

Chem. J. Chinese Universities ›› 2015, Vol. 36 ›› Issue (6): 1033.doi: 10.7503/cjcu20150044
• Articles: Inorganic Chemistry • Previous Articles Next Articles
WEN Jinyan1, LÜ Biaobiao2, ZHANG Yang1, WANG Jiamin1, YING Xiao2, WANG Hui3, JI Liangnian3,4, LIU Haiyang1,3,*( )
)
Received:2015-01-14
Online:2015-06-10
Published:2015-05-22
Contact:
LIU Haiyang
E-mail:chhyliu@scut.edu.cn
Supported by:CLC Number:
TrendMD:
WEN Jinyan, LÜ Biaobiao, ZHANG Yang, WANG Jiamin, YING Xiao, WANG Hui, JI Liangnian, LIU Haiyang. Interaction of Human Serum Albumin and Water Soluble Cationic Pyridyl Corrole Gallium Complex†[J]. Chem. J. Chinese Universities, 2015, 36(6): 1033.
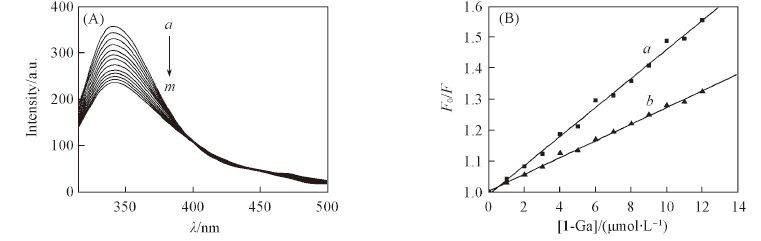
Fig.2 Fluorescence spectra of HSA(5 μmol/L) in the absence and presence of 1-Ga in Tris-HCl buffer(A) and the plots of F0/F vs. [1-Ga] before(a) and after(b) IFE correction(B)(A) [1-Ga]/(μmol·L-1) from a to m: 1—12, step 1.
| Complex | Stern-Volmer method | Scatchard method | ||
|---|---|---|---|---|
| 10-4KSV/(L·mol-1) | 10-12Kq/(L·mol-1·s-1) | 10-4KA/(L·mol-1) | n | |
| 298 | 2.69 | 2.69 | 2.82 | 0.94 |
| 304 | 2.53 | 2.53 | 2.71 | 0.97 |
| 310 | 2.39 | 2.39 | 2.59 | 1.01 |
Table 1 Binding parameters of 1-Ga and HSA at pH=7.4
| Complex | Stern-Volmer method | Scatchard method | ||
|---|---|---|---|---|
| 10-4KSV/(L·mol-1) | 10-12Kq/(L·mol-1·s-1) | 10-4KA/(L·mol-1) | n | |
| 298 | 2.69 | 2.69 | 2.82 | 0.94 |
| 304 | 2.53 | 2.53 | 2.71 | 0.97 |
| 310 | 2.39 | 2.39 | 2.59 | 1.01 |
| Complex | ΔH/(kJ·mol-1) | ΔG/(kJ·mol-1) | ΔS/(J·mol-1·K-1) |
|---|---|---|---|
| 298 | -5.465 | -25.391 | 66.865 |
| 304 | -25.792 | ||
| 310 | -26.193 |
Table 2 Thermodynamic parameters of 1-Ga-HSA interaction at pH=7.4
| Complex | ΔH/(kJ·mol-1) | ΔG/(kJ·mol-1) | ΔS/(J·mol-1·K-1) |
|---|---|---|---|
| 298 | -5.465 | -25.391 | 66.865 |
| 304 | -25.792 | ||
| 310 | -26.193 |
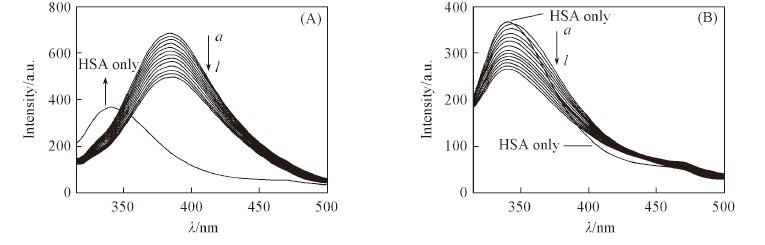
Fig.4 Fluorescence spectra changes of HSA-warfarin(A) and HSA-ibuprofen systems(B) with the addition of 1-Ga in Tris-HCl buffer [HSA]=[warfarin]=[ibuprofen]=5 μmol/L, [1-Ga]/(μmol·L-1) from a to l: 1—12, step 1.
| Site marker | Stern-Volmer method | Scatchard method | ||
|---|---|---|---|---|
| 10-4KSV/(L·mol-1) | 10-12Kq/(L·mol-1·s-1) | 10-4KA/(L·mol-1) | n | |
| Blank | 2.69 | 2.69 | 2.82 | 0.94 |
| Warfarin | 2.03 | 2.03 | 2.53 | 1.09 |
| Ibuprofen | 1.35 | 1.35 | 1.47 | 1.05 |
Table 3 Binding parameters for site competitive experiments of 1-Ga at pH=7.4, 298 K
| Site marker | Stern-Volmer method | Scatchard method | ||
|---|---|---|---|---|
| 10-4KSV/(L·mol-1) | 10-12Kq/(L·mol-1·s-1) | 10-4KA/(L·mol-1) | n | |
| Blank | 2.69 | 2.69 | 2.82 | 0.94 |
| Warfarin | 2.03 | 2.03 | 2.53 | 1.09 |
| Ibuprofen | 1.35 | 1.35 | 1.47 | 1.05 |
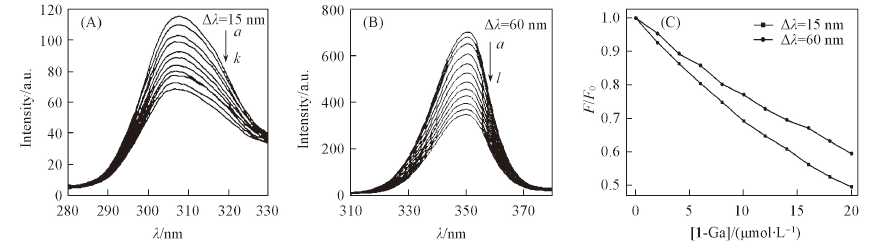
Fig.8 Synchronous fluorescence spectra of tyrosine(A) and tryptophan(B) in HSA, and F/F0 changes of HSA(5 μmol/L) upon the addition of 1-Ga in Tris-HCl buffer(C)(A) [1-Ga]/(μmol·L-1) from a to k: 0—20, step 2. (B) [1-Ga]/(μmol·L-1) from a to l: 0—22, step 2.
| [1] | He X. M., Carter D. C., Nature, 1992, 358(6383), 209—215 |
| [2] | Kragh-Hansen U., Pharmacol. Rev., 1981, 33(1), 17—53 |
| [3] | Ruiz J., Vicente C., de Haro C., Bautista D., Inorg. Chem., 2013, 52(2), 974—982 |
| [4] | Liu Y., Long M., Xie M. X., Acta Phys. Chim. Sin., 2013, 29(12), 2647—2654 |
| (刘媛, 龙梅, 谢孟峡.物理化学学报, 2013,29(12), 2647—2654) | |
| [5] | Zhou B., Zhang Z., ZhangY., Li R., Xiao Q., Liu Y., Li Z. Y., J. Pharm. Sci., 2009, 98(1), 105—113 |
| [6] | Zhang Y., Chen H., Wen J. Y., Wang X. L., Wang H., Ji L. N., Liu H. Y., Chem. J. Chinese Universities, 2013, 34(11), 2462—2469 |
| (张阳, 陈欢, 闻金燕, 王湘利, 王惠, 计亮年, 刘海洋.高等学校化学学报, 2013,34(11), 2462—2469) | |
| [7] | Huang J. T., Zhang Y., Wang X. L., Ji L. N., Liu H. Y., Chinese J. Inorg. Chem., 2013, 29(8), 1649—1656 |
| (黄俊腾, 张阳, 王湘利, 计亮年, 刘海洋.无机化学学报, 2013,29(8), 1649—1656) | |
| [8] | Lu J., Liu H. Y., Shi L., Wang X. L., Ying X., Zhang L., Ji L. N., Zang L. Q., Chang C. K., Chin. Chem. Lett., 2011, 22(1), 101—104 |
| [9] | Huang J. T., Wang X. L., Zhang Y., Mahmood M. H. R., Huang Y. Y., Ying X., Ji L. N., Liu H. Y., Trans. Metal. Chem., 2013, 38(3), 283—289 |
| [10] | Zhang Y., Wang Q., Wen J. Y., Wang X. L., Mahmood M. H. R., Ji L. N., Liu H. Y., Chin. J. Chem., 2013, 31(10), 1321—1328 |
| [11] | Zhang Y., Wen J. Y., Wang X. L., Mahmood M. H. R., Liu Z. Y., Wang H., Ji L. N., Liu H. Y., Appl. Organomet. Chem., 2014, 28(7), 559—566 |
| [12] | Zhai Q. Q., Liang X., Ge Y. S., Tian T., Wu W. D., Yan S. Y., Zhou Y. Y., Deng M. G., Liu Y., Zhou X., Chem. Eur. J., 2011, 17(32), 8890—8895 |
| [13] | Fu B. Q., Zhang D., Weng X. C., Zhang M., Ma H., Ma Y. Z., Zhou X., Chem. Eur. J., 2008, 14(30), 9431—9441 |
| [14] | Fu B. Q., Huang J., Ren L. G., Weng X. C., Zhou Y. Y., Du Y. H., Wu X. J., Zhou X., Yang G. F., Chem. Commun., 2007, 31, 3264—3266 |
| [15] | Mahammed A., Gray H. B., Weaver J. J., Sorasaenee K., Gross Z., Bioconjugate Chem., 2004, 15(4), 738—746 |
| [16] | Haber A., Agadjanian H., Medina-Kauwe L. K., Gross Z., J. Inorg. Biochem., 2008, 102(3), 446—457 |
| [17] | Lim P., Mahammed A., Okun Z., Saltsman I., Gross Z., Gray H. B., Termini J., Chem. Res. Toxicol., 2012, 25(2), 400—409 |
| [18] | Agadjanian H., Weaver J. J., Mahammed A., Rentsendorj A., Bass S., Kim J., Dmochowski I. J., Margalit R., Gray H. B., Gross Z., Medina-Kauwe L. K., Pharmacol. Res., 2006, 23(2), 367—377 |
| [19] | Agadjanian H., Ma J., Rentsendorj A., Valluripalli V., Hwang J. Y., Mahammed A., Farkas D. L., Gray H. B., Gross Z., Medina-Kauwe L. K., Proc. Natl. Acad. Sci. USA, 2009, 106(15), 6105—6110 |
| [20] | Hwang J. Y., Gross Z., Gray H. B., Medina-Kauwe L. K., Farkas D. L., J. Biomed. Opt., 2011, 16(6), 0660071—0660076 |
| [21] | Aviv-Harel I., Gross Z., Coord. Chem. Rev., 2011, 255(7/8), 717—736 |
| [22] | Haber A., Mahammed A., Fuhrman B., Volkova N., Coleman R., Hayek T., Aviram M., Gross Z., Angew. Chem. Int. Ed., 2008, 47(41), 7896—7900 |
| [23] | Haber A., Angel I., Mahammed A., Gross Z., J. Diabetes. Complicat., 2013, 27(4), 316—321 |
| [24] | Mahammed A., Gross Z., J. Am. Chem. Soc., 2005, 127(9), 2883—2887 |
| [25] | Lessa J. A., Parrilha G. L., Beraldo H., Inorg. Chim. Acta, 2012, 393, 53—63 |
| [26] | Lu J., Wang X.L., Chen W. J., Liu H. Y., Ji L. N., Chinese Journal of Synthetic Chemistry, 2010, (S1), 165—169(陆俊, 王湘利, 陈伟健, 刘海洋, 计亮年. 合成化学, 2010, (S1), 165—169) |
| [27] | Samari F., Hemmateenejad B., Shamsipur M., Rashidi M., Samouei H., Inorg. Chem., 2012, 51(6), 3454—3464 |
| [28] | Tabassum S., Al-Asbahy W. M., Afzal M., Arjmand F., J. Photochem. Photobiol. B, 2012, 114, 132—139 |
| [29] | Tabassum S., Al-Asbahy W. M., Afzal M., Arjmand F., Khan R. H., Mol. BioSyst., 2012, 8(9), 2424—2433 |
| [30] | Wang C. X., Yan Y. Y., Zhang Y. X., Ye L., J. Photochem. Photobiol. A, 2007, 192(1) 23—28 |
| [31] | Kang J. W., Wu H. X., Lu X. Q., Wang Y. S., Zhou L., Spectrochim. Acta Part A, 2005, 61(9), 2041—2047 |
| [32] | Gauthler T. D., Shane E. C., Guerln W. F., Seltz W. R., Grant C. L., Environ. Sci. Technol., 1986, 20(11), 1162—1166 |
| [33] | Shahabadi N., Khorshidi A., Moghadam N. H., Spectrochim. Acta Part A, 2013, 114, 627—632 |
| [34] | Ross P. D., Subramanian S., Biochemistry, 1981, 20(11), 3096—3102 |
| [35] | Tian F. F., Li J. H., Jiang F. L., Han X. L., Xiang C., Ge Y. S., Li L. L., Liu Y., RSC Adv., 2012, 2(2), 501—513 |
| [36] | Ferenc Z., Mol. Pharmaceut., 2013, 10(5), 1668—1682 |
| [37] | Cheng Z. J., Spectrochim. Acta, Part A, 2012, 93, 321—330 |
| [38] | Wang Y. Q., Wang X. Y., Wang J., Zhao Y. M., He W. J., Guo Z. J., Inorg. Chem., 2011, 50(24), 12661—12668 |
| [39] | Sudhamalla B., Gokara M., Ahalawat N., Amooru D. G., Subramanyam R., J. Phys. Chem. B, 2010, 114(27), 9054—9062 |
| [1] | REN Shijie, QIAO Sicong, LIU Chongjing, ZHANG Wenhua, SONG Li. Synchrotron Radiation X-Ray Absorption Spectroscopy Research Progress on Platinum Single-atom Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220466. |
| [2] | ZHOU Leilei, CHENG Haiyang, ZHAO Fengyu. Research Progress of CO2 Hydrogenation over Pd-based Heterogeneous Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220279. |
| [3] | PENG Kuilin, LI Guilin, JIANG Chongyang, ZENG Shaojuan, ZHANG Xiangping. Research Progress for the Role of Electrolytes in the CO2 Electrochemical Reduction [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220238. |
| [4] | HU Huimin, CUI Jing, LIU Dandan, SONG Jiaxin, ZHANG Ning, FAN Xiaoqiang, ZHAO Zhen, KONG Lian, XIAO Xia, XIE Zean. Influence of Different Transition Metal Decoration on the Propane Dehydrogenation Performance over Pt/M-DMSN Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210815. |
| [5] | LIU Yang, LI Wangchang, ZHANG Zhuxia, WANG Fang, YANG Wenjing, GUO Zhen, CUI Peng. Theoretical Exploration of Noncovalent Interactions Between Sc3C2@C80 and [12]Cycloparaphenylene Nanoring [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220457. |
| [6] | JIANG Shan, SHEN Qianqian, LI Qi, JIA Husheng, XUE Jinbo. Pd-loaded Defective TiO2 Nanotube Arrays for Enhanced Photocatalytic Hydrogen Production Performance [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220206. |
| [7] | XU Wenzhe, ZHANG Hao. Supramolecular Interactions-mediated Nanodrug Nucleation [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220264. |
| [8] | WU Ji, ZHANG Hao, LUO Yuhui, GENG Wuyue, LAN Yaqian. A Microporous Cationic Ga(III)-MOF with Fluorescence Properties for Selective sensing Fe3+ Ion and Nitroaromatic Compounds [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210617. |
| [9] | PAN Xiaojun, BAO Rongrong, PAN Caofeng. Research Progress of Flexible Tactile Sensors Applied to Wearable Electronics [J]. Chem. J. Chinese Universities, 2021, 42(8): 2359. |
| [10] | WEN Wei, HUANG Dading, BAO Jingxiao, ZHANG John Z. H.. Residue Specific Binding Mechanisms of PD-1 to Its Monoclonal Antibodies by Computational Alanine Scanning [J]. Chem. J. Chinese Universities, 2021, 42(7): 2161. |
| [11] | ZHANG Jun, LIU Yixuan, DU Xiaohui, YANG Hui. Highly Adhesive and Stretchable Polymers for the Interface of Cyber-human Interaction [J]. Chem. J. Chinese Universities, 2021, 42(4): 1093. |
| [12] | MA Zhuoyuan, WANG Dayang. Status and Prospect of Surface Wettability of Molecular Self-assembled Monolayers [J]. Chem. J. Chinese Universities, 2021, 42(4): 1031. |
| [13] | WANG Yupeng, ZHAO Yang, LI Mohan, SHI Suqing, GONG Yongkuan. Fabrication of Antifouling-antibacterial Dual Functional Polymer Coating via Dopamine-based Multiple Interactions [J]. Chem. J. Chinese Universities, 2021, 42(3): 811. |
| [14] | LI Mengshuo, ZHANG Jing, LIU Dan, ZHU Yaxian, ZHANG Yong. Interactions of Pyrene with Human Serum Albumin and Bovine Serum Albumin: Microenvironmental Polarity Differences at Binding Sites [J]. Chem. J. Chinese Universities, 2021, 42(3): 731. |
| [15] | CHEN Binggang, LIU Sanrong, YU Xifei, JIANG Zijiang. Preparation and Properties Characterization of Polysiloxane Skin Tissue Adhesive [J]. Chem. J. Chinese Universities, 2021, 42(12): 3746. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||