

Chem. J. Chinese Universities ›› 2015, Vol. 36 ›› Issue (5): 919.doi: 10.7503/cjcu20140906
• Organic Chemistry • Previous Articles Next Articles
XU Jian1,3, WEI Mengxue1,2, LI Guoming3, LI Xueqiang1,2,*( )
)
Received:2014-10-11
Online:2015-05-10
Published:2015-04-13
Contact:
LI Xueqiang
E-mail:lixq@nxu.edu.cn
Supported by:CLC Number:
TrendMD:
XU Jian, WEI Mengxue, LI Guoming, LI Xueqiang. Synthesis and Anti-tumor Activities of Novel Artemisone-piperazine-sulfonamide Derivatives†[J]. Chem. J. Chinese Universities, 2015, 36(5): 919.
| Compd. | Appearance | Yield* (%) | m. p./℃ | [α (c=1 , CH2Cl2) | ESI-TOF-MS, m/z | IR(KBr ), |
|---|---|---|---|---|---|---|
| 2 | Pale yellow viscous solid | 78.5 | ||||
| 3 | White solid | 77.9 | 123.1—123.4 | -3 | 424.1764 [M+Na]+ | 2928, 2881, 1647, 1467, 1383, 1307, 1258, 1126, 1025, 931, 850, 750 |
| 4 | Brown yellow viscous solid | 31.6 | ||||
| 5 | Pale yellow viscous solid | 86.2 | +11.2 | 524.2650 [M+Na]+ | 2929, 2873, 1651, 1447, 1383, 1261, 1120, 1113, 1039, 987, 865, 750 | |
| 6 | Brown yellow viscous solid | 87.9 | ||||
| 7 | Pale yellow solid | 82.6 | 58.1—60.4 | +16.5 | 570.3579 [M+H]+ | 2931, 2830, 1662, 1454, 1367, 1274, 1124, 1040, 974, 858, 748 |
| 8a | Pale yellow solid | 93.2 | 103.5—103.7 | +3.2 | 710.3500 [M+H]+ | 2928, 2855, 1456, 1303, 1170, 1120, 1028, 940, 884, 742 |
| 8b | Yellow solid | 88.7 | 151.4—151.6 | +20.6 | 755.3353 [M+H]+ | 2927, 2862, 1532, 1454, 1350, 1297, 1170, 1120, 1015, 930, 838, 742 |
| 8c | Yellow solid | 85.6 | 93.1—93.3 | +3.6 | 755.3353 [M+H]+ | 2928, 2852, 1716, 1651, 1543, 1454, 1373, 1287, 1126, 1025, 960, 750 |
| 8d | Pale yellow solid | 75.8 | 119.9—120.1 | +8.6 | 724.3663 [M+H]+ | 2927, 2858, 1739, 1647, 1557, 1456, 1375, 1261, 1124, 1010, 927, 820, 736 |
| 8e | Pale yellow solid | 78.4 | 98.5—99.8 | +6.6 | 724.3674 [M+H]+ | 2926, 2852, 1724, 1542, 1463, 1360, 1303, 1166, 1120, 1025, 938, 858, 734 |
| 8f | White solid | 84.6 | 100.5—100.7 | +4 | 740.3616 [M+H]+ | 2926, 2866, 1606, 1499, 1454, 1353, 1261, 1163, 1011, 924, 873, 748 |
Table 1 Appearance, yields, melting points, optical rotation, ESI-TOF-MS and IR data for all the compounds
| Compd. | Appearance | Yield* (%) | m. p./℃ | [α (c=1 , CH2Cl2) | ESI-TOF-MS, m/z | IR(KBr ), |
|---|---|---|---|---|---|---|
| 2 | Pale yellow viscous solid | 78.5 | ||||
| 3 | White solid | 77.9 | 123.1—123.4 | -3 | 424.1764 [M+Na]+ | 2928, 2881, 1647, 1467, 1383, 1307, 1258, 1126, 1025, 931, 850, 750 |
| 4 | Brown yellow viscous solid | 31.6 | ||||
| 5 | Pale yellow viscous solid | 86.2 | +11.2 | 524.2650 [M+Na]+ | 2929, 2873, 1651, 1447, 1383, 1261, 1120, 1113, 1039, 987, 865, 750 | |
| 6 | Brown yellow viscous solid | 87.9 | ||||
| 7 | Pale yellow solid | 82.6 | 58.1—60.4 | +16.5 | 570.3579 [M+H]+ | 2931, 2830, 1662, 1454, 1367, 1274, 1124, 1040, 974, 858, 748 |
| 8a | Pale yellow solid | 93.2 | 103.5—103.7 | +3.2 | 710.3500 [M+H]+ | 2928, 2855, 1456, 1303, 1170, 1120, 1028, 940, 884, 742 |
| 8b | Yellow solid | 88.7 | 151.4—151.6 | +20.6 | 755.3353 [M+H]+ | 2927, 2862, 1532, 1454, 1350, 1297, 1170, 1120, 1015, 930, 838, 742 |
| 8c | Yellow solid | 85.6 | 93.1—93.3 | +3.6 | 755.3353 [M+H]+ | 2928, 2852, 1716, 1651, 1543, 1454, 1373, 1287, 1126, 1025, 960, 750 |
| 8d | Pale yellow solid | 75.8 | 119.9—120.1 | +8.6 | 724.3663 [M+H]+ | 2927, 2858, 1739, 1647, 1557, 1456, 1375, 1261, 1124, 1010, 927, 820, 736 |
| 8e | Pale yellow solid | 78.4 | 98.5—99.8 | +6.6 | 724.3674 [M+H]+ | 2926, 2852, 1724, 1542, 1463, 1360, 1303, 1166, 1120, 1025, 938, 858, 734 |
| 8f | White solid | 84.6 | 100.5—100.7 | +4 | 740.3616 [M+H]+ | 2926, 2866, 1606, 1499, 1454, 1353, 1261, 1163, 1011, 924, 873, 748 |
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(100 MHz, CDCl3), δ | |
|---|---|---|---|
| 2 | 0.780(d, J=7.2 Hz, 3H), 0.938(d, J=6 Hz, 3H), 0.969—1.018(m, 1H), 1.194—1.354(m, 3H), 1.372—1.391(m, 3H), 1.421—1.440(m, 1H), 1.475—1.534(m, 1H), 1.581—1.599(m, 2H), 1.663—1.716(m, 1H), 1.821—1.903(m, 1H), 2.331—2.407(m, 1H), 2.568—2.699(m, 5H), 2.891—2.947(m, 2H), 3.242—3.298(m, 2H), 3.951(d, J=10.2 Hz, 1H), 5.257(s, 1H) | 13.86, 20.25, 21.80, 24.30, 25.25, 29.60, 34.25, 35.95, 37.21, 44.59, 47.20, 51.81, 52.85, 80.46, 91.26, 93.69, 105.35 | |
| 3 | 0.772(d, J=7.2 Hz, 3H), 0.925(d, J=6 Hz, 3H), 0.976—1.005(m, 1H), 1.210—1.318(m, 3H), 1.344(s, 3H), 1.376—1.485(m, 1H), 1.530—1.564(m, 1H), 1.665—1.715(m, 2H), 1.724—1.849(m, 1H), 1.962—1.998(m, 1H), 2.278—2.348(m, 1H), 2.529—2.584(m, 1H), 3.180(s, 4H), 3.317—3.379(m, 2H), 3.435—3.483(m, 2H), 4.193(d, J=10.4 Hz, 1H), 5.259(s, 1H) | 13.46, 20.23, 21.60, 24.80, 25.91, 29.05, 34.18, 36.15, 37.45, 45.59, 46.96, 51.41, 51.87, 80.18, 91.00, 92.09, 104.27 | |
| 4 | 0.042(s, 6H), 0.819(d, J=3.4 Hz, 3H), 0.886(s, 9H), 0.951(d, J=3 Hz, 3H), 0.974—1.055(m, 1H), 1.273—1.407(m, 11H), 1.468—1.601(m, 6H), 1.691—1.751(m, 2H), 1.841—1.908(m, 1H), 1.975—2.057(m, 2H), 2.302—2.382(m, 1H), 2.515—2.604(m, 1H), 2.912—2.939(m, 1H), 3.081—3.107(m, 1H), 3.299—3.475(m, 5H), 3.584(t, J=6.6 Hz, 2H), 4.202(d, J=10.4 Hz, 1H), 5.287(s, 1H) | -5.20, 13.53, 18.46, 20.21, 21.65, 23.32, 24.83, 25.58, 25.90, 26.04, 26.75, 29.02, 29.30, 32.70, 34.22, 36.16, 37.44, 45.65, 51.46, 51.77, 60.66, 63.13, 76.64, 80.02, 90.95, 91.87, 104.08 | |
| 5 | 0.769(d, J=7.2 Hz, 3H), 0.919(d, J=6 Hz, 3H), 0.968—0.999(m, 1H), 1.198—1.339(m, 11H), 1.389—1.555(m, 6H), 1.666—1.708(m, 2H), 1.818—1.860(m, 1H), 1.954—1.991(m, 2H), 2.276—2.310(m, 1H), 2.509—2.527(m, 1H), 2.875(d, J=11.6 Hz, 1H), 2.985(t, J=12.2 Hz, 1H), 3.316—3.415(m, 5H), 3.588(t, J=6.4 Hz, 2H), 4.168(d, J=10 Hz, 1H), 5.255(s, 1H), 5.259(s, 1H) | 13.58, 20.33, 21.68, 23.26, 24.86, 25.44, 26.00, 26.71, 29.12, 29.17, 32.63, 34.24, 36.23, 37.52, 45.69, 51.50, 51.77, 60.51, 62.88, 80.24, 91.14, 91.95, 104.31 | |
| 6 | 0.815(d, J=7.1 Hz, 3H), 0.952(d, J=6.1 Hz, 3H), 0.992—1.056(m, 1H), 1.301—1.422(m, 11H), 1.497—1.591(m, 6H), 1.699—1.732(m, 2H) 1.851—1.901(m, 1H), 1.987—2.069(m, 2H), 2.302—2.382(m, 1H), 2.558—2.585(m, 1H), 2.917(d, J=11.6 Hz, 1H), 3.319—3.472(m, 5H), 3.634(t, J=6.5 Hz, 2H), 4.215(d, J=10.4 Hz, 1H), 5.287(s, 1H) | 7.55, 13.92, 20.61, 22.03, 23.62, 25.24, 26.35, 26.96, 28.77, 29.43, 30.52, 33.73, 34.64, 36.55, 37.86, 46.02, 51.81, 52.13, 60.74, 77.65, 80.46, 91.37, 92.24, 104.03 | |
| 7 | 0.781(d, J=7.2 Hz, 3H), 0.939(d, J=6 Hz, 3H), 0.981—1.019(m, 1H), 1.184—1.353(m, 11H), 1.399—1.568(m, 6H), 1.685—1.725(m, 2H), 1.843—1.851(m, 1H), 1.972—2.009(m, 2H), 2.289—2.350(m, 3H), 2.538(s, 5H), 2.873—2.896(m, 1H), 3.022(t, J=4.8 Hz, 5H), 3.283—3.422(m, 5H), 4.171(d, J=10.4 Hz, 1H), 5.267(s, 1H) | 13.60, 20.35, 21.72, 23.40, 24.91, 26.05, 26.53, 26.76, 27.17, 29.15, 29.44, 29.81, 34.31, 36.30, 37.57, 45.08, 45.76, 51.59, 51.89, 52.62, 58.84, 60.71, 80.22, 91.17, 92.04, 104.31 | |
| 8a | 0.756(d, J=6.8 Hz, 3H), 0.928(d, J=6.4 Hz, 3H), 0.973—1.002(m, 1H), 1.183—1.325(m, 11H), 1.413—1.522(m, 6H), 1.686(d, J=10.4 Hz, 2H), 1.832—1.900(m, 1H), 1.944—1.993(m, 2H), 2.254—2.323(m, 3H), 2.480—2.534(m, 5H), 2.861(d, J=10.8 Hz, 1H), 2.962—3.049(m, 5H), 3.261—3.408(m, 5H), 4.157(d, J=10 Hz, 1H), 5.249(s, 1H), 7.518(d, J=7.6 Hz, 2H), 7.568(d, J=7.2 Hz, 1H), 7.724(d, J=8.4 Hz, 2H) | 13.55, 20.31, 21.66, 23.33, 24.85, 26.00, 26.64, 26.99, 29.10, 29.28, 29.76, 34.24, 36.24, 37.50, 45.69, 45.86, 51.52, 51.83, 52.15, 58.11, 60.55, 80.19, 91.08, 91.96, 104.22, 127.87, 129.16, 133.01, 135.27 | |
| 8b | 0.740(d, J=7.2 Hz, 3H), 0.912(d, J=6 Hz, 3H), 0.952—0.987(m, 1H), 1.167—1.326(m, 11H), 1.344—1.528(m, 6H), 1.672(d, J=10.8 Hz, 2H), 1.817—1.834(m, 1H), 1.918—1.973(m, 2H), 2.234—2.295(m, 3H), 2.480(s, 5H), 2.847(d, J=11.6 Hz, 1H), 2.902—2.965(m, 1H), 3.048(s, 4H), 3.282—3.392(m, 5H), 4.147(d, J=10.4 Hz, 1H), 5.235(s, 1H), 7.905(d, J=8.8 Hz, 2H), 8.351(d, J=8.8 Hz, 2H) | 13.47, 20.24, 21.57, 23.27, 24.77, 25.92, 26.45, 26.58, 26.81, 29.02, 29.23, 29.68, 34.16, 36.16, 37.43, 45.62, 46.02, 51.44, 51.74, 52.03, 57.88, 60.48, 80.13, 91.00, 91.88, 104.14, 124.33, 128.99, 141.52, 150.18 | |
| 8c | 0.756(d, J=7.2 Hz, 3H), 0.921(d, J=6 Hz, 3H), 0.968—0.998(m, 1H), 1.165—1.325(m, 11H), 1.415—1.545(m, 6H), 1.681(d, J=10 Hz, 2H), 1.827—1.861(m, 1H), 1.954(d, J=4.4 Hz, 2H), 1.986—2.326(m, 3H), 2.484(s, 5H), 2.856(d, J=11.2 Hz, 1H), 2.958(t, J=12.3 Hz, 1H), 3.281(s, 5H), 3.341—3.406(m, 4H), 4.161(d, J=10 Hz, 1H), 5.248(s, 1H), 7.571(d, J=8.4 Hz, 1H), 7.644—7.710(m, 2H), 7.907(d, J=8.8 Hz, 1H) | 13.54, 20.30, 21.64, 23.30, 24.83, 25.99, 26.42, 26.65, 26.98, 29.09, 29.29, 29.74, 34.33, 36.23, 37.49, 45.49, 45.92, 51.49, 51.81, 52.42, 58.13, 60.54, 80.19, 91.08, 91.95, 104.22, 124.09, 130.63, 130.91, 131.53, 133.87, 148.59 | |
| 8d | 0.765(d, J=7.2 Hz, 3H), 0.937(d, J=6.4 Hz, 3H), 0.982—1.012(m, 1H), 1.192—1.383(m, 11H), 1.403—1.554(m, 6H), 1.695(d, J=10 Hz, 2H), 1.882—1.833(m, 1H), 1.954—1.996(m, 2H), 2.265—2.343(m, 3H), 2.408(s, 3H), 2.508(s, 5H), 2.868(d, J=10.8 Hz, 1H), 2.973—3.021(m, 5H), 3.345—3.422(m, 5H), 4.164(d, J=10.4 Hz, 1H), 5.257(s, 1H), 7.376—7.393(m, 2H), 7.527(s, 2H) | 13.57, 20.34, 21.48, 21.69, 23.38, 24.88, 26.02, 26.45, 26.70, 27.07, 29.12, 29.37, 29.79, 34.27, 36.27, 37.54, 45.72, 46.06, 51.54, 51.85, 52.27, 58.21, 60.60, 80.21, 91.11, 92.00, 104.26, 125.14, 128.20, 129.00, 133.76, 135.06, 139.34 | |
| 8e | 0.762(d, J=7.2 Hz, 3H), 0.933(d, J=6 Hz, 3H), 0.978—1.008(m, 1H), 1.172—1.395(m, 11H), 1.413—1.551(m, 6H), 1.691(d, J=10.8 Hz, 2H), 1.837—1.845(m, 1H), 1.950—1.992(m, 2H), 2.271—2.330(m, 3H), 2.401(s, 3H), 2.497(s, 5H), 2.865(d, J=10.8 Hz, 1H), 2.935—3.087(m, 5H), 3.344—3.454(m, 5H), 4.162(d, J=10.4 Hz, 1H), 5.254(s, 1H), 7.296(d, J=8 Hz, 2H), 7.607(d, J=8 Hz, 2H) | 13.56, 20.33,21.60, 21.67, 23.36, 24.87, 26.01, 26.45, 26.69, 27.06, 29.11, 29.36, 29.78, 34.26, 36.26, 37.52, 45.71, 46.06, 51.53, 51.84, 52.25, 58.18, 60.59, 80.20, 91.10, 91.99, 104.25, 127.97, 129.73, 132.23, 143.75 | |
| 8f | 0.758(d, J=7.2 Hz, 3H), 0.929(d, J=6 Hz, 3H), 0.974—1.003(m, 1H), 1.167—1.395(m, 11H), 1.409—1.545(m, 6H), 1.686(d, J=11.2 Hz, 2H), 1.825—1.849(m, 1H), 1.945—1.987(m, 2H), 2.256—2.325(m, 3H), 2.493(s, 5H), 2.858(d, J=10.8 Hz, 1H), 2.923—2.991(m, 5H), 3.304—3.409(m, 5H), 3.840(s, 3H), 4.158(d, J=10.4 Hz, 1H), 5.248(s, 1H), 6.961(d, J=9.2 Hz, 2H), 7.657(d, J=8.8 Hz, 2H) | 13.56, 20.32, 21.67, 23.36, 24.86, 26.00, 26.45, 26.68, 27.05, 29.10, 29.35, 29.77, 34.25, 36.25, 37.52, 45.70, 46.06, 51.53, 51.83, 52.23, 55.70, 58.16, 60.56, 80.18, 91.08, 91.97, 104.23, 114.27, 126.86, 130.03, 163.15 | |
Table 2 1H NMR and 13C NMR data for all compounds
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(100 MHz, CDCl3), δ | |
|---|---|---|---|
| 2 | 0.780(d, J=7.2 Hz, 3H), 0.938(d, J=6 Hz, 3H), 0.969—1.018(m, 1H), 1.194—1.354(m, 3H), 1.372—1.391(m, 3H), 1.421—1.440(m, 1H), 1.475—1.534(m, 1H), 1.581—1.599(m, 2H), 1.663—1.716(m, 1H), 1.821—1.903(m, 1H), 2.331—2.407(m, 1H), 2.568—2.699(m, 5H), 2.891—2.947(m, 2H), 3.242—3.298(m, 2H), 3.951(d, J=10.2 Hz, 1H), 5.257(s, 1H) | 13.86, 20.25, 21.80, 24.30, 25.25, 29.60, 34.25, 35.95, 37.21, 44.59, 47.20, 51.81, 52.85, 80.46, 91.26, 93.69, 105.35 | |
| 3 | 0.772(d, J=7.2 Hz, 3H), 0.925(d, J=6 Hz, 3H), 0.976—1.005(m, 1H), 1.210—1.318(m, 3H), 1.344(s, 3H), 1.376—1.485(m, 1H), 1.530—1.564(m, 1H), 1.665—1.715(m, 2H), 1.724—1.849(m, 1H), 1.962—1.998(m, 1H), 2.278—2.348(m, 1H), 2.529—2.584(m, 1H), 3.180(s, 4H), 3.317—3.379(m, 2H), 3.435—3.483(m, 2H), 4.193(d, J=10.4 Hz, 1H), 5.259(s, 1H) | 13.46, 20.23, 21.60, 24.80, 25.91, 29.05, 34.18, 36.15, 37.45, 45.59, 46.96, 51.41, 51.87, 80.18, 91.00, 92.09, 104.27 | |
| 4 | 0.042(s, 6H), 0.819(d, J=3.4 Hz, 3H), 0.886(s, 9H), 0.951(d, J=3 Hz, 3H), 0.974—1.055(m, 1H), 1.273—1.407(m, 11H), 1.468—1.601(m, 6H), 1.691—1.751(m, 2H), 1.841—1.908(m, 1H), 1.975—2.057(m, 2H), 2.302—2.382(m, 1H), 2.515—2.604(m, 1H), 2.912—2.939(m, 1H), 3.081—3.107(m, 1H), 3.299—3.475(m, 5H), 3.584(t, J=6.6 Hz, 2H), 4.202(d, J=10.4 Hz, 1H), 5.287(s, 1H) | -5.20, 13.53, 18.46, 20.21, 21.65, 23.32, 24.83, 25.58, 25.90, 26.04, 26.75, 29.02, 29.30, 32.70, 34.22, 36.16, 37.44, 45.65, 51.46, 51.77, 60.66, 63.13, 76.64, 80.02, 90.95, 91.87, 104.08 | |
| 5 | 0.769(d, J=7.2 Hz, 3H), 0.919(d, J=6 Hz, 3H), 0.968—0.999(m, 1H), 1.198—1.339(m, 11H), 1.389—1.555(m, 6H), 1.666—1.708(m, 2H), 1.818—1.860(m, 1H), 1.954—1.991(m, 2H), 2.276—2.310(m, 1H), 2.509—2.527(m, 1H), 2.875(d, J=11.6 Hz, 1H), 2.985(t, J=12.2 Hz, 1H), 3.316—3.415(m, 5H), 3.588(t, J=6.4 Hz, 2H), 4.168(d, J=10 Hz, 1H), 5.255(s, 1H), 5.259(s, 1H) | 13.58, 20.33, 21.68, 23.26, 24.86, 25.44, 26.00, 26.71, 29.12, 29.17, 32.63, 34.24, 36.23, 37.52, 45.69, 51.50, 51.77, 60.51, 62.88, 80.24, 91.14, 91.95, 104.31 | |
| 6 | 0.815(d, J=7.1 Hz, 3H), 0.952(d, J=6.1 Hz, 3H), 0.992—1.056(m, 1H), 1.301—1.422(m, 11H), 1.497—1.591(m, 6H), 1.699—1.732(m, 2H) 1.851—1.901(m, 1H), 1.987—2.069(m, 2H), 2.302—2.382(m, 1H), 2.558—2.585(m, 1H), 2.917(d, J=11.6 Hz, 1H), 3.319—3.472(m, 5H), 3.634(t, J=6.5 Hz, 2H), 4.215(d, J=10.4 Hz, 1H), 5.287(s, 1H) | 7.55, 13.92, 20.61, 22.03, 23.62, 25.24, 26.35, 26.96, 28.77, 29.43, 30.52, 33.73, 34.64, 36.55, 37.86, 46.02, 51.81, 52.13, 60.74, 77.65, 80.46, 91.37, 92.24, 104.03 | |
| 7 | 0.781(d, J=7.2 Hz, 3H), 0.939(d, J=6 Hz, 3H), 0.981—1.019(m, 1H), 1.184—1.353(m, 11H), 1.399—1.568(m, 6H), 1.685—1.725(m, 2H), 1.843—1.851(m, 1H), 1.972—2.009(m, 2H), 2.289—2.350(m, 3H), 2.538(s, 5H), 2.873—2.896(m, 1H), 3.022(t, J=4.8 Hz, 5H), 3.283—3.422(m, 5H), 4.171(d, J=10.4 Hz, 1H), 5.267(s, 1H) | 13.60, 20.35, 21.72, 23.40, 24.91, 26.05, 26.53, 26.76, 27.17, 29.15, 29.44, 29.81, 34.31, 36.30, 37.57, 45.08, 45.76, 51.59, 51.89, 52.62, 58.84, 60.71, 80.22, 91.17, 92.04, 104.31 | |
| 8a | 0.756(d, J=6.8 Hz, 3H), 0.928(d, J=6.4 Hz, 3H), 0.973—1.002(m, 1H), 1.183—1.325(m, 11H), 1.413—1.522(m, 6H), 1.686(d, J=10.4 Hz, 2H), 1.832—1.900(m, 1H), 1.944—1.993(m, 2H), 2.254—2.323(m, 3H), 2.480—2.534(m, 5H), 2.861(d, J=10.8 Hz, 1H), 2.962—3.049(m, 5H), 3.261—3.408(m, 5H), 4.157(d, J=10 Hz, 1H), 5.249(s, 1H), 7.518(d, J=7.6 Hz, 2H), 7.568(d, J=7.2 Hz, 1H), 7.724(d, J=8.4 Hz, 2H) | 13.55, 20.31, 21.66, 23.33, 24.85, 26.00, 26.64, 26.99, 29.10, 29.28, 29.76, 34.24, 36.24, 37.50, 45.69, 45.86, 51.52, 51.83, 52.15, 58.11, 60.55, 80.19, 91.08, 91.96, 104.22, 127.87, 129.16, 133.01, 135.27 | |
| 8b | 0.740(d, J=7.2 Hz, 3H), 0.912(d, J=6 Hz, 3H), 0.952—0.987(m, 1H), 1.167—1.326(m, 11H), 1.344—1.528(m, 6H), 1.672(d, J=10.8 Hz, 2H), 1.817—1.834(m, 1H), 1.918—1.973(m, 2H), 2.234—2.295(m, 3H), 2.480(s, 5H), 2.847(d, J=11.6 Hz, 1H), 2.902—2.965(m, 1H), 3.048(s, 4H), 3.282—3.392(m, 5H), 4.147(d, J=10.4 Hz, 1H), 5.235(s, 1H), 7.905(d, J=8.8 Hz, 2H), 8.351(d, J=8.8 Hz, 2H) | 13.47, 20.24, 21.57, 23.27, 24.77, 25.92, 26.45, 26.58, 26.81, 29.02, 29.23, 29.68, 34.16, 36.16, 37.43, 45.62, 46.02, 51.44, 51.74, 52.03, 57.88, 60.48, 80.13, 91.00, 91.88, 104.14, 124.33, 128.99, 141.52, 150.18 | |
| 8c | 0.756(d, J=7.2 Hz, 3H), 0.921(d, J=6 Hz, 3H), 0.968—0.998(m, 1H), 1.165—1.325(m, 11H), 1.415—1.545(m, 6H), 1.681(d, J=10 Hz, 2H), 1.827—1.861(m, 1H), 1.954(d, J=4.4 Hz, 2H), 1.986—2.326(m, 3H), 2.484(s, 5H), 2.856(d, J=11.2 Hz, 1H), 2.958(t, J=12.3 Hz, 1H), 3.281(s, 5H), 3.341—3.406(m, 4H), 4.161(d, J=10 Hz, 1H), 5.248(s, 1H), 7.571(d, J=8.4 Hz, 1H), 7.644—7.710(m, 2H), 7.907(d, J=8.8 Hz, 1H) | 13.54, 20.30, 21.64, 23.30, 24.83, 25.99, 26.42, 26.65, 26.98, 29.09, 29.29, 29.74, 34.33, 36.23, 37.49, 45.49, 45.92, 51.49, 51.81, 52.42, 58.13, 60.54, 80.19, 91.08, 91.95, 104.22, 124.09, 130.63, 130.91, 131.53, 133.87, 148.59 | |
| 8d | 0.765(d, J=7.2 Hz, 3H), 0.937(d, J=6.4 Hz, 3H), 0.982—1.012(m, 1H), 1.192—1.383(m, 11H), 1.403—1.554(m, 6H), 1.695(d, J=10 Hz, 2H), 1.882—1.833(m, 1H), 1.954—1.996(m, 2H), 2.265—2.343(m, 3H), 2.408(s, 3H), 2.508(s, 5H), 2.868(d, J=10.8 Hz, 1H), 2.973—3.021(m, 5H), 3.345—3.422(m, 5H), 4.164(d, J=10.4 Hz, 1H), 5.257(s, 1H), 7.376—7.393(m, 2H), 7.527(s, 2H) | 13.57, 20.34, 21.48, 21.69, 23.38, 24.88, 26.02, 26.45, 26.70, 27.07, 29.12, 29.37, 29.79, 34.27, 36.27, 37.54, 45.72, 46.06, 51.54, 51.85, 52.27, 58.21, 60.60, 80.21, 91.11, 92.00, 104.26, 125.14, 128.20, 129.00, 133.76, 135.06, 139.34 | |
| 8e | 0.762(d, J=7.2 Hz, 3H), 0.933(d, J=6 Hz, 3H), 0.978—1.008(m, 1H), 1.172—1.395(m, 11H), 1.413—1.551(m, 6H), 1.691(d, J=10.8 Hz, 2H), 1.837—1.845(m, 1H), 1.950—1.992(m, 2H), 2.271—2.330(m, 3H), 2.401(s, 3H), 2.497(s, 5H), 2.865(d, J=10.8 Hz, 1H), 2.935—3.087(m, 5H), 3.344—3.454(m, 5H), 4.162(d, J=10.4 Hz, 1H), 5.254(s, 1H), 7.296(d, J=8 Hz, 2H), 7.607(d, J=8 Hz, 2H) | 13.56, 20.33,21.60, 21.67, 23.36, 24.87, 26.01, 26.45, 26.69, 27.06, 29.11, 29.36, 29.78, 34.26, 36.26, 37.52, 45.71, 46.06, 51.53, 51.84, 52.25, 58.18, 60.59, 80.20, 91.10, 91.99, 104.25, 127.97, 129.73, 132.23, 143.75 | |
| 8f | 0.758(d, J=7.2 Hz, 3H), 0.929(d, J=6 Hz, 3H), 0.974—1.003(m, 1H), 1.167—1.395(m, 11H), 1.409—1.545(m, 6H), 1.686(d, J=11.2 Hz, 2H), 1.825—1.849(m, 1H), 1.945—1.987(m, 2H), 2.256—2.325(m, 3H), 2.493(s, 5H), 2.858(d, J=10.8 Hz, 1H), 2.923—2.991(m, 5H), 3.304—3.409(m, 5H), 3.840(s, 3H), 4.158(d, J=10.4 Hz, 1H), 5.248(s, 1H), 6.961(d, J=9.2 Hz, 2H), 7.657(d, J=8.8 Hz, 2H) | 13.56, 20.32, 21.67, 23.36, 24.86, 26.00, 26.45, 26.68, 27.05, 29.10, 29.35, 29.77, 34.25, 36.25, 37.52, 45.70, 46.06, 51.53, 51.83, 52.23, 55.70, 58.16, 60.56, 80.18, 91.08, 91.97, 104.23, 114.27, 126.86, 130.03, 163.15 | |
| Compd. | IC50*/(μmol·mL-1) | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| Artemisinin | 0.72 | 0.57 | 0.44 |
| 1 | 0.65 | 0.45 | 0.40 |
| 3 | 0.52 | 0.31 | 0.30 |
| 8a | 0.22 | 0.15 | 0.10 |
| 8b | 0.24 | 0.14 | 0.11 |
| 8c | 0.23 | 0.15 | 0.12 |
| 8d | 0.21 | 0.16 | 0.09 |
| 8e | 0.25 | 0.15 | 0.10 |
| 8f | 0.24 | 0.14 | 0.11 |
Table 3 In vitro anticancer activities against SMMC-7721cell lines with artemisinin and compounds 1, 3 and 8a—8f
| Compd. | IC50*/(μmol·mL-1) | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| Artemisinin | 0.72 | 0.57 | 0.44 |
| 1 | 0.65 | 0.45 | 0.40 |
| 3 | 0.52 | 0.31 | 0.30 |
| 8a | 0.22 | 0.15 | 0.10 |
| 8b | 0.24 | 0.14 | 0.11 |
| 8c | 0.23 | 0.15 | 0.12 |
| 8d | 0.21 | 0.16 | 0.09 |
| 8e | 0.25 | 0.15 | 0.10 |
| 8f | 0.24 | 0.14 | 0.11 |
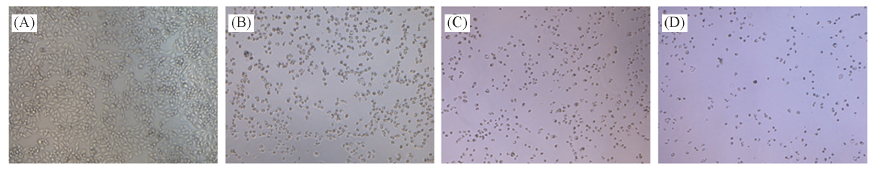
Fig.1 Morphological changes of SMMC-7721 cells induced by compound 8d in different intervals of time (A) Normal SMMC-7721 cells; (B)—(D) morphological changes of cells induced by compound 8d for 24, 48 and 72h, respectively.
| [1] | Tu Y. Y., Drugs of Artemisia Annua and Artemisinin Chemical Industry Press, Beijing, 2009, 1— 4 |
| ( 屠呦呦. 青蒿及青蒿素类药物, 北京: 化学工业出版社, 2009, 1— 4) | |
| [2] | Klayman D., L. , Science, 1985, 228( 4703), 1049- 1055 |
| [3] | 徐振海. 安徽预防医学杂志, 2004, 10( 3), 181- 182 |
| Xu Z., H. , Anhui Journal of Preventive Medicine, 2004, 10( 3), 181- 182 | |
| [4] | 刘宁, 杨腊虎, 张正行, 俞如英. 药物分析杂志, 2002, 22( 4), 303- 306 |
| Liu, N. , Yang L., H. , Zhang Z., X. , Yu R., Y. , Chinese J. Pharmac. Anal., 2002, 22( 4), 303- 306 | |
| [5] | Pacorel, B. , Leung S., C. , Stachulski A., V. , Davies, J. , Vivas, L. , Lander, H. , Ward S., A. , Kaiser, M. , Brun, R. , O’, Neill P. M. , J. Med. Chem., 2010, 53( 2), 633- 640 |
| [6] | Hindley, S. , Ward S., A. , Storr R., C. , Searle N., L. , Bray P., G. , Park B., K. , Davies, J. , O’, Neill P. M. , J. Med. Chem., 2002, 45( 5), 1052- 1063 |
| [7] | Ribeiro I., R. , Olliaro, P. , Med. Trap., 1998, 58( 3), 50- 53 |
| [8] | Lin A., J. , Klayman D., L. , Milhous W., K. , J. Med. Chem., 1987, 30( 11), 2147- 2150 |
| [9] | 杜幼芹, 肖长义. 临床和实验医学杂志, 2009, 8( 7), 8- 10 |
| Du Y., Q. , Xiao C., Y. , Journal of Clinical and Experimental Medicine, 2009, 8( 7), 8- 10 | |
| [10] | 王勤, 吴理茂, 李爱媛, 赵一, 王乃平. 中国中药杂志, 2001, 26, 707- 708 |
| Wang, Q. , Wu L., M. , Li A., Y. , Zhao, Y. , Wang N., P. , Chinese Medicine Traditional, 2001, 26, 707- 708 | |
| [11] | Li, Y. , Shan, F. , Wu J., M. , Wu G., S. , Ding, J. , Xiao, D. , Yang W., Y. , Atassi, G. , Leonce, L. , Caignard D., H. , Renard, P. , Bioorg. Med. Chem. Lett., 2001, 11, 5- 8 |
| [12] | Li, Y. , Wu J., M. , Shan, F. , Wu G., S. , Ding, J. , Xiao, D. , Han J., X. , Atassi, G. , Leonce, L. , Caignard D., H. , Renard, P. , Bioorg. Med. Chem., 2003, 11, 977- 980 |
| [13] | 孙玮辰, 韩家娴, 杨蔚怡, 邓定安, 乐秀芳. 中国药理学报, 1992, 13( 6), 541- 543 |
| Sun W., C. , Han J., X. , Yang W., Y. , Deng D., A. , Le X., F. , Chinese Journal of Pharmacology, 1992, 13( 6), 541- 543 | |
| [14] | Singh N., P. , Lai, H. , Life Science, 2001, 70( 1), 49- 56 |
| [15] | Zhou H., J. , Wang, Z. , Li, A. , Anticancer Drugs, 2008, 19( 3), 247- 255 |
| [16] | Genovese R., F. , Newman D., B. , Arch. Toxicol., 2008, 82, 379- 385 |
| [17] | Wesche M., A. , DeCoster F., C. , Tortella F., C. , Brewer T., G. , Antimicrob. Agents Chemother., 1994, 38( 8), 1813- 1819 |
| [18] | Fishwick, J. , McLean W., G. , Edwards, G. , Ward S., A. , Chem. Biol. Interact., 1995, 96( 3), 263- 271 |
| [19] | Nontprasert, A. , Pukrittayakamee, S. , Prakongpan, S. , Supanaranond, W. , Looareesuwan, S. , White N., J. , Trans, R. , Soc. Trop. Med. Hyg., 2002, 96( 1), 99- 101 |
| [20] | Nontprasert, A. , Pukrittayakamee, S. , Nosten-Bertrand, M. , Vanijanonta, S. , White N., J. , Am. J. Trop. Med. Hyg., 2000, 62( 3), 409- 412 |
| [21] | Nontprasert, A. , Nosten-Bertrand, M. , Pukrittayakamee, S. , Vanijanonta, S. , Angus B., J. , White N., J. , Am. J. Trop. Med. Hyg., 1998, 59( 4), 519- 522 |
| [22] | Haynes R., K. , Fugmann, B. , Stetter, J. , Rieckmann, K. , Heilmann H., D. , Chan H., W. , Cheung M., K. , Lam W., L. , Wong H., N. , Croft S., L. , Vivas, L. , Ra-tray, L. , Stewart, L. , Peters, W. , Robinson B., L. , Edstein M., D. , Kotecka, B. , Kyle D., E. , Beckermann, B. , Gerisch, M. , Radtke, M. , Schmuck, G. , Steinke, W. , Wollborn, U. , Schmeer, K. , Axel R., J. , Angew. Chem. Int. Ed., 2006, 45( 12), 2082- 2088 |
| [23] | Nagelschmitz, J. , Voith, B. , Wensing, G. , Roemer, A. , Fugmann, B. , Haynes R., K. , Kotecka B., M. , Rieckmann K., H. , Edstein M., D. , Antimicrob. Agents Chemother., 2008, 52( 9), 3085- 3091 |
| [24] | Schmuck, G. , Temerowski, M. , Haynes R., K. , Fugmann, B. , Recent. Res. Dev. Antimicrob. Agents. Chemother., 2003, 3, 35- 40 |
| [25] | Waknine-Grinberg J., H. , Hunt, N. , Bentura-Marciano, A. , McQuillan J., A. , Chan H., W. , Chan W., C. , Barenholz, Y. , Haynes R., K. , Golenser, J. , Malaria. Journal, 2010, 9, 227- 230 |
| [26] | Ildiko, R. , Chan W., C. , Haynes R., K. , Sibley L., D. , Antimicrobial Agents and Chemotherapy, 2009, 53( 10), 4450- 4456 |
| [27] | Haynes R., K. , Chan W., C. , Lung C., M. , Uhlemann A. X., C. , Eckstein, U. , Taramelli, D. , Parapini, S. , Monti, D. , Krishna, S. , ChemMedChem, 2007, 2( 10), 1480- 1497 |
| [28] | Nandurdikar R., S. , Maciag A., E. , Citro M., L. , Shami P., J. , Keefer L., K. , Saavedra J., E. , Chakrapani, H. , Bioorg. Med. Chem. Lett., 2009, 19, 2760- 2762 |
| [29] | Gillet, R. , Jeannesson, P. , Sefraoui, H. , Arnould-GueArin M., L. , Kirkiacharian, S. , Jardillier J., C. , Pieri, F. , Cancer. Chemother. Pharmaco., 1998, 41( 3), 252- 255 |
| [30] | Gabriel F., E. , Gu, J. , Slater L., M. , Hara, K. , Jacobs J., W. , Cancer. Chemother. Pharmacol., 2000, 45( 3), 183- 191 |
| [31] | Vullo, D. , Franchi, M. , Gallori, E. , Pastorek, J. , Scozzafava, A. , Pastorekova, S. , Supuran C., T. , Bioorg. Med. Chem. Lett., 2003, 13, 1005- 1009 |
| [32] | Owa, T. , Yoshino, H. , Okauchi, T. , Okabe, T. , Ozawa Y. N., H. , Yoshimatsu, K. , Nagasu, T. , Koyanagi, N. , Kitoh, K. , Bioorg. Med. Chem. Lett., 2002, 12( 16), 2097- 2100 |
| [33] | Chang J., Y. , Hsieh H., P. , Chang C., Y. , Hsu K., S. , Chiang Y., F. , Chen C., M. , Kuo C., C. , Liou J., P. , J. Med. Chem., 2006, 49( 23), 6656- 6659 |
| [34] | Kamal, A. , Khan M. N., A. , Raddy K., S. , Rohini, K. , Bioorg. Med. Chem., 2007, 15( 2), 1004- 1013 |
| [35] | Hu G., Q. , Wang G., Q. , Duan N., N. , Wen X., Y. , Cao T., Y. , Xie S., Q. , Huang W., L. , Chem. Res. Chinese Universities, 2012, 28( 6), 980- 984 |
| [36] | Zhao, J. , Xuan L., N. , Zhao H., C. , Cheng, J. , Fu X., Y. , Li, S. , Jing, F. , Liu Y., M. , Chen B., Q. , Chem. Res. Chinese Universities, 2014, 30( 5), 764- 769 |
| [37] | 杨洪亮, 徐国兴, 宝梅英, 张大鹏, 李志伟, 裴亚中. 高等学校化学学报, 2014, 35( 12), 2584- 2592 |
| Yang H., L. , Xu G., X. , Bao M., Y. , Zhang D., P. , Li Z., W. , Pei Y., Z. , Chem. J. Chinese Universities, 2014, 35( 12), 2584- 2592 | |
| [38] | Oh, S. , Shin W., S. , Ham, J. , Lee, S. , Bioorg. Med. Chem. Lett., 2010, 20( 14), 4112- 4115 |
| [39] | Wei M., X. , Feng, L. , Li X., Q. , Zhou X., Z. , Shao Z., H. , Eur. J. Med. Chem., 2009, 44( 8), 3340- 3344 |
| [40] | 赵晨阳, 邱嵘, 郑荣梁. 兰州大学学报(自然科学版), 2000, 36( 4), 66- 68 |
| Zhao C., Y. , Qiu, R. , Zheng R., L. , Journal of Lanzhou University (Natural Sciences), 2000, 36( 4), 66- 68 |
| [1] | RUAN Xianghui, ZHAO Hongju, ZHANG Cheng, CHEN Lijuan, LI Pu, WANG Yihui, HE Ming, XUE Wei. Syntheses and Bioactivities of Myricetin Derivatives Containing Piperazine Acidamide Moiety† [J]. Chem. J. Chinese Universities, 2018, 39(6): 1197. |
| [2] | LIU Li, MA Yangyang, WANG Kuan, JIA Yunjing, LI Wan, ZHU Huajie. Anti-tumor and Antimicrobial Activities of β-Carbolines† [J]. Chem. J. Chinese Universities, 2018, 39(4): 674. |
| [3] | MIAO Ruixiang,LIU Dongqing,REN Shun,ZHU Zexian,CHEN Yingbo,ZHANG Yufeng. Foams of Supramolecular Network Constructed by Poly(calixarene-piperazine) Amide and 2,2'-Bipyridine† [J]. Chem. J. Chinese Universities, 2018, 39(10): 2327. |
| [4] | YAN Chang, ZOU Yu, FU Junjie, HUANG Zhangjian, ZHANG Dayong, ZHANG Yihua. Design, Synthesis and Biological Evaluation of Novel O2-(2,4-Dinitrophenyl)diazeniumdiolates as Anti-tumor Agents† [J]. Chem. J. Chinese Universities, 2017, 38(4): 591. |
| [5] | ZHAO Lun, BAI Helong, SUN Erjun, WANG Xiaofeng, WANG Zichen. Synthesis and Characterization of Novel Ni(Ⅱ) Metal-organic Frameworks with Helical Crystal Structure† [J]. Chem. J. Chinese Universities, 2016, 37(7): 1250. |
| [6] | ZHANG Yan, WANG Baolei, ZHAN Yizhou, ZHANG Liyuan, LI Yonghong, LI Zhengming. Synthesis and Biological Activities of Novel 5-(Pyridine-3-yl)-1,2,4-triazole Mannich Bases and Bis-Mannich Bases Containing Piperazine Moiety† [J]. Chem. J. Chinese Universities, 2016, 37(6): 1100. |
| [7] | SHI Lei, YANG Wencong, ZENG Shuying, MO Tingting, ZHANG Zhao, CAO Manli, LIU Haiyang. DNA-binding and Anti-tumor Activities of Cobalt Corrole Complexes† [J]. Chem. J. Chinese Universities, 2016, 37(6): 1059. |
| [8] | ZHAO Lun, ZHAO Changjiang. Syntheses, Characterizations and Properties of Two Unusual (3,4,6) and (3,6)-Connected Polynuclear 3D Metal-organic Frameworks† [J]. Chem. J. Chinese Universities, 2016, 37(10): 1763. |
| [9] | ZHAO Lun, ZHANG Min, SUN Erjun, WANG Xiaofeng, WANG Zichen. Synthesis, Structures and Photochemical Properties of Two New Cd(II) Metal-organic Frameworks Isolated from the Same Reaction System† [J]. Chem. J. Chinese Universities, 2015, 36(12): 2380. |
| [10] | WANG Gang, HAN Leiqiang, FANG Hao. Syntheses and Antitumor Activities of Phenylpiperazine Derivatives† [J]. Chem. J. Chinese Universities, 2015, 36(12): 2435. |
| [11] | ZHENG Yongbiao, PANG Haiyue, WANG Jifeng, CHEN Danxia, SHI Guowei, HUANG Jianzhong. Novel Diketopiperazine and Ten-membered Macrolides from the Entomogenous Fungus Paecilomyces tenuipes† [J]. Chem. J. Chinese Universities, 2014, 35(8): 1665. |
| [12] | TONG Chunyi, TANG Fengxia, LIU Bin, LIAO Hongdong, LIU Xuanming. Preparation of Micro-spheres Co-carried Anti-tumor Drug and Sensitizer(5-Fu-COS/SeNP) and Their Inhibiting Tumorous Cellular Growths Activity† [J]. Chem. J. Chinese Universities, 2014, 35(7): 1603. |
| [13] | WANG Junhua, WANG Quande, DUN Yanyan, FANG Hao. Syntheses and Antitumor Activities of Purine-sulfonamides Derivatives† [J]. Chem. J. Chinese Universities, 2014, 35(6): 1189. |
| [14] | LI Dan, FANG Wen-Jun, LIU Li, WU Qian, ZHANG Hong-Yan, SUN Xiao-Ri. Volumetric and Viscous Properties for Binary Mixtures of N-Methylpiperazine with Ethyl Acetate or Butyl Acetate from 298.15 K to 313.15 K [J]. Chem. J. Chinese Universities, 2013, 34(8): 1924. |
| [15] | XU Jun-Ying, DONG Wei-Li, XIONG Li-Xia, LI Zheng-Ming. Design, Synthesis and Biological Activities of Amides(Sulfonamides) Containing N-Pyridylpyrazole [J]. Chem. J. Chinese Universities, 2012, 33(02): 298. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||