

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (2): 362.doi: 10.7503/cjcu20130599
• Physical Chemistry • Previous Articles Next Articles
ZHAO Lifang1, ZHANG Qian2, HE Shici3, LIU Weiwei2, YANG Yusheng4, ZHENG Junwei2, PAN Qinmin1, LI Decheng2,*( )
)
Received:2013-06-27
Online:2014-02-10
Published:2013-09-02
Contact:
LI Decheng
E-mail:lidecheng@suda.edu.cn
Supported by:CLC Number:
TrendMD:
ZHAO Lifang, ZHANG Qian, HE Shici, LIU Weiwei, YANG Yusheng, ZHENG Junwei, PAN Qinmin, LI Decheng. Structure and Electrochemical Properties of Li-rich Li2+4xMn0.6+2xNi0.6-6xCr0.8O4 Spinel Prepared by Hydrothermal Method†[J]. Chem. J. Chinese Universities, 2014, 35(2): 362.
| Aimed product | Obtained product |
|---|---|
| LMn0.667NCr0.8O4 | Li1.077Mn0.617Ni0.399Cr0.82O4 |
| LMn0.700NCr0.8O4 | Li1.149Mn0.675Ni0.293Cr0.896O4 |
| LMn0.733NCr0.8O4 | Li1.241Mn0.751Ni0.211Cr0.899O4 |
| LMn0.767Ni0.1Cr0.8O4 | Li1.925Mn0.697Ni0.129Cr0.880O4 |
Table 1 Results of the composition analysis for the Li2+4xMn0.6+2xNi0.6-6xCr0.8O4(x=1/30, 1/20, 1/15, 1/12)
| Aimed product | Obtained product |
|---|---|
| LMn0.667NCr0.8O4 | Li1.077Mn0.617Ni0.399Cr0.82O4 |
| LMn0.700NCr0.8O4 | Li1.149Mn0.675Ni0.293Cr0.896O4 |
| LMn0.733NCr0.8O4 | Li1.241Mn0.751Ni0.211Cr0.899O4 |
| LMn0.767Ni0.1Cr0.8O4 | Li1.925Mn0.697Ni0.129Cr0.880O4 |
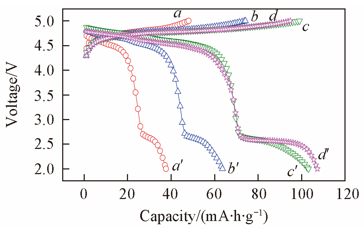
Fig.6 Initial charge(a—d) and discharge(a'—d') curves of Li2+4xMn0.6+2xNi0.6-6xCr0.8O4 at a current density of 20 mA/g in 2.0—5.0 V voltage rangex: a, a'. 1/30; b, b'. 1/20; c, c'. 1/15;d, d'. 1/12.
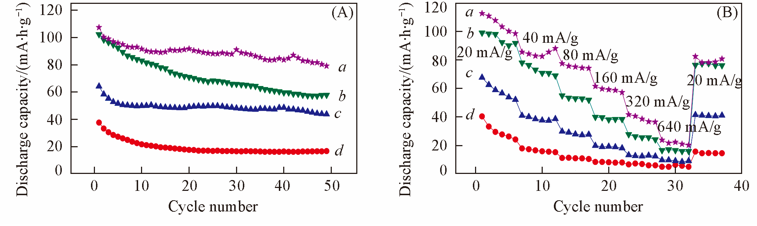
Fig.8 Cyclic performance of Li2+4xMn0.6+2xNi0.6-6xCr0.8O4 at a current density of 20 mA/g in 2.0—5.0 V voltage range(A) and rate capabilities of as-prepared Li2+4xMn0.6+2xNi0.6-6xCr0.8O4(B)x: a. 1/12; b. 1/15; c. 1/20; d. 1/30.
| [1] | Yang M. C., Xu B., Cheng J. H., Pan C. J., Hwang B. J., Meng Y. S., Chem. Mater., 2011, 23, 2832—2841 |
| [2] | Benedek R., Johnson C. S., Thackeray M. M., Electrochemical and Solid-State Letters, 2006, 9(6), A289—A291 |
| [3] | Lu H. Q., Wu F., Su Y. F., Li N., Chen S., Bao L. Y., Chem. J. Chinese Universities, 2011, 32(4), 946—951 |
| (卢华权, 吴锋, 苏岳锋, 李宁, 陈实, 包丽颖.高等学校化学学报,2011, 32(4), 946—951) | |
| [4] | Fang H. S., Wang Z. X., Zhang B., Li X. H., Li G. S., Electrochem. Commun., 2007, 9, 1077—1082 |
| [5] | Hagh N. M., Amatucci G. G., J. Power Sources, 2010, 195, 5005—5012 |
| [6] | He S. C., Zhang Q., Liu W. W., Fang G. Q., Sato Y., Zheng J. W., Li D. C., Chem. Res. Chinese Universities, 2013, 29(2), 329—332 |
| [7] | Wang S. J., Zhao Y. J., Zhao C. S., Xia D. G., Chem. J. Chinese Universities, 2009, 30(12), 2358—2362 |
| (王绥军, 赵煜娟, 赵春松, 夏定国.高等学校化学学报,2009, 30(12), 2358—2362) | |
| [8] | Ohzuku T., Makimura Y., Chem. Lett., 2001, 30, 744—745 |
| [9] | Ohzuku T., Ueda A., Nagayama M., Iwakoshi Y., Komori H., Electrochimica Acta, 1993, 38, 1159—1167 |
| [10] | Kim H. S., Kong M., Kim K., Kim I. J., Gu H. B., J. Power Sources, 2007, 171, 917—921 |
| [11] | Ito A., Li D., Ohsawa Y., Sato Y., J. Power Sources, 2008, 183, 344—346 |
| [12] | Feng C. Q., Zhang K. L., Sun J. T., Spectroscopy and Spectral Analysis, 2003, 23(2), 279—281 |
| (冯传启, 张克立, 孙聚堂.光谱学与光谱分析,2003, 23(2), 279—281) | |
| [13] | Aitchison P., Ammundsen B., Jones D. J., Burns G., Rozière J., J. Mater. Chem., 1999, 9, 3125—3130 |
| [14] | Ammundsen B., Burns G. R., Islam M. S., Kanoh H., Rozière J., J. Phys. Chem. B, 1999, 103, 5175—5180 |
| [15] | Wei Y., Nam K. W., Kim K. B., Chen G., Solid State Ionics, 2006, 177, 29—35 |
| [16] | Liu D., Han J., Dontigny M., Charest P., Guerfi A., Zaghib K., Goodenough J. B., J. Electrochem. Soc., 2010, 157, A770—A775 |
| [17] | Ohzuku T., Tatsumi K., Matoba N., Sawai K., J. Electrochem. Soc., 2000, 147, 3592—3597 |
| [18] | Zhang Q., Liu W. W., Fang G. Q., Xia B. B., Sun H. D., Kaneko S., Yang Y. S., Zheng J. W., Li D. C., J. Ionrg. Mater., 2013, 28, 616—622 |
| (张茜, 刘伟伟, 方国清, 夏丙波, 孙洪丹, 金子信悟, 杨裕生, 郑军伟, 李德成.无机材料学报, 2013, 28, 616—622) | |
| [19] | Bardi U., Atrei A., Rovida G., Surf. Sci., 1992, 268, 87—97 |
| [20] | Yu D. C., Cao W. J., Wu H. Y., Zhao J. F., Acta Physico-Chimica Sinica, 2007, 23, 683—687 |
| (余德才, 曹文娟, 吴海玉, 赵剑锋.物理化学学报, 2007, 23, 683—687) | |
| [21] | Stranick M. A., Surf. Sci. Spec., 1999, 6, 39—46 |
| [22] | Jang S. H., Kang T., Kim H. J., Kim K.Y., Appl. Phys. Lett., 2002, 81, 105—107 |
| [23] | Zhang Q., He S. C., Liu W. W., Fang G. Q., Sato Y., Yang Y. S., Zheng J. W., Li D. C., Chin. J. Inorg. Chem., 2012, 28(12), 2501—2507 |
| (张茜, 贺诗词, 刘伟伟, 方国清, 金子信悟, 杨裕生, 郑军伟, 李德成.无机化学学报,2012, 28(12), 2501—2507) | |
| [24] | Yoon W. S., Iannopollo S., Grey C. P., Carlier D., Gorman J., Reed J., Ceder G., Electrochem. Solid-State Lett., 2004, 7, A167—A171 |
| [25] | Komaba S., Takei C., Nakayama T., Ogata A., Yabuuchi N., Electrochem. Commun., 2010, 12, 355—358 |
| [26] | Park Y. J., Hong Y. S., Wu X., Ryu K. S., Chang S. H., J. Power Sources, 2004, 129, 288—295 |
| [27] | Lu C. H., Lee W. C., Liou S. J., Ting-Kuo F. G., J. Power Sources, 1999, 81, 696—699 |
| [28] | Myung S. T., Komaba S., Hirosaki N., Kumagai N., Electrochem. Commun., 2002, 4, 397—401 |
| [1] | ZHANG Shiyu, HE Runhe, LI Yongbing, WEI Shijun, ZHANG Xingxiang. Fabrication of Lithium-sulfur Battery Cathode with Radiation Crosslinked Low Molecular Weight of Polyacrylonitrile and the Mechanism of Sulfur Storage [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210632. |
| [2] | LI Xiaohui, WEI Aijia, MU Jinping, HE Rui, ZHANG Lihui, WANG Jun, LIU Zhenfa. Effects of SmPO4 Coatingon Electrochemical Performance of High-voltage LiNi0.5Mn1.5O4 Cathode Materials [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210546. |
| [3] | BAO Junquan, ZHENG Shibing, YUAN Xuming, SHI Jinqiang, SUN Tianjiang, LIANG Jing. An Organic Salt PTO(KPD)2 with Enhanced Performance as a Cathode Material in Lithium-ion Batteries [J]. Chem. J. Chinese Universities, 2021, 42(9): 2911. |
| [4] | GAO Xiaole, WANG Jiaxin, LI Zhifang, LI Yanchun, YANG Donghua. Synthesis of NiOx-ZSM-5 Composite Materials and Its Electrocatalytic Hydrogen Evolution Performance in Microbial Electrolysis Cell [J]. Chem. J. Chinese Universities, 2021, 42(9): 2886. |
| [5] | LI Huiyang, ZHU Siying, LI Sha, ZHANG Qiaobao, ZHAO Jinbao, ZHANG Li. Influencing Factors and Promotion Strategies of the First-cycle Coulombic Efficiency of Silicon Suboxide Anodes in Lithium-ion Batteries [J]. Chem. J. Chinese Universities, 2021, 42(8): 2342. |
| [6] | WANG Yimeng, LIU Kai, WANG Baoguo. Coating Strategies of Ni-rich Layered Cathode in LIBs [J]. Chem. J. Chinese Universities, 2021, 42(5): 1514. |
| [7] | LIU Tiefeng, ZHANG Ben, SHENG Ouwei, NAI Jianwei, WANG Yao, LIU Yujing, TAO Xinyong. Research Progress of the Binders for the Silicon Anode [J]. Chem. J. Chinese Universities, 2021, 42(5): 1446. |
| [8] | WANG Renheng, XIAO Zhe, LI Yan, SUN Yiling, FAN Shuting, ZHENG Junchao, QIAN Zhengfang, HE Zhenjiang. Synthesis of Li2FeP2O7 Cathode Material at Different Temperatures and Its Electrochemical Performance for Lithium Ion Batteries [J]. Chem. J. Chinese Universities, 2021, 42(4): 1299. |
| [9] | ZHANG Huishuang, GAO Yanxiao, WANG Qiuxian, LI Xiangnan, LIU Wenfeng, YANG Shuting. High-low Temperature Properties of Ni-rich LiNi0.6Co0.2Mn0.2O2 Cathode Material by Hydrothermal Synthesis with CTAB Assisted [J]. Chem. J. Chinese Universities, 2021, 42(3): 819. |
| [10] | HAN Muyao, ZHAO Lina, SUN Jie. Advances in Silicon and Silicon-based Anode Materials [J]. Chem. J. Chinese Universities, 2021, 42(12): 3547. |
| [11] | LU Di,ZHENG Chunman,CHEN Yufang,LI Yujie,ZHANG Hongmei. Synthesis of Li-rich Layers/Spinel/Carbon Composite Cathode Materials with Phenol Formaldehyde Resin and Its Electrochemical Performance† [J]. Chem. J. Chinese Universities, 2020, 41(7): 1684. |
| [12] | ZHANG Chenyang,WEN Yuehua,ZHAO Pengcheng,CHENG Jie,QIU Jingyi,SUN Yanzhi. Effect of Organic Carbon Source on Performance of LiTi2(PO4)3/C Composite Electrodes in Aqueous Solutions † [J]. Chem. J. Chinese Universities, 2020, 41(6): 1352. |
| [13] | JI Tianyi, LIU Xiaoxu, ZHAO Jiupeng, LI Yao. Synthesis and Lithium-storage Characteristics of Three-dimensional Cross-linked Graphene Nanofibers † [J]. Chem. J. Chinese Universities, 2020, 41(4): 821. |
| [14] | RONG Hua, WANG Chungang, ZHOU Ming. Synthesis and Electrochemical Performance of FeS2 Microspheres as an Anode for Li-ion Batteries † [J]. Chem. J. Chinese Universities, 2020, 41(3): 447. |
| [15] | LI Xin, CHEN Liang, MA Xiaotao, ZHANG Ding, XU Shoudong, ZHOU Xianxian, DUAN Donghong, LIU Shibin. Preparation of V2O3 Hollow Spheres for Lithium Sulfur Batteries † [J]. Chem. J. Chinese Universities, 2019, 40(9): 1972. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||