

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (3): 482.doi: 10.7503/cjcu20131049
• Analytical Chemistry • Previous Articles Next Articles
XIN Hua1, CHEN Libo1, SHI Hongyan2, SONG Wenbo2, LIU Tiemei1,*( )
)
Received:2013-10-28
Online:2014-03-10
Published:2019-08-01
Contact:
LIU Tiemei
E-mail:liutiemei777@163.com
Supported by:CLC Number:
TrendMD:
XIN Hua, CHEN Libo, SHI Hongyan, SONG Wenbo, LIU Tiemei. Electrodeposition of Nanostructured Cu Electrode and Its Glucose Assay Performance†[J]. Chem. J. Chinese Universities, 2014, 35(3): 482.
| Electrodeposition method | Start oxidation current/V | 0.45 V Oxidation current/μA |
|---|---|---|
| Constant current electrodeposition(I=-1.20 mA, t=10 s) | 0.32 | 550.32 |
| Constant potential electrodeposition(E=-1.20 V, t=10 s) | 0.30 | 746.78 |
| Potential step electrodeposition(E1=-1.50 V, t1=2 s; E2=-1.00 V, t2=8 s) | 0.32 | 431.25 |
Table 1 Comparison of electrocatalytical properties towards glucose oxidation(1.0 mmol/L) at Cu/PSS/PDDA/ITO electrodes prepared by different electrodeposition methods
| Electrodeposition method | Start oxidation current/V | 0.45 V Oxidation current/μA |
|---|---|---|
| Constant current electrodeposition(I=-1.20 mA, t=10 s) | 0.32 | 550.32 |
| Constant potential electrodeposition(E=-1.20 V, t=10 s) | 0.30 | 746.78 |
| Potential step electrodeposition(E1=-1.50 V, t1=2 s; E2=-1.00 V, t2=8 s) | 0.32 | 431.25 |
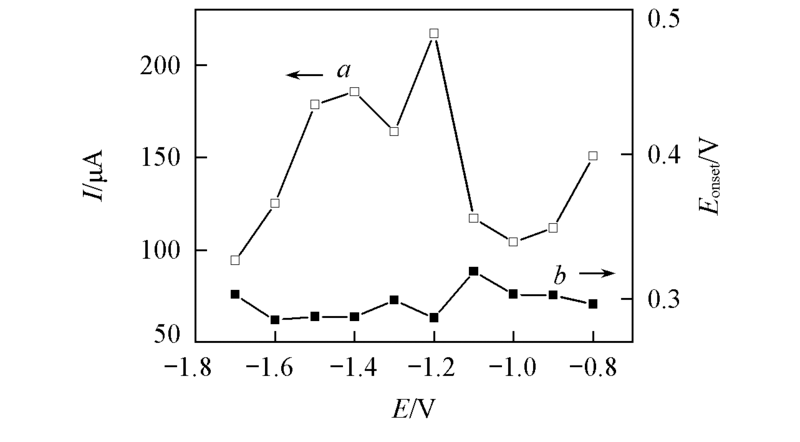
Fig.1 Evolution of oxidative peak current(a) at 0.40 V and onset potential(b) of 0.2 mmol/L glucose at Cu/PSS/PDDA/ITO deposited at different potentials for 10 s in 0.5 mol/L H2SO4 containing 0.1 mol/L CuSO4
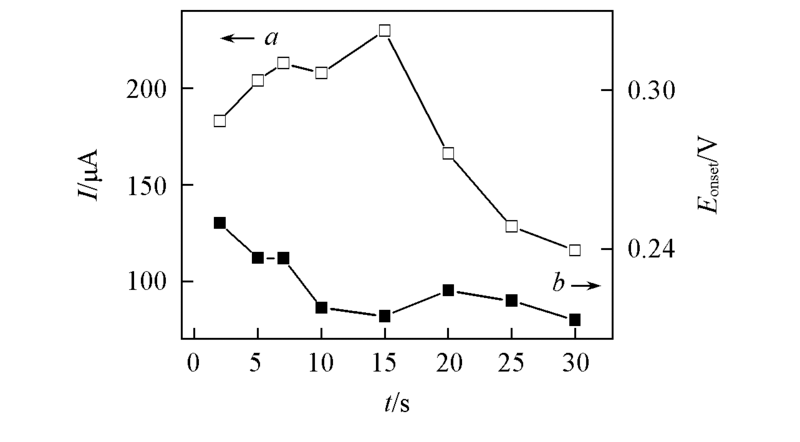
Fig.2 Evolution of oxidative peak current(a) at 0.40 V and onset potential(b) of 0.2 mmol/L glucose at Cu/PSS/PDDA/ITO deposited at -1.20 V for different time in 0.5 mol/L H2SO4 containing 0.1 mol/L CuSO4
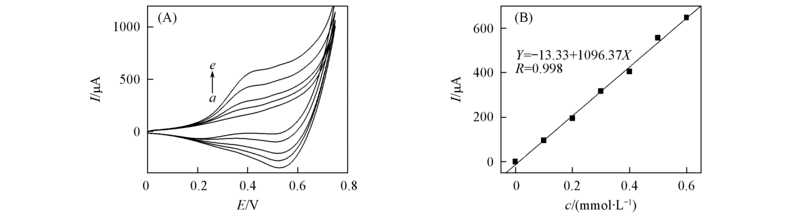
Fig.4 Current responses of glucose with different concentrations at Cu/PSS/PDDA/ITO electrode(A) and its calibration curve(B) (A) c(Glucose)/(mmol·L-1): a. 0; b. 0.1; c. 0.2; d. 0.3; e. 0.4.
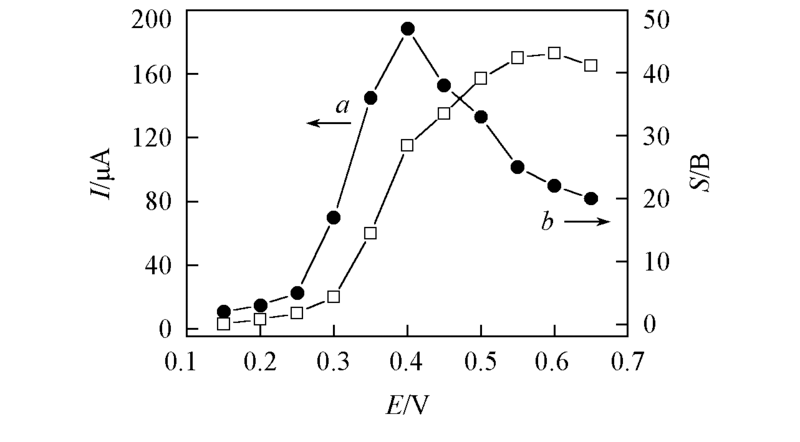
Fig.5 Plots of oxidation current density(a) and signal-to-background ratio(b) to the applied potential at Cu/PSS/PDDA/ITO electrode obtained by chronoamperometry
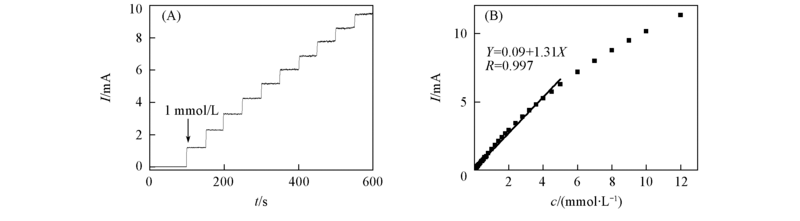
Fig.6 Steady-state current-time response of Cu/PSS/PDDA/ITO electrode with successive addition of 1.0 mmol/L glucose into 0.1 mol/L NaOH at 0.40 V(A) and calibration curve for amperome-tric response to glucose at Cu/PSS/PDDA/ITO electrode(B)
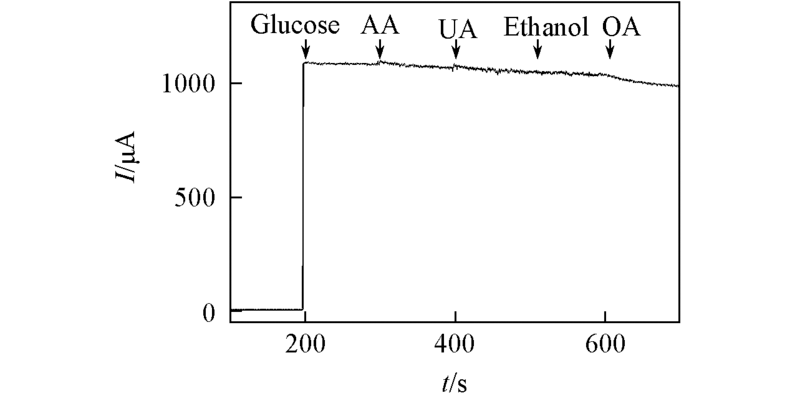
Fig.7 Ampermetric current response of Cu/PSS/PDDA/ITO electrode to 1.0 mmol/L glucose and other interferences in 0.1 mol/L NaOH at +0.40 Vc(AA)=c(UA)=0.1 mmol/L; c(Ethanol)=c(OA)=10 mmol/L.
| Sample | Glucose concentration/ (mmol·L-1) | Detected results on Cu/PSS/PDDA/ITO/(mmol·L-1) | Recovery(%) | Detected results by standard spectrophotometry/(mmol·L-1) |
|---|---|---|---|---|
| 1 | 0.20 | 0.2055 | 102.80 | 0.2025 |
| 2 | 0.50 | 0.5013 | 100.30 | 0.5026 |
| 3 | 0.80 | 0.7973 | 99.66 | 0.8019 |
| 4 | 1.00 | 0.9904 | 99.04 | 1.0350 |
| 5 | 2.10 | 2.1420 | 102.00 | 2.1020 |
| 6 | 3.50 | 3.4630 | 98.94 | 3.5080 |
Table 2 Results of analysis of standard samples with different concentrations of glucose at 0.40 V
| Sample | Glucose concentration/ (mmol·L-1) | Detected results on Cu/PSS/PDDA/ITO/(mmol·L-1) | Recovery(%) | Detected results by standard spectrophotometry/(mmol·L-1) |
|---|---|---|---|---|
| 1 | 0.20 | 0.2055 | 102.80 | 0.2025 |
| 2 | 0.50 | 0.5013 | 100.30 | 0.5026 |
| 3 | 0.80 | 0.7973 | 99.66 | 0.8019 |
| 4 | 1.00 | 0.9904 | 99.04 | 1.0350 |
| 5 | 2.10 | 2.1420 | 102.00 | 2.1020 |
| 6 | 3.50 | 3.4630 | 98.94 | 3.5080 |
| [1] | Rappaport F., Eichhorn E., Am. J. Clin. Path., 1950, 20, 834—836 |
| [2] | Wang J., J. Pharm. Biomed. Anal., 1999, 19, 47—53 |
| [3] | Park S., Chung T. D. Kim H. C., Anal. Chem., 2003, 75, 3046—3049 |
| [4] | Jiang L. C., Zhang W. D., Biosens. Bioelectron., 2010, 25, 1402—1407 |
| [5] | Yang J., Jiang L. C., Zhang W. D., Gunasekaran S., Talanta,2010, 82, 25—33 |
| [6] | Ding Y., Wang Y., Su L. A., Bellagamba M., Zhang H., Lei Y., Biosens. Bioelectron., 2010, 26, 542—548 |
| [7] | Song S. Q., Rao R. C., Yang H. X., Zhang A. M., J. Phys. Chem. C,2010, 114, 13998—14003 |
| [8] | Xia Y., Yang P., Sun Y., Wu Y., Mayers B., Gates B., Yin Y., Kim F., Yan H., Adv. Mater., 2003, 15, 353—389 |
| [9] | Wang X., Hu C., Liu H., Du G., He X., Xi Y., Sensors and Actuators B: Chemical,2010, 144, 220—225 |
| [10] | Qu J. Y., Kang S. P., Lou T. F., Du X. P., Chem. J. Chinese Universities,2013, 34(9), 2079—2101 |
| (屈建莹, 康世平, 娄童芳, 杜学萍. 高等学校化学学报, 2013, 34(9), 2079—2101) | |
| [11] | Shiju N. R., Guliants V. V., Appl. Catal. A: Gen., 2009, 356, 1—17 |
| [12] | Murray R. W., Chem. Rev., 2008, 108, 2688—2720 |
| [13] | Kang X., Mai Z., Zou X., Cai P., Mo J., Anal. Biochem., 2007, 363, 143—150 |
| [14] | Zhou S., Feng X., Shi H., Chen J., Zhang F., Song W., Sensors and Actuators B: Chemical,2013, 177, 445—452 |
| [15] | Zhao J., Wei L., Peng C., Su Y., Yang Z., Zhang L., Wei H., Zhang Y., Biosens. Bioelectron., 2013, 47, 86—91 |
| [16] | Li R. Y., Xia Q. F., Li Z. J., Sun X. L., Liu J. K., Biosens. Bioelectron., 2013, 44, 235—240 |
| [17] | Tong S., Wang W., Li X., Xu Y., Song W., J. Phy. Chem. C,2009, 113, 6832—6838 |
| [18] | Qin S., Chen L. S., Shi Y., Liao Q., Jin X. G., Chem. J. Chinese Universities,2005, 26(6), 1183—1185 |
| (秦胜, 陈柳生, 史燚, 廖琦, 金熹高. 高等学校化学学报, 2005, 26(6), 1183—1185) | |
| [19] | Meng F., Shi W., Sun Y., Zhu X., Wu G., Ruan C., Liu X., Ge D., Biosens. Bioelectron., 2013, 42, 141—147 |
| [20] | Luo S., Su F., Liu C., Li J., Liu R., Xiao Y., Li Y., Liu X., Cai Q., Talanta,2011, 86, 157—163 |
| [21] | Batchelor C., Du Y., Wildgoose G. G., Compton R. G., Sensors and Actuators B: Chemical,2008, 135, 230—235 |
| [22] | Zhao Y., Zhao J., Ma D., Li Y., Hao X., Li L., Yu C., Zhang L., Lu Y., Wang Z., Colloids and Surfaces A-Physicochemical and Engineering Aspects,2012, 409, 105—111 |
| [23] | Kumar S. A., Cheg H. W., Chen S. M., Wang S. F., Materials Science & Engineering C-Materials for Biological Applications,2010, 30, 86—91 |
| [1] | QIN Yongji, LUO Jun. Applications of Single-atom Catalysts in CO2 Conversion [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220300. |
| [2] | YAO Qing, YU Zhiyong, HUANG Xiaoqing. Progress in Synthesis and Energy-related Electrocatalysis of Single-atom Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220323. |
| [3] | LIN Gaoxin, WANG Jiacheng. Progress and Perspective on Molybdenum Disulfide with Single-atom Doping Toward Hydrogen Evolution [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220321. |
| [4] | WANG Sicong, PANG Beibei, LIU Xiaokang, DING Tao, YAO Tao. Application of XAFS Technique in Single-atom Electrocatalysis [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220487. |
| [5] | ZHENG Anni, JIN Lei, YANG Jiaqiang, WANG Zhaoyun, LI Weiqing, YANG Fangzu, ZHAN Dongping, TIAN Zhongqun. Effects of 5,5-Dimethylhydantoin on Electroless Copper Plating [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220191. |
| [6] | HAN Fuchao, LI Fujin, CHEN Liang, HE Leiyi, JIANG Yunan, XU Shoudong, ZHANG Ding, QI Lu. Enhance of CoSe2/C Composites Modified Separator on Electrochemical Performance of Li-S Batteries at High Sulfur Loading [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220163. |
| [7] | WANG Ruhan, JIA Shunhan, WU Limin, SUN Xiaofu, HAN Buxing. CO2-involved Electrochemical C—N Coupling into Value-added Chemicals [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220395. |
| [8] | WANG Lijun, LI Xin, HONG Song, ZHAN Xinyu, WANG Di, HAO Leiduan, SUN Zhenyu. Efficient Electrocatalytic CO2 Reduction to CO by Tuning CdO-Carbon Black Interface [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220317. |
| [9] | YANG Lijun, YU Yang, ZHANG Lei. Construction of Dual-functional 2D/3D Hydrid Co2P-CeO x Heterostructure Integrated Electrode for Electrocatalytic Urea Oxidation Assisted Hydrogen Production [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220082. |
| [10] | XIA Tian, WAN Jiawei, YU Ranbo. Progress of the Structure-property Correlation of Heteroatomic Coordination Structured Carbon-based Single-atom Electrocatalysts [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220162. |
| [11] | ZHANG Hongwei, CHEN Wen, ZHAO Meiqi, MA Chao, HAN Yunhu. Research Progress of Single Atom Catalysts in Electrochemistry [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220129. |
| [12] | WU Jun, HE Guanchao, FEI Huilong. Self-supported Film Electrodes Decorated with Single Atoms for Energy Electrocatalysis [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220051. |
| [13] | CHEN Changli, MI Wanliang, LI Yujing. Research Progress of Single Atom Catalysts in Electrochemical Hydrogen Cycling [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220065. |
| [14] | CHEN Zhaoyang, XUE Yurui, LI Yuliang. Synthesis and Applications of Graphdiyne Based Zerovalent Atomic Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220063. |
| [15] | FAN Xiaoyong, ZHU Yongqiang, WU Yan, ZHANG Shuai, XU Lei, GOU Lei, LI Donglin. Three-dimensional Porous Sn-Zn Alloy Towards Uniform Zn Plating/stripping [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210861. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||