

高等学校化学学报 ›› 2021, Vol. 42 ›› Issue (6): 1816.doi: 10.7503/cjcu20200896
收稿日期:2020-12-26
出版日期:2021-06-10
发布日期:2021-06-08
通讯作者:
樊小勇,李东林
E-mail:xyfan@chd.edu.cn;dlli@chd.edu.cn
基金资助:
FAN Xiaoyong( ), WU Yan, SUN Ruibo, GOU Lei, LI Donglin(
), WU Yan, SUN Ruibo, GOU Lei, LI Donglin( )
)
Received:2020-12-26
Online:2021-06-10
Published:2021-06-08
Contact:
FAN Xiaoyong,LI Donglin
E-mail:xyfan@chd.edu.cn;dlli@chd.edu.cn
Supported by:摘要:
锰基氧化物作为锌离子电池正极具有高比容量和低成本等优点, 但在电化学循环过程中不可逆相变、 锰的溶解和电极/电解质界面不稳定导致其在小电流密度、 深度放电条件下的循环性能差. 针对以上问题, 合成了三维(3D)多孔MnOx立方盒子, 并在其表面包覆In2O3层, 获得3D多孔MnOx@In2O3立方盒子. 结果显示, MnOx@In2O3立方盒子具有大量孔径约10 nm左右的孔, 有利于H+和Zn2+的快速传输; In2O3包覆层均匀包覆于3D多孔MnOx立方盒子的孔壁上, 有利于抑制MnOx在电化学循环过程中的不可逆相变和锰的溶解, 稳定电极/电解质界面. 电化学测试结果表明, 该3D多孔MnOx@In2O3电极在0.3 A/g的小电流密度、 深度放电条件下能稳定循环400次以上, 容量保持260 mA·h/g; 在1. 8 A/g电流密度下可稳定循环4000次以上, 容量保持81 mA·h/g; 即使在高电流密度6.0 A/g下仍保持73.4 mA·h/g的高可逆容量. 恒电流间隙滴定(GITT)和循环伏安测试结果表明, 3D多孔MnOx@In2O3电极比3D多孔MnOx具有更高的离子扩散速率, 有利于提升其高倍率容量. 电化学阻抗谱结果表明, 3D多孔MnOx@In2O3电极具有比3D多孔MnOx更稳定的电极/电解质界面, 有利于提升其循环寿命. 2000次循环后的扫描电子显微镜(SEM)结果表明, MnOx@In2O3电极表面仍分布少量In2O3, 以确保电极/电解质界面和循环的稳定性.
中图分类号:
TrendMD:
樊小勇, 毋妍, 孙瑞波, 苟蕾, 李东林. 三维多孔MnOx@In2O3立方盒子的构筑及储锌性能. 高等学校化学学报, 2021, 42(6): 1816.
FAN Xiaoyong, WU Yan, SUN Ruibo, GOU Lei, LI Donglin. Construction and Zn Storage Performance of Three Dimensional Porous MnOx@In2O3 Cubes. Chem. J. Chinese Universities, 2021, 42(6): 1816.
| Material | Cycling performance(low rate) | Cycling performance(high rate) | Rate capability | Ref. |
|---|---|---|---|---|
| MnO@NGS | 215 mA·h/g after 50 cycles at 100 mA/g | 114.6 mA·h/g after 300 cycles at 500 mA/g | 18.9 mA·h/g at 1.0 A/g | [ |
| V?MnO2 | 131 mA·h/g after 100 cycles at 66 mA/g | — | 64 mA·h/g at 1.064 A/g | [ |
| α?MnO2 | 147 mA·h/g after 50 cycles at 83 mA/g | — | 16 mA·h/g at 1.666 A/g | [ |
| Cu?MnO | 288 mA·h/g after 200 cycles at 150 mA/g | 100 mA·h/g after 1000 cycles at 900 mA/g | 156 mA·h/g at 0.9 A/g | [ |
| Mn2O3 | 233 mA·h/g after 120 cycles at 308 mA/g | 146 mA·h/g after 3000 cycles at 3080 mA/g | 162 mA·h/g at 3.080 A/g | [ |
| Mn2O3@PPy | 230 mA·h/g after 130 cycles at 100 mA/g | — | 75.6 mA·h/g at 2 A/g | [ |
| Mn2O3/Al2O3 | 289 mA·h/g after 125 cycles at 300 mA/g | 118 mA·h/g after 1100 cycles at 1500 mA/g | [ | |
| 3D?NVO | 487 mA·h/g after 50 cycles at 100 mA/g | 135 mA·h/g after 3000 cycles at 10000 mA/g | 142 mA·h/g at 10 A/g | [ |
| Mn3O4@NC | 280 mA·h/g after 80 cycles at 100 mA/g | 97 mA·h/g after 700 cycles at 1000 mA/g | [ | |
| SSWM@Mn3O4 | 290 mA·h/g after 50 cycles at 100 mA/g | 110 mA·h/g after 500 cycles at 500 mA/g | 125 mA·h/g at 0.5 A/g | [ |
| Mn3O4@C | 390 mA·h/g after 50 cycles at 200 mA/g | 84.1 mA·h/g after 12000 cycles at 50000 mA/g | 133 mA·h/g at 5 A/g | [ |
| MnOx@NC | 305 mA·h/g after 600 cycles at 500 mA/g | 100 mA·h/g after 1600 cycles at 2000 mA/g | [ | |
| 3D MnOx@In2O3 | 260 mA·h/g after 400 cycles at 300 mA/g | 81 mA·h/g after 4000 cycles at 1.8 A/g | 73.4 mA·h/g at 6.0 A/g | This work |
Table 1 Comparison of electrochemical performance of reported Mn-based oxides and this work
| Material | Cycling performance(low rate) | Cycling performance(high rate) | Rate capability | Ref. |
|---|---|---|---|---|
| MnO@NGS | 215 mA·h/g after 50 cycles at 100 mA/g | 114.6 mA·h/g after 300 cycles at 500 mA/g | 18.9 mA·h/g at 1.0 A/g | [ |
| V?MnO2 | 131 mA·h/g after 100 cycles at 66 mA/g | — | 64 mA·h/g at 1.064 A/g | [ |
| α?MnO2 | 147 mA·h/g after 50 cycles at 83 mA/g | — | 16 mA·h/g at 1.666 A/g | [ |
| Cu?MnO | 288 mA·h/g after 200 cycles at 150 mA/g | 100 mA·h/g after 1000 cycles at 900 mA/g | 156 mA·h/g at 0.9 A/g | [ |
| Mn2O3 | 233 mA·h/g after 120 cycles at 308 mA/g | 146 mA·h/g after 3000 cycles at 3080 mA/g | 162 mA·h/g at 3.080 A/g | [ |
| Mn2O3@PPy | 230 mA·h/g after 130 cycles at 100 mA/g | — | 75.6 mA·h/g at 2 A/g | [ |
| Mn2O3/Al2O3 | 289 mA·h/g after 125 cycles at 300 mA/g | 118 mA·h/g after 1100 cycles at 1500 mA/g | [ | |
| 3D?NVO | 487 mA·h/g after 50 cycles at 100 mA/g | 135 mA·h/g after 3000 cycles at 10000 mA/g | 142 mA·h/g at 10 A/g | [ |
| Mn3O4@NC | 280 mA·h/g after 80 cycles at 100 mA/g | 97 mA·h/g after 700 cycles at 1000 mA/g | [ | |
| SSWM@Mn3O4 | 290 mA·h/g after 50 cycles at 100 mA/g | 110 mA·h/g after 500 cycles at 500 mA/g | 125 mA·h/g at 0.5 A/g | [ |
| Mn3O4@C | 390 mA·h/g after 50 cycles at 200 mA/g | 84.1 mA·h/g after 12000 cycles at 50000 mA/g | 133 mA·h/g at 5 A/g | [ |
| MnOx@NC | 305 mA·h/g after 600 cycles at 500 mA/g | 100 mA·h/g after 1600 cycles at 2000 mA/g | [ | |
| 3D MnOx@In2O3 | 260 mA·h/g after 400 cycles at 300 mA/g | 81 mA·h/g after 4000 cycles at 1.8 A/g | 73.4 mA·h/g at 6.0 A/g | This work |
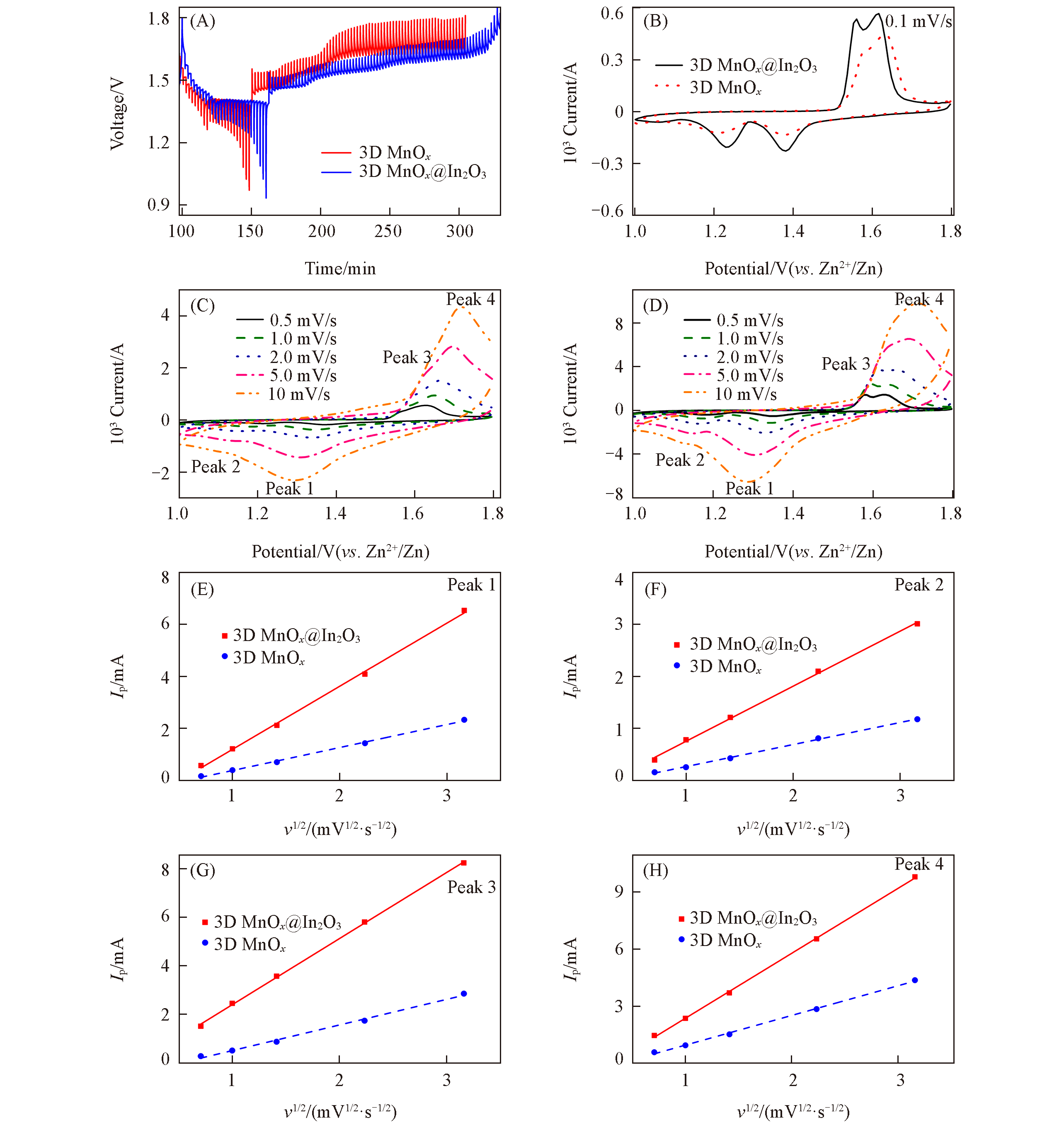
Fig.8 GITT profiles(A) and CV profiles(B) at 0.1 mV/s of 3D MnOxand 3D MnOx@In2O3 cubes, the CV profiles of 3D MnOx(C)and 3D MnOx@In2O3 cubes(D) at different scanning rates and their Ip?v1/2 profiles(E―H)
| Peak | MnOx | MnOx@In2O3 |
|---|---|---|
| 1 | 0.42396 | 1.05873 |
| 2 | 0.88535 | 2.43143 |
| 3 | 1.05504 | 2.72165 |
| 4 | 1.55795 | 3.40846 |
Table 2 Slopes of Ip-v1/2 profiles
| Peak | MnOx | MnOx@In2O3 |
|---|---|---|
| 1 | 0.42396 | 1.05873 |
| 2 | 0.88535 | 2.43143 |
| 3 | 1.05504 | 2.72165 |
| 4 | 1.55795 | 3.40846 |
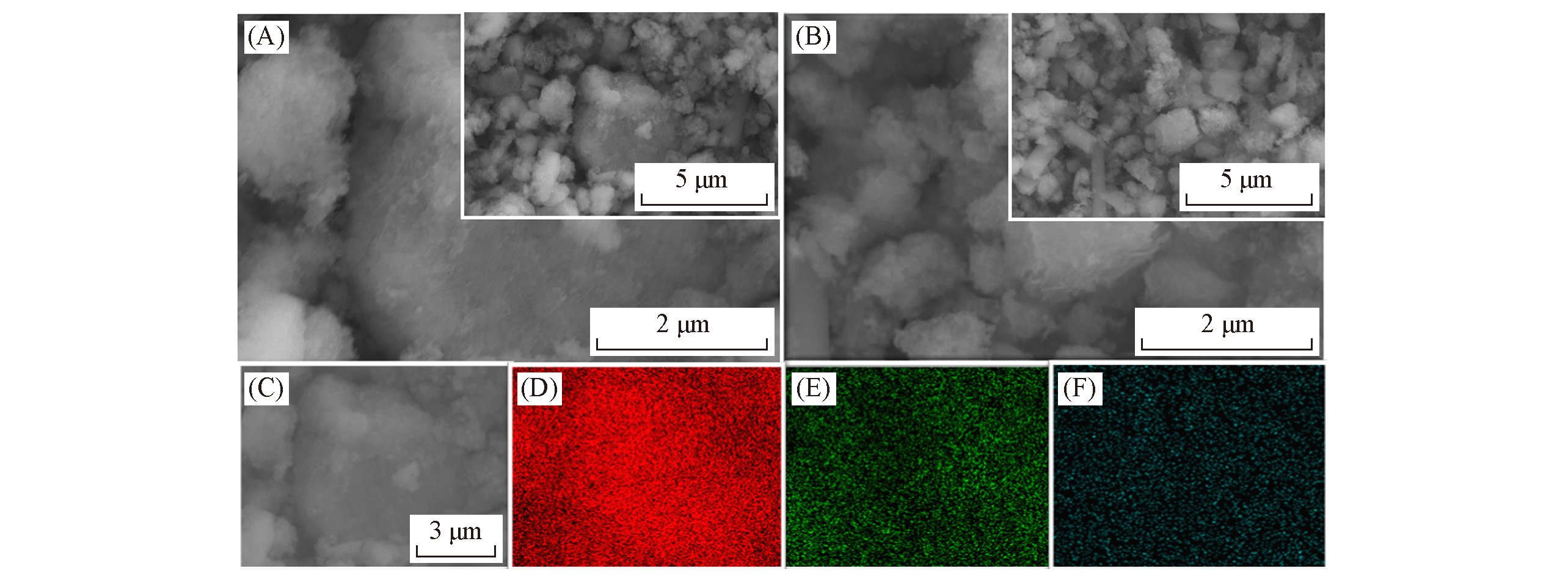
Fig.10 SEM(A, C) and corresponding EDS mapping(D―F) images of 3D MnOx@In2O3 cubes suffered 2000 cycles, SEM image(B) of 3D MnOxcubes suffered 2000 cycles(D) Mn; (E) Zn; (F) In.
| 1 | Fan X. Y., Han J., Ding Y. L., Deng Y. P., Luo D., Zeng X., Jiang Z., Gou L., Li D. L., Chen Z., Adv. Energy Mater., 2019, 9(28), 20344—20353 |
| 2 | Rahman M. A., Wong Y. C., Song G., Wen C., J. Porous Mat., 2015, 22(5), 1313—1343 |
| 3 | Qiu J. X., Jiang Q., Gao Y. K., Peng J. Q., Duan Z. H., Lu X. Y., Chem. J. Chinese Universities, 2018, 39(10), 2238—2244(邱家欣, 江奇, 高艺珂, 彭俊棋, 段志虹, 卢晓英. 高等学校化学学报, 2018, 39(10), 2238—2244 ) |
| 4 | Feng D. Y., Guo D., Liu X. X., Chem. J. Chinese Universities, 2018, 39(10), 2280—2288(冯东阳, 郭迪, 刘晓霞. 高等学校化学学报, 2018, 39(10), 2280—2288) |
| 5 | Yang J. G., Li Y. J., Lu D.,Cheng Y. F., Sun W. W., Zheng C. M., Chem. J. Chinese Universities, 2019, 40(7), 1495—1500(杨金戈, 李宇杰, 陆地, 陈宇方, 孙巍巍, 郑春满. 高等学校化学学报, 2019, 40(7), 1495—1500) |
| 6 | Fan X. Y., Jiang Z., Huang L., Wang X., Han J., Sun R., Gou L., Li D. L., Ding Y. L., ACS Appl. Mater. Inter., 2020, 12(18), 20344—20353 |
| 7 | Li G. D., Cheng W., Zhang H., Gong Y., Shi F. F., Wang J. Y., Zhang R. F., Chen G., Jin Y., Wu T., Tang Z., Cui Y., Adv. EnergyMater.,2020, 10(9), 1902085 |
| 8 | Jia H., Wang Z., Tawiah B., Wang Y., Chan C. Y., Fei B., Pan F., Nano Energy, 2020, 70, 104523—104535 |
| 9 | Shi Y., Chen Y., Shi L., Wang K., Wang B., Li L., Ma Y., Li Y., Sun Z., Ali W., Ding S., Small, 2020, 16(23), e2000730 |
| 10 | Zeng X. H., Mao J. F., Hao J. N., Wang Z. J., Zhou S., Ling C. D., Guo Z. P., Adv. Energy Mater., 2020, 10(32), 1904163 |
| 11 | Li J., Chen Y., Guo J., Wang F., Liu H., Li Y., Adv. Funct. Mater., 2020, 30(42), 2004115 |
| 12 | Fan X., Yang H., Ni K., Han J., Wu Y., Sun R., Gou L., Li D., Funct. Mater. Lett., 2020, 13(3), 2050011—2050014 |
| 13 | Jiang Y., Ba D., Li Y., Liu J., Adv. Sci., 2020, 7(6), 1902795—1902806 |
| 14 | Liu N., Wu X., Yin Y., Chen A., Zhao C., Guo Z., Fan L., Zhang N., ACS Appl. Mater. Inter., 2020, 12(25), 28199—28205 |
| 15 | Gou L., Mou K. L., Fan X. Y., Zhao M. J., Wang Y., Xue D., Li D. L., Dalton Trans., 2020, 49(3), 711—718 |
| 16 | Fan X. Y., Cui Y., Liu P., Gou L., Xu L., Li D. L., Phys. Chem. Chem. Phys., 2016, 18(32), 22224—22234 |
| 17 | Ma L., Li L. P., Liu Y. N., Zhu J. H., Meng T., Zhang H., Jiang J., Li C. M., Chem. Commun., 2018, 54(77), 10835—10838 |
| 18 | Fang G., Zhou J., Pan A., Liang S., ACS Energy Lett., 2018, 3(10), 2480—2501 |
| 19 | Li H., Ma L., Han C., Wang Z., Liu Z., Tang Z., Zhi C., Nano Energy, 2019, 62, 550—587 |
| 20 | Wang M., Zheng X., Zhang X., Chao D., Qiao S. Z., Alshareef H. N., Cui Y., Chen W., Adv. Energy Mater., 2020,5(3), 2002904 |
| 21 | Xu D., Li B., Wei C., He Y. B., Du H., Chu X., Qin X., Yang Q. H., Kang F., Electrochimica Acta, 2014, 133, 254—261 |
| 22 | Wang C., Wang M., He Z., Liu L., Huang Y., ACS Appl. Energy Mater., 2020, 3(2), 1742—1748 |
| 23 | Wang K., Zhang X., Han J., Zhang X., Sun X., Li C., Liu W., Li Q., Ma Y., ACS Appl. Mater. Inter., 2018, 10(29), 24573—24582 |
| 24 | Mao J., Wu F. F., Shi W. H., Liu W. X., Xu X. L., Cai G. F., Li Y. W., Cao X. H., Chinese J. Polymer Sci., 2019, 38(5), 514—521 |
| 25 | Liu Y., Zhou X., Liu R., Li X., Bai Y., Xiao H., Wang Y., Yuan G., ACS Appl. Mater. Interfaces,2019, 11(21), 19191—19199 |
| 26 | Fu Y., Wei Q., Zhang G., Wang X., Zhang J., Hu Y., Wang D., Zuin L., Zhou T., Wu Y., Sun S., Adv. Energy Mater., 2018, 8(26), 1801445 |
| 27 | Gou L., Xue D., Mou K. L., Zhao S. P., Wang Y., Fan X. Y., Li D. L., J. Electrochem. Soc., 2019, 166(13), A3362—A3368 |
| 28 | Fan X., Ni K., Han J., Wang S., Gou L., Li D. L., Funct. Mater. Lett., 2019, 12(3),1950073 |
| 29 | Wu B., Zhang G., Yan M., Xiong T., He P., He L., Xu X., Mai L., Small, 2018, 14(13), e1703850 |
| 30 | Huang J., Wang Z., Hou M., Dong X., Liu Y., Wang Y., Xia Y., Nat. Commun., 2018, 9(1), 2906 |
| 31 | Fan X., Li S., Lu L., Electrochimica Acta,2016,200, 152—160 |
| 32 | Wang Q., Sun J., Wang Q., Zhang D., Xing L. L., Xue X., J. Mater. Chem. A,2015,3, 5083—5091 |
| 33 | Jiang B., Xu C., Wu C., Dong L., Li J., Kang F., Electrochimica Acta,2017,229, 422—428 |
| 34 | Li W., Gao X., Chen Z., Guo R., Zou G., Hou H., Deng W., Ji X., Zhao J., Chem. Eng. J., 2020, 402, 125509 |
| 35 | Alfaruqi M. H., Islam S., Mathew V., Song J., Kim S., Tung D. P., Jo J., Kim S., Baboo J. P., Xiu Z., Kim J., Appl. Surf. Sci., 2017, 404, 435—442 |
| 36 | Alfaruqi M. H., Gim J., Kim S., Song J., Jo J., Kim S., Mathew V., Kim J., J.Power Sources, 2015, 288, 320—327 |
| 37 | Fenta F. W., Olbasa B. W., Tsai M. C., Weret M. A., Zegeye T. A., Huang C. J., Huang W. H., Zeleke T. S., Sahalie N. A., Pao C. W., Wu S. h., Su W. N., Dai H., Hwang B. J., J. Mater. Chem. A, 2020, 8, 17595—17607 |
| 38 | Feng D., Gao T. N., Zhang L., Guo B., Song S., Qiao Z. A., Dai S., Nanomicro Lett., 2019, 12(1), 1—13 |
| 39 | Liu Y., Zhou X., Liu R., Li X., Bai Y., Xiao H., Wang Y., Yuan G., ACS Appl. Mater. Interfaces, 2019, 11(21), 19191—19199 |
| 40 | Gou L., Mou K. L., Fan X. Y., Zhao M. J., Wang Y., Xue D., Li D. L., Dalton Trans., 2020, 49(3), 711—718 |
| 41 | Li Q., Rui X., Chen D., Feng Y., Xiao N., Gan L., Zhang Q., Yu Y., Huang S., Nanomicro Lett., 2020, 12(1), 1—12 |
| 42 | Sun M., Li D. S., Wang Y. F., Liu W. L., Ren M. M., Kong F. G., Wang S. J., Guo Y. Z., Liu Y. M., Chem. Electro. Chem., 2019, 6(9), 2510—2516 |
| 43 | Zhu C., Fang G., Zhou J., Guo J., Wang Z., Wang C., Li J., Tang Y., Liang S., J. Mater. Chem. A, 2018, 6(20), 9677—9683 |
| 44 | Tan Q., Li X., Zhang B., Chen X., Tian Y., Wan H., Zhang L., Miao L., Wang C., Gan Y., Jiang J., Wang Y., Wang H., Adv. Energy Mater., 2020, 10(38), 2001050 |
| 45 | Fu Y., Wei Q., Zhang G., Wang X., Zhang J., Hu Y., Wang D., Zuin L., Zhou T., Wu Y., Sun S., Adv. Energy Mater., 2018, 8(26), 1801445 |
| [1] | 樊小勇, 朱永强, 毋妍, 张帅, 许磊, 苟蕾, 李东林. 三维多孔Sn-Zn合金电极助力Zn的均匀沉积/剥离[J]. 高等学校化学学报, 2022, 43(4): 20210861. |
| [2] | 张诗昱, 何润合, 李永兵, 魏士俊, 张兴祥. 辐照交联制备低分子量聚丙烯腈纤维锂硫电池正极材料及其储硫机理[J]. 高等学校化学学报, 2022, 43(3): 20210632. |
| [3] | 李晓辉, 魏爱佳, 穆金萍, 何蕊, 张利辉, 王军, 刘振法. 磷酸钐包覆对高电压镍锰酸锂正极材料电化学性能的影响[J]. 高等学校化学学报, 2022, 43(2): 20210546. |
| [4] | 姜宝正, 黄文婷, 刘文宝, 郭荣胜, 徐成俊, 康飞宇. 纳米铜修饰三维锌网电极的制备及锌离子电池负极的电化学性能[J]. 高等学校化学学报, 2022, 43(10): 20220257. |
| [5] | 鲍俊全, 郑仕兵, 苑旭明, 史金强, 孙田将, 梁静. 有机盐PTO(KPD)2作为高性能锂离子电池正极材料的研究[J]. 高等学校化学学报, 2021, 42(9): 2911. |
| [6] | 王弈艨, 刘凯, 王保国. 高镍三元正极材料的表面包覆策略[J]. 高等学校化学学报, 2021, 42(5): 1514. |
| [7] | 王任衡, 肖哲, 李艳, 孙一翎, 范姝婷, 郑俊超, 钱正芳, 贺振江. 固相烧结法制备锂离子电池正极材料Li2FeP2O7及其电化学性能研究[J]. 高等学校化学学报, 2021, 42(4): 1299. |
| [8] | 张会双, 高延晓, 王秋娴, 李向南, 刘文凤, 杨书廷. CTAB辅助水热合成高镍三元材料LiNi0.6Co0.2Mn0.2O2及其高低温性能研究[J]. 高等学校化学学报, 2021, 42(3): 819. |
| [9] | 黄永烽, 黄文婷, 刘文宝, 刘岳峰, 刘伟, 徐成俊. 锌离子电池正极材料V2O5的储能机理和容量衰减原因[J]. 高等学校化学学报, 2020, 41(8): 1859. |
| [10] | 陆地,郑春满,陈宇方,李宇杰,张红梅. 以酚醛树脂为碳源原位合成富锂层状相/尖晶石/碳核壳结构正极材料及其电化学性能[J]. 高等学校化学学报, 2020, 41(7): 1684. |
| [11] | 李新, 陈良, 马晓涛, 张鼎, 徐守冬, 周娴娴, 段东红, 刘世斌. V2O3空心球的制备及在锂硫电池中的应用[J]. 高等学校化学学报, 2019, 40(9): 1972. |
| [12] | 姚枫楠, 李瑀, 封伟. 碳包覆氟化亚铁纳米复合材料的制备及电化学性能[J]. 高等学校化学学报, 2019, 40(7): 1418. |
| [13] | 杨金戈, 李宇杰, 陆地, 陈宇方, 孙巍巍, 郑春满. 微纳结构富锂锰基层状正极材料的形貌调控与储锂性能[J]. 高等学校化学学报, 2019, 40(7): 1495. |
| [14] | 马东玮, 田润赛, 刘振江, 冯源源, 丁泓宇, 冯季军. Na掺杂Li2-xNaxMnSiO4/C正极材料的微波辅助合成与电化学性能[J]. 高等学校化学学报, 2019, 40(6): 1280. |
| [15] | 陈红, 杜勇慧, 张鑫, 刘文闫, 周晓明. 聚(3-己基噻吩)包覆富锂层状正极材料Li1.18Ni0.15Co0.15Mn0.52O2的制备与电化学性能[J]. 高等学校化学学报, 2019, 40(4): 777. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||