

Chem. J. Chinese Universities ›› 2015, Vol. 36 ›› Issue (10): 1961.doi: 10.7503/cjcu20150303
• Physical Chemistry • Previous Articles Next Articles
HUANG Qinghong1,2, ZOU Liangliang2, ZHOU Yi2, ZOU Zhiqing2, ZHANG Xiaogang1,*( ), YANG Hui2,*(
), YANG Hui2,*( )
)
Received:2015-04-16
Online:2015-10-10
Published:2015-09-18
Contact:
ZHANG Xiaogang,YANG Hui
E-mail:azhangxg@163.com;yangh@sari.ac.cn
Supported by:CLC Number:
TrendMD:
HUANG Qinghong, ZOU Liangliang, ZHOU Yi, ZOU Zhiqing, ZHANG Xiaogang, YANG Hui. Electrocatalytic Performance of Highly Loaded PtNi Intermetallic Nanoparticles for Oxygen Reduction†[J]. Chem. J. Chinese Universities, 2015, 36(10): 1961.
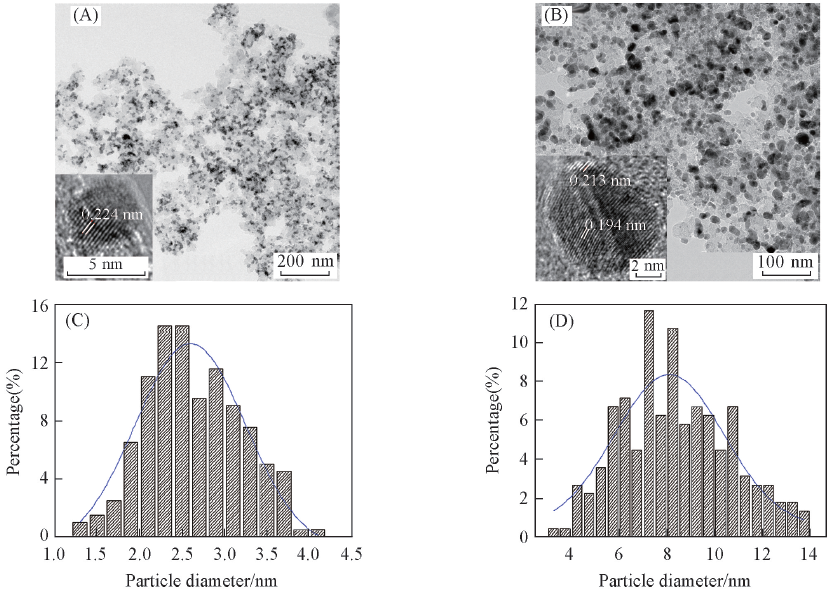
Fig.3 TEM images(A, B) and particles size distributions(C, D) of PtNi/C(A, C) and PtNi/C-450(B, D)Insets in (A) and (B) show the HRTEM images of the corresponding samples.
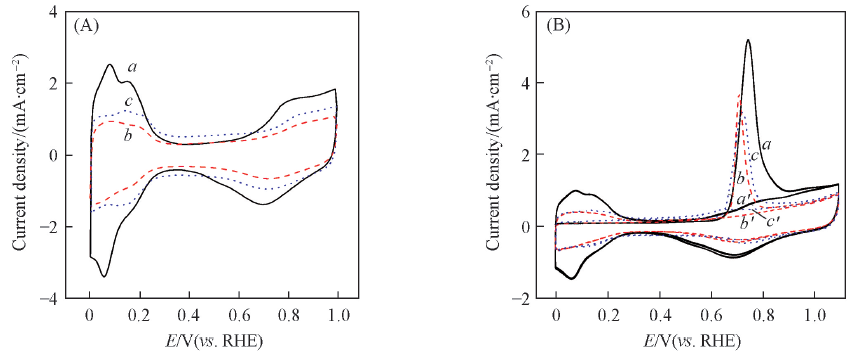
Fig.5 Cyclic voltammograms at a scan rate of 50 mV/s(A) and CO stripping voltammograms at a scan rate of 20 mV/s(B) of the JM Pt/C(a, a'), PtNi/C(b, b') and PtNi/C-450(c, c') catalysts in N2-saturated 0.5 mol/L HClO4 solution(B) a—c. the first cycle; a'—c'. the second cycle.
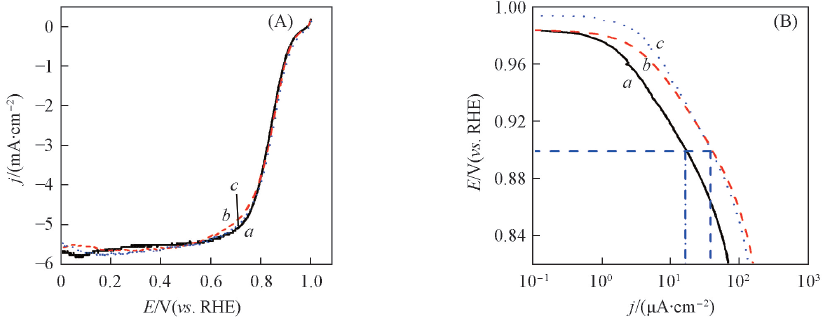
Fig.6 Linear scan voltammograms of the JM Pt/C(a), PtNi/C(b) and PtNi/C-450(c) catalysts in O2-saturated 0.5 mol/L HClO4 solution at a scan rate of 5 mV/s with a rotating speed of 1600 r/min(A) and Tafel plots for the ORR(B)
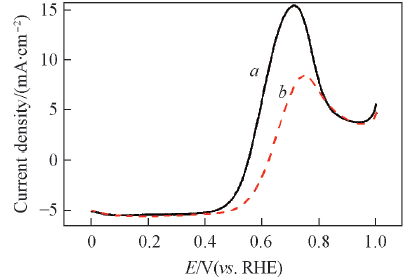
Fig.7 LSVs of JM Pt/C(a) and PtNi/C-450(b) in O2-saturated 0.5 mol/L HClO4 +0.5 mol/L CH3OH solution at a scan rate of 5 mV/s with a rotating speed of 1600 r/minCurrent densities are normalized to the geometric surface area.
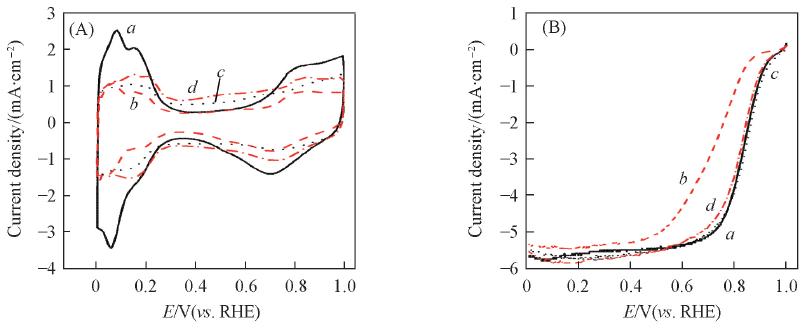
Fig.8 CVs at 50 mV/s in N2-saturated 0.5 mol/L HClO4(A) and LSVs at 5 mV/s with a rotating speed of 1600 r/min in O2-saturated 0.5 mol/L HClO4(B) of the JM Pt/C(a, b) and PtNi/C-450(c, d) before(a, c) and after(b, d) ASTs for 3000 cyclesCurrent densities are normalized to the geometric surface area.
| [1] | Wu J. B., Zhang J. L., Peng Z. M., Yang S. C., Wagner F. T., Yang H., J. Am. Chem. Soc., 2010, 132(14), 4984—4985 |
| [2] | Ghosh T., Leonard B. M., Zhou Q., Disalvo F., J. Chem. Mater., 2010, 22(7), 2190—2202 |
| [3] | Wang Y. E., Tang Y. W., Zhou Y. M., Gao Y., Liu C. P., Lu T. H., Chem. J. Chinese Universities, 2007, 28(4), 743—746 |
| (王彦恩, 唐亚文, 周益明, 高颖, 刘长鹏, 陆天虹. 高等学校化学学报,2007, 28(4), 743—746) | |
| [4] | Liu F., Lee J. Y., Zhou W. J., Small, 2006, 2(1), 121—128 |
| [5] | Huang Q. H., Yang H., Tang Y. W., Lu T. H., Akins D. L., Electrochem. Commun., 2006, 8(8), 1220—1224 |
| [6] | Kang J., Wang R. F., Wang H., Liao S. J., Key J. L., Linkov V., Ji S., Materials, 2013, 6(7), 2689—2700 |
| [7] | Yang H., Vogel W., Lamy C., Alonso V. N., J. Phys. Chem. B, 2004, 108(30), 11024—11034 |
| [8] | Yano H. S., Kataoka M., Yamashita H., Uchida H., Watanabe M., Langmuir, 2007, 23(11), 6438—6445 |
| [9] | Qian Y., Wen W., Adcock P. A., Jiang Z., Hakim N., Saha M. S., Mukerjee S., J. Phys. Chem. C, 2008, 112(4), 1146—1157 |
| [10] | Ammam M., Easton E. B., J. Power Sources, 2013, 236, 311—320 |
| [11] | Samjeské G., Nagamatsu S., Takao S., Nagasawa K., Imaizumi Y., Sekizawa O., Yamamoto T., Uemura Y., Uruga T., Iwasawa Y., Phys. Chem. Chem. Phys., 2013, 15(40), 17208—17218 |
| [12] | Drillet J. F., Ee A., Friedemann J., Kötz R., Schnyder B., Schmidt V. M., Electrochim. Acta, 2002, 47(12), 1983—1988 |
| [13] | Strasser P., Koh S., Anniyev T., Greeley J., More K., Yu C. F, Liu Z. C., Kaya S., Nordlund D., Ogasawara H., Toney M. F., Nilsson A., Nat. Chem., 2010, 2(6), 454—460 |
| [14] | Wang D. L., Xin H. L., Hovden R., Wang H. S., Yu Y. C., Muller D. A., Disalvo F. J., Abruña H. D., Nat. Mater., 2013, 12(1), 81—87 |
| [15] | Yang H., Coutanceau C., Léger J. M., Alonso V. N., Claude L., J. Electroanal. Chem., 2005, 576(2), 305—313 |
| [16] | Antolini E., Lopes T., Gonzalez E. R., J. Alloys Compd., 2008, 461(1/2), 253—262 |
| [17] | Salgado J. R. C., Antolini E., Gonzalez E. R., Appl. Catal., B, 2005, 57(4), 283—290 |
| [18] | Chen G., Xia D. G., Nie Z. R., Wang Z. Y., Wang L., Zhang L., Zhang J. J., Chem. Mater., 2007, 19(7), 1840—1844 |
| [19] | Mazumder V., Chi M. F., More K. L., Sun S. H., J. Am. Chem. Soc., 2010, 132(23), 7848—7849 |
| [20] | Chen Y. M., Yang F., Dai Y., Wang W. Q., Chen S. L., J. Phys. Chem. C, 2008, 112(5), 1645—1649 |
| [21] | Ding L. X., Li G. R., Wang Z. L., Liu Z. Q., Liu H., Tong Y. X., Chem. Eur. J., 2012, 18(27), 8386—8391 |
| [22] | Wang L. L., Johnson D. D., J. Am. Chem. Soc., 2009, 131(39), 14023—14029 |
| [23] | Alayoglu S., Nilekar A. U., Mavrikakis M., Eichhorn B., Nature Materials, 2008, 7(4), 333—338 |
| [24] | Liu B., Zeng H. C., Small, 2005, 1(5), 566—571 |
| [25] | Zhang H., Yin Y. J., Hu Y. J., Li C. Y., Wu P., Wei S. H., Cai C. X., J. Phys. Chem. C, 2010, 114(27), 11861—11867 |
| [26] | Lim B., Jiang M. J., Camargo P. H. C., Cho E. C., Tao J., Lu X. M., Zhu Y. M., Xia Y. N., Science, 2009, 324(5932), 1302—1305 |
| [27] | Cheng D. J., Qiu X. G., Yu H. Y., Phys. Chem. Chem. Phys., 2014, 16(38), 20377—20381 |
| [28] | Yang H., Angew. Chem. Int. Ed., 2011, 50(12), 2674—2676 |
| [29] | Xu C. X., Wang L. Q., Wang R. Y., Wang K., Zhang Y., Tian F., Ding Y., Adv. Mater., 2009, 21(21), 2165—2169 |
| [30] | Stamenkovic V. R., Fowler B., Mun B. S., Wang G. F., Ross P. N., Lucas C. A., Markovic' N. M., Science, 2007, 315(5811), 493—497 |
| [31] | Chen J. Y., Lim B., Lee E. P., Xia Y., Nano Today, 2009, 4(1), 81—95 |
| [32] | Fan Z. J., Yan J., Zhi L. J., Zhang Q., Wei T., Feng J., Zhang M. l., Qian W. Z., Wei F., Adv. Mater., 2010, 22(33), 3723—3728 |
| [33] | Tian N., Zhou Z. Y., Sun S. G., Ding Y., Wang Z. L., Science, 2007, 316(5828), 732—735 |
| [34] | Wang D. L., Yu Y. C., Xin H. L., Hovden R., Ercius P., Mundy J. A., Chen H., Richard J. H., Muller D. A., Disalvo F. J., Abruña H. D., Nano Lett., 2012, 12(10), 5230—5238 |
| [35] | Cui C. H., Gan L., Li H. H., Yu S. H., Heggen M., Strasser P., Nano Lett., 2012, 12(11), 5885—5889 |
| [36] | Chen C., Kang Y., Huo Z., Zhu Z., Huang W., Xin H. L., Snyder J. D., Li D., Herron J. A., Mavrikakis M., Chi M., More K. L., Li Y., Markovic N. M., Somorjai G. A., Yang P., Stamenkovic V. R., Science, 2014, 343(6177), 1339—1343 |
| [37] | Kim J., Lee Y., Sun S., J. Am. Chem. Soc., 2010, 132(14), 4996—4997 |
| [38] | Zou L. L., Li J., Yuan T., Zhou Y., Li X. M., Yang H., Nanoscale, 2014, 6(18), 10686—10692 |
| [39] | Burkert T., Eriksson O., Simak I., Ruban A. V., Sanyal B., Nordström L., Wills J. M., Phys. Rev. B, 2005, 71(13), 134411 |
| [40] | Staunton J. B., Ostanin S., Razee S. S. A., Gyorffy B. L., Szunyogh L., Ginatempo B., Bruno E., Phys. Rev. Lett., 2004, 93(25), 257204 |
| [41] | Chen S. G., Wei Z. D., Qi X. Q., Dong L. C., Guo Y. G., Wan L.J., Shao Z. G., Li L., J. Am. Chem. Soc., 2012, 134(32), 13252—13255 |
| [42] | Zhang Y., Huang Q. H., Zou Z. Q., Yang J. F., Vogel W., Yang H., J. Phys. Chem. C, 2010, 114(14), 6860—6868 |
| [43] | Luo B. M, Xu S., Yan X. B., Xue Q. J., J. Electrochem. Soc., 2013, 160(3), F262—F268 |
| [44] | Cao J. Y., Du C., Wang S. C., Mercier P., Zhang X. G., Yang H., Akins D. L., Electrochem. Commun., 2007, 9(4), 735—740 |
| [45] | Xiong L., Manthiram A., J. Electrochem. Soc., 2005, 152(4), A697—A703 |
| [46] | Eberhardt W., Fayet P., Cox D. M., Fu Z., Kaldor A., Sherwood R., Sondericker D., Phys. Rev. Lett., 1990, 64(7), 780—783 |
| [47] | Koh S., Strasser P., J. Am. Chem. Soc., 2007, 129(42), 12624—12625 |
| [48] | Hogarth M. P., Hards G. A., Platinum Met. Rev., 1996, 40(4), 150—159 |
| [49] | Dinh H. N., Ren X. M., Garzon F. H., Zelenay P., Gottesfeld S., J. Electroanal. Chem., 2000, 491(1), 222—233 |
| [50] | Müller J., Frank G., Colbow K., Wilkinson D., Hand Book of Fuel Cells, John Wiley & Sons, Chichester, West Sussex, 2010 |
| [1] | FAN Jianling, TANG Hao, QIN Fengjuan, XU Wenjing, GU Hongfei, PEI Jiajing, CEHN Wenxing. Nitrogen Doped Ultra-thin Carbon Nanosheet Composited Platinum-ruthenium Single Atom Alloy Catalyst for Promoting Electrochemical Hydrogen Evolution Process [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220366. |
| [2] | CHENG Qian, YANG Bolong, WU Wenyi, XIANG Zhonghua. S-doped Fe-N-C as Catalysts for Highly Reactive Oxygen Reduction Reactions [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220341. |
| [3] | CHU Yuyi, LAN Chang, LUO Ergui, LIU Changpeng, GE Junjie, XING Wei. Single-atom Cerium Sites Designed for Durable Oxygen Reduction Reaction Catalyst with Weak Fenton Effect [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220294. |
| [4] | WONG Honho, LU Qiuyang, SUN Mingzi, HUANG Bolong. Rational Design of Graphdiyne-based Atomic Electrocatalysts: DFT and Self-validated Machine Learning [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220042. |
| [5] | GU Yu, XI Baojuan, LI Jiangxiao, XIONG Shenglin. Structure Regulation of Single-atom Catalysts in Oxygen Reduction Reactions [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220036. |
| [6] | JIN Xiangyuan, ZHANG Libing, SUN Xiaofu, HAN Buxing. Electrocatalytic CO2 Reduction over Single-atom Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220035. |
| [7] | ZHANG Xiaoyu, XUE Dongping, DU Yu, JIANG Su, WEI Yifan, YAN Wenfu, XIA Huicong, ZHANG Jianan. MOF-derived Carbon-based Electrocatalysts Confinement Catalyst on O2 Reduction and CO2 Reduction Reactions [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210689. |
| [8] | HE Yujing, LI Jiale, WANG Dongyang, WANG Fuling, XIAO Zuoxu, CHEN Yanli. Zinc-based Activated Fe/Co/N Doped Biomass Carbon Electrocatalysts with High Oxygen Reduction Activity [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220475. |
| [9] | WU Yaqiang, LIU Siming, JIN Shunjin, YAN Yongqing, WANG Zhao, CHEN Lihua, SU Baolian. Synthesis of Zn-Doped NiCoP Catalyst with Porous Double-layer Nanoarray Structure and Its Electrocatalytic Properties for Hydrogen Evolution [J]. Chem. J. Chinese Universities, 2021, 42(8): 2483. |
| [10] | YANG Tao, YAO Huiying, LI Qing, HAO Wei, CHI Lifeng, ZHU Jia. Density Functional Theoretical Studies on the Promising Electrocatalyst of M-BHT(M=Co or Cu) for CO2 Reduction to CH4 [J]. Chem. J. Chinese Universities, 2021, 42(4): 1268. |
| [11] | MA Jun, ZHONG Yang, ZHANG Shanshan, HUANG Yijun, ZHANG Lipeng, LI Yaping, SUN Xiaoming, XIA Zhenhai. Design and Theoretical Calculation of Heteroatoms Doped Graphdiyne Towards Efficiently Catalyzing Oxygen Reduction and Evolution Reactions [J]. Chem. J. Chinese Universities, 2021, 42(2): 624. |
| [12] | WANG Yuemin, MENG Qinglei, WANG Xian, GE Junjie, LIU Changpeng, XING Wei. Enhancement of Performance of Fe-N-C Catalysts by Copper and Sulfur Doping for the Oxygen Reduction Reaction [J]. Chem. J. Chinese Universities, 2020, 41(8): 1843. |
| [13] | ZHAO Guoqing, YUAN Zhao, WANG Lian, GUO Zhuo. Preparation of Ni2P/N, S co-Doped Reduced Graphene Oxide Composites and Their Electrocatalytic Properties for Hydrogen Evolution† [J]. Chem. J. Chinese Universities, 2020, 41(7): 1575. |
| [14] | JIANG Yuanyuan, LI Boyu, LU Yizhong, WU Tongshun, HAN Dongxue. Oxygen Evolution Reaction Electrocatalytic Performance Analysis of Electroless Plated Ni-Bx [J]. Chem. J. Chinese Universities, 2020, 41(12): 2774. |
| [15] | YIN Wenjing, LIU Xiao, QIAN Huidong, ZOU Zhiqing. Preparation and Oxygen Reduction Performance of Fe, N co-Doped arbon Nanoplate with High Density of Active Sites† [J]. Chem. J. Chinese Universities, 2019, 40(7): 1480. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||