) | |
| [11] | Pasaogullari N., Icil H., Demuth M., Dyes Pigments,2006, 69(3), 118—127 |
| [12] | Nagao Y., Naito T., Abe Y., Misono T., Dyes Pigments,1996, 32(2), 71—83 |
| [13] | Asir S., Demir A. S., Icil H., Dyes Pigments,2010, 84(1), 1—13 |
| [14] | Zhang K., Niu H. J., Wang C., Bai X. D., Lian Y. F., Wang W., J. Electroanal. Chem., 2012, 682(6), 101—109 |
| [15] | Li G. Y., Zhang B., Yan J., Wang Z. G., J. Mater. Chem. A,2014, 2(44), 18881—18888 |
| [16] | Iverson I. K., Tam-Chang S. W., J. Am. Chem. Soc., 1999, 121(24), 5801—5802 |
| [17] | Wang W., Li L. S., Helms G., Zhou H. H., Li A. D., J. Am. Chem. Soc., 2003, 125(5), 1120—1121 |
| [18] | Liu Y., Wang K. R., Guo D. S., Jiang B. P., Adv. Funct. Mater., 2009, 19(14), 2230—2235 |
| [19] | Wang W., Han J. J., Wang L. Q., Li L. S., Shaw W. J., Li A. D., Nano Lett., 2003, 3(4), 455—458 |
| [20] | Rybtchinski B., Sinks L. E., Wasielewski M. R., J. Phys. Chem. A,2004, 108(37), 7497—7505 |
| [21] | Adachi M., Murata Y., Nakamura S., J. Phys. Chem., 1995, 99(39), 14240—14246 |
| [22] | Iverson I. K., Casey S. M., Seo W., Tam-Chang S. W., Pindzola B. A., Langmuir, 2002, 18(9), 3510—3516 |
| [23] | Li X., Sinks L. E., Rybtchinski B., Wasielewski M. R., J. Am. Chem. Soc., 2004, 126(35), 10810—10811 |
| [1] | LI Lin, QI Fenglian, QIU Lili, MENG Zihui. Dynamic Amorphous Photonic Structure Patterns Assembled by Hexagonal Magnetic Nanosheets [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220123. |
| [2] | WU Yushuai, SHANG Yingxu, JIANG Qiao, DING Baoquan. Research Progress of Controllable Self-assembled DNA Origami Structure as Drug Carrier [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220179. |
| [3] | CHEN Xiaolu, YUAN Zhenyan, ZHONG Yingchun, REN Hao. Preparation of Triphenylamine Based PAF-106s via Mechanical Ball Milling and C2 Hydrocarbons Adsorption Property [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210771. |
| [4] | YU Bin, CHEN Xiaoyan, ZHAO Yue, CHEN Weichang, XIAO Xinyan, LIU Haiyang. Graphene Oxide-based Cobalt Porphyrin Composites for Electrocatalytic Hydrogen Evolution Reaction [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210549. |
| [5] | LI Bo, MENG Yuxi, WANG Wenwen, ZANG Hongying. Synthesis and Proton Conductivity of Polynuclear Polyoxothiomolybdate Compound [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210657. |
| [6] | DU Shunfu, WANG Wenjing, EL⁃SAYED El⁃Sayed M., SU Kongzhao, YUAN Daqiang, HONG Maochun. A Chemiluminescent Zirconocene Coordination Tetrahedron [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210628. |
| [7] | XUE Jin, CAO Xiaowei, LIU Yifan, WANG Min. Preparation of Paper Hollow Gold Nanocage SERS Sensor and Its Rapid and Highly Sensitive Detection for miRNAs in Sputum of Patients with Non-small Cell Lung Cancer [J]. Chem. J. Chinese Universities, 2021, 42(8): 2393. |
| [8] | ZHANG Juan, HU Xinyue, WANG Hongbo, LIAN Ying, LE Jinyu, YANG Zihao. Crystal-like Hydrogels Consisting of Parallel Hexahedrons Obtained from the Self-assembly ofβ⁃Cyclodextrin/perfluorononanoic Acid Inclusion Complexes [J]. Chem. J. Chinese Universities, 2021, 42(10): 3187. |
| [9] | TANG Wentao, LI Shengkai, WANG Shen, CHEN Long, CHEN Zhuo. Laser-mediated Enrichment Based Surface Enhanced Raman Analysis [J]. Chem. J. Chinese Universities, 2021, 42(10): 3054. |
| [10] | WANG Linshuo, LI Kunjie, LIU Yumin, ZHAO Ruihong, LI Qing, QIAN Xin, ZHANG Fan, XUE Zhiwei. Theoretical Studies of Triphenyl-s-triazine Groups Regulating Photoelectric Properties of Sensitizing Dyes† [J]. Chem. J. Chinese Universities, 2020, 41(7): 1653. |
| [11] | HOU Chunxi, LI Yijia, WANG Tingting, LIU Shengda, YAN Tengfei, LIU Junqiu. Application of Elastin-like Polypeptides in Supramolecular Assembly † [J]. Chem. J. Chinese Universities, 2020, 41(6): 1163. |
| [12] | BAI Ruonan, LI Qing, QIAO Shanlin, ZHANG Chunhuan, ZHAO Yongsheng. Controlled Preparation and Optical Waveguide Property of 1,4-Dicarbazolidinylbenzene Microwires † [J]. Chem. J. Chinese Universities, 2020, 41(5): 967. |
| [13] | WANG Jun, WANG Tie. Recent Progress in Functional Nanomaterials Based on Self-assembly Technology † [J]. Chem. J. Chinese Universities, 2020, 41(3): 377. |
| [14] | LI Dong,SUN Yinghui,WANG Zhongshun,HUANG Jing,Lü Nan,JIANG Lin. Large-scale Multiplexed Surface Plasmonic Gold Nanostructures Based on Nanoimprint and Self-assembly † [J]. Chem. J. Chinese Universities, 2020, 41(2): 221. |
| [15] | GAO Miaomiao,WANG Chenglong,DOU Hongjing,XU Guoxiong. One-step Self-assembly/polymerization Fabrication and Biomedical Application of Carboplatin@Dextran Nanocarrier† [J]. Chem. J. Chinese Universities, 2019, 40(6): 1301. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||