

高等学校化学学报 ›› 2014, Vol. 35 ›› Issue (1): 37.doi: 10.7503/cjcu20130721
魏王慧1, 王青1, 储艳秋1( ), 汪日志2(
), 汪日志2( ), 丁传凡1
), 丁传凡1
收稿日期:2013-07-25
出版日期:2014-01-10
发布日期:2013-12-13
作者简介:联系人简介: 储艳秋, 男, 博士, 副教授, 主要从事质谱法研究有机分子和生物大分子及其相互作用的研究. E-mail:基金资助:
WEI Wanghui1, WANG Qing1, CHU Yanqiu1,*( ), WANG Rizhi2,*(
), WANG Rizhi2,*( ), DING Chuanfan1
), DING Chuanfan1
Received:2013-07-25
Online:2014-01-10
Published:2013-12-13
Contact:
CHU Yanqiu,WANG Rizhi
E-mail:chuyq@fudan.edu.cn;rizhi9752@hotmail.com
Supported by:摘要:
为了探索金属离子对含有不同侧链的多肽气相解离的影响, 采用质谱法研究了碱金属离子Li+, Na+, K+, Rb+和Cs+分别与丝氨酸、 亮氨酸和赖氨酸五肽(分别简写为S5, L5和K5)形成的复合物的裂解反应. 质谱定性结果表明, 5种碱金属离子均可以在气相中与丝氨酸、 亮氨酸和赖氨酸五肽形成配合比为1∶1 和2∶1的非共价复合物; 竞争反应结果表明, 随着碱金属离子半径的增加, 它们与3种五肽的结合能力逐渐减弱. 质谱定量结果表明, K+与丝氨酸、 亮氨酸和赖氨酸五肽复合物的结合常数分别为8.94×104, 2.83×104和2.50×103 L/mol, 表明K+与五肽复合物的结合强度按照丝氨酸、 亮氨酸和赖氨酸的顺序依次减小. 含不同侧链碱金属离子-五肽复合物的碰撞诱导解离结果表明, 复合物的碎裂主要发生在骨架上, 丝氨酸五肽复合物最易碎裂, 亮氨酸五肽复合物其次, 赖氨酸五肽复合物则较难碎裂, 且3种复合物的侧链断裂情况也呈现明显差异. 此外, 研究了Na+与亮氨酸五肽复合物所产生的碎片离子, 分析了不同离子之间的来源关系, 并以Dunbar的复合物理论模型为依据, 推测在碎裂过程中, 碱金属离子可能向五肽的碳端或氮端偏移. 质谱碎片分析结果表明, 在2∶1的非共价复合物中, 第一个碱金属离子与五肽上4个酰胺键的羰基结合, 第二个碱金属离子与五肽的羧基氧原子结合.
中图分类号:
TrendMD:
魏王慧, 王青, 储艳秋, 汪日志, 丁传凡. 气相中碱金属离子与丝氨酸、 亮氨酸和赖氨酸五肽复合物的裂解反应. 高等学校化学学报, 2014, 35(1): 37.
WEI Wanghui, WANG Qing, CHU Yanqiu, WANG Rizhi, DING Chuanfan. Fragmentation Reactions of Complexes of Alkali Metal Ions with Pentaserine, Pentaleucine and Pentalysine in Gas Phase†. Chem. J. Chinese Universities, 2014, 35(1): 37.
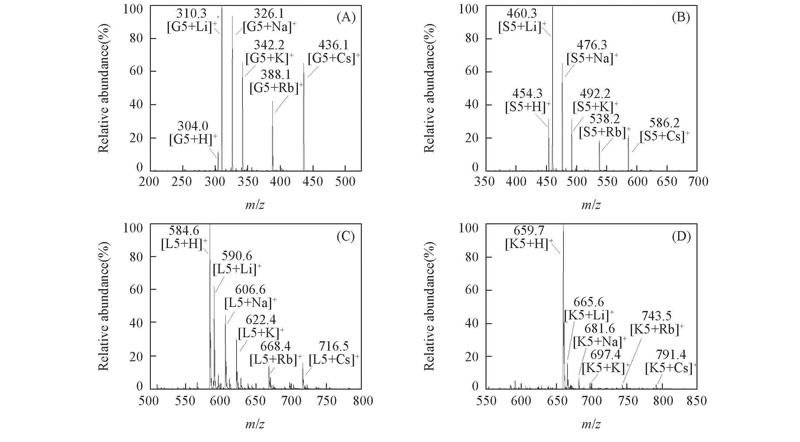
Fig.1 Mass spectra for competition reaction of pentapeptides with alkali metal ions in a molar ratio of 1∶1∶1∶1∶1∶1∶1(A) G5; (B) S5; (C) L5; (D) K5. c(Pentapeptide)=c(Alkali metal ion)=1.0×10-4 mol/L.
| 105 [G | 105 [K+]0/(mol·L-1) | IP | IPM | IPM2 | a1 | a2 | 10-5 Ka1/(L·mol-1) |
|---|---|---|---|---|---|---|---|
| 5.00 | 10.00 | 7.54 | 100.00 | 97.94 | 0.487 | 0.477 | 4.84 |
| 5.00 | 15.00 | 3.17 | 100.00 | 98.03 | 0.497 | 0.487 | 4.06 |
| 5.00 | 20.00 | 1.92 | 100.00 | 98.35 | 0.499 | 0.491 | 3.96 |
| 5.00 | 25.00 | 1.68 | 100.00 | 98.42 | 0.499 | 0.492 | 3.15 |
| 5.00 | 30.00 | 1.32 | 100.00 | 99.31 | 0.498 | 0.495 | 3.15 |
| Average | 3.83 | ||||||
| SD | 0.70 | ||||||
| RSD(%) | 18.2 |
Table 1 Ka1 values of the complexes of G5 with K+
| 105 [G | 105 [K+]0/(mol·L-1) | IP | IPM | IPM2 | a1 | a2 | 10-5 Ka1/(L·mol-1) |
|---|---|---|---|---|---|---|---|
| 5.00 | 10.00 | 7.54 | 100.00 | 97.94 | 0.487 | 0.477 | 4.84 |
| 5.00 | 15.00 | 3.17 | 100.00 | 98.03 | 0.497 | 0.487 | 4.06 |
| 5.00 | 20.00 | 1.92 | 100.00 | 98.35 | 0.499 | 0.491 | 3.96 |
| 5.00 | 25.00 | 1.68 | 100.00 | 98.42 | 0.499 | 0.492 | 3.15 |
| 5.00 | 30.00 | 1.32 | 100.00 | 99.31 | 0.498 | 0.495 | 3.15 |
| Average | 3.83 | ||||||
| SD | 0.70 | ||||||
| RSD(%) | 18.2 |
| 105 [S | 105 [K+]0/(mol·L-1) | IP | IPM | IPM2 | a1 | a2 | 10-4 |
|---|---|---|---|---|---|---|---|
| 5.00 | 10.00 | 23.99 | 100.00 | 51.09 | 0.571 | 0.292 | 9.86 |
| 5.00 | 15.00 | 12.80 | 100.00 | 70.47 | 0.546 | 0.384 | 9.25 |
| 5.00 | 20.00 | 8.15 | 100.00 | 97.10 | 0.487 | 0.473 | 9.49 |
| 5.00 | 25.00 | 6.97 | 100.00 | 97.57 | 0.489 | 0.477 | 8.09 |
| 5.00 | 30.00 | 5.47 | 100.00 | 98.15 | 0.491 | 0.482 | 8.00 |
| Average | 8.94 | ||||||
| SD | 0.84 | ||||||
| RSD(%) | 9.4 |
Table 2 Ka1 values of the complexes of S5 with K+
| 105 [S | 105 [K+]0/(mol·L-1) | IP | IPM | IPM2 | a1 | a2 | 10-4 |
|---|---|---|---|---|---|---|---|
| 5.00 | 10.00 | 23.99 | 100.00 | 51.09 | 0.571 | 0.292 | 9.86 |
| 5.00 | 15.00 | 12.80 | 100.00 | 70.47 | 0.546 | 0.384 | 9.25 |
| 5.00 | 20.00 | 8.15 | 100.00 | 97.10 | 0.487 | 0.473 | 9.49 |
| 5.00 | 25.00 | 6.97 | 100.00 | 97.57 | 0.489 | 0.477 | 8.09 |
| 5.00 | 30.00 | 5.47 | 100.00 | 98.15 | 0.491 | 0.482 | 8.00 |
| Average | 8.94 | ||||||
| SD | 0.84 | ||||||
| RSD(%) | 9.4 |
| 105 [L | 105 [K+]0/(mol·L-1) | IP | IPM | IPM2 | a1 | a2 | 10-4 |
|---|---|---|---|---|---|---|---|
| 5.00 | 10.00 | 51.03 | 100.00 | 8.19 | 0.628 | 0.051 | 3.08 |
| 5.00 | 15.00 | 33.63 | 100.00 | 13.53 | 0.680 | 0.092 | 2.79 |
| 5.00 | 20.00 | 21.85 | 100.00 | 15.31 | 0.729 | 0.112 | 3.01 |
| 5.00 | 25.00 | 18.68 | 100.00 | 17.09 | 0.737 | 0.126 | 2.68 |
| 5.00 | 30.00 | 15.71 | 100.00 | 20.75 | 0.733 | 0.152 | 2.57 |
| Average | 2.83 | ||||||
| SD | 0.21 | ||||||
| RSD(%) | 7.4 |
Table 3 Ka1 values of the complexes of L5 with K+
| 105 [L | 105 [K+]0/(mol·L-1) | IP | IPM | IPM2 | a1 | a2 | 10-4 |
|---|---|---|---|---|---|---|---|
| 5.00 | 10.00 | 51.03 | 100.00 | 8.19 | 0.628 | 0.051 | 3.08 |
| 5.00 | 15.00 | 33.63 | 100.00 | 13.53 | 0.680 | 0.092 | 2.79 |
| 5.00 | 20.00 | 21.85 | 100.00 | 15.31 | 0.729 | 0.112 | 3.01 |
| 5.00 | 25.00 | 18.68 | 100.00 | 17.09 | 0.737 | 0.126 | 2.68 |
| 5.00 | 30.00 | 15.71 | 100.00 | 20.75 | 0.733 | 0.152 | 2.57 |
| Average | 2.83 | ||||||
| SD | 0.21 | ||||||
| RSD(%) | 7.4 |
| 105 [K | 105 [K+]0/(mol·L-1) | IP | IPM | IPM2 | a1 | a2 | 10-3 |
|---|---|---|---|---|---|---|---|
| 5.00 | 10.00 | 100.00 | 22.88 | 7.29 | 0.176 | 0.056 | 2.68 |
| 5.00 | 15.00 | 100.00 | 34.33 | 14.11 | 0.231 | 0.095 | 2.66 |
| 5.00 | 20.00 | 100.00 | 42.90 | 22.47 | 0.259 | 0.136 | 2.47 |
| 5.00 | 25.00 | 100.00 | 51.93 | 31.90 | 0.282 | 0.174 | 2.37 |
| 5.00 | 30.00 | 100.00 | 61.13 | 40.60 | 0.303 | 0.201 | 2.31 |
| Average | 2.50 | ||||||
| SD | 0.17 | ||||||
| RSD(%) | 6.8 |
Table 4 Ka1 values of the complexes of K5 with K+
| 105 [K | 105 [K+]0/(mol·L-1) | IP | IPM | IPM2 | a1 | a2 | 10-3 |
|---|---|---|---|---|---|---|---|
| 5.00 | 10.00 | 100.00 | 22.88 | 7.29 | 0.176 | 0.056 | 2.68 |
| 5.00 | 15.00 | 100.00 | 34.33 | 14.11 | 0.231 | 0.095 | 2.66 |
| 5.00 | 20.00 | 100.00 | 42.90 | 22.47 | 0.259 | 0.136 | 2.47 |
| 5.00 | 25.00 | 100.00 | 51.93 | 31.90 | 0.282 | 0.174 | 2.37 |
| 5.00 | 30.00 | 100.00 | 61.13 | 40.60 | 0.303 | 0.201 | 2.31 |
| Average | 2.50 | ||||||
| SD | 0.17 | ||||||
| RSD(%) | 6.8 |
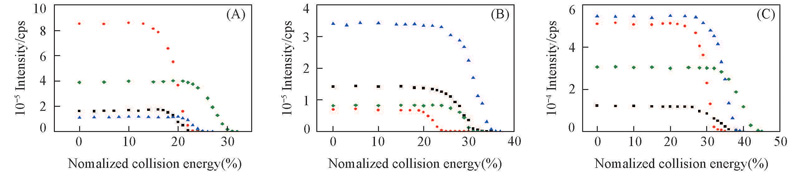
Fig.2 Relationship between intensity of mass spectrometric peak and normalized collision energy The precursors are the complexes of G5(■), S5(●), L5(▲) or K5(◆) with H+(A), Li+(B) and 2Li+(C).
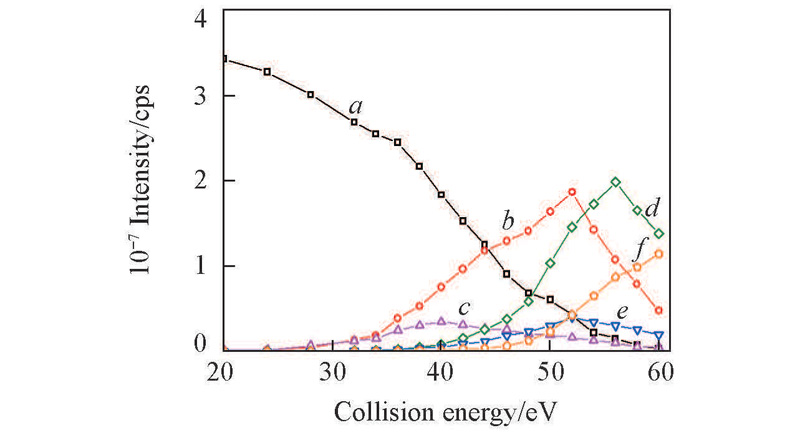
Fig.4 CID breakdown curves for the complexes of Na+ with pentaleucinea. Precursor ion [L5+Na]+; b. [b4+Na+OH]+; c. [b4+Na-H]+; d. [y3+Na+H]+; e. [b3+Na-H]+; f. [y2+Na+H]+.
| Precursor ion(#) | an/an*/an** | Other | ||
|---|---|---|---|---|
| [S5+H]+ | a4, a3, a2, a1, a4-H2O, a4-2H2O, a3-H2O, a3-2H2O, a2-H2O | b4, b3, b2, b4-H2O, b3-H2O, b2-H2O, b3-2H2O | y3, y2, y1 | [#-H2O]+, [#-2H2O]+, [#-3H2O]+, [#-4H2O]+ |
| [S5+Li]+ | a4*, a4*-CH2O, a4*-H2O, a3*, a3*-CH2O, a3*-H2O, a2*, a2*-CH2O, a2*-H2O, a1* | [b4+Li+OH]+, [b4+Li+OH- CH2O]+, b4*-H2O, b4*-CO2, [b3+Li+OH]+, b4*-H2O-CH2O, b3*-H2O, b4*, b3*-CO2, [b3+ Li+OH-CH2O]+, b3*, b2*, b3*-H2O-CH2O, b2*-H2O, b2*-H2O-CH2O | y2*, y2*-H2O, y1* | [#-H2O]+, [#-CH2O]+, [#-H2O-CH2O]+ , [#-H2O-CO2]+, [#-H2O-2CH2O]+, [#-2H2O-CH2O]+ |
| [S5+Na]+ | a4*, a4*-CH2O, a3*, a1* | [b4+Na+OH]+, [b4+Na+OH- CH2O]+, b4*, b4*-H2O, [b3+ Na+OH]+, b3*, b2*, [b3+Na+ OH-CH2O]+ | y2* | [#-H2O]+, [#-CH2O]+, [#-H2O-CH2O]+, [#-H2O-CO2]+ |
| [S5+K]+ | a4*, a4*-CH2O | [b4+K+OH]+, [b4+K+OH- CH2O]+, b4*, b4*-H2O, [b3+ K+OH]+, [b3+K+OH-CH2O]+, b3* | K+, [#-H2O]+, [#-CH2O]+, [#-2CH2O]+ | |
| [S5+Rb]+ | Rb+ | |||
| [S5+Cs]+ | Cs+ | |||
| [S5+2Li-H]+ | a4**, a3**, a4*, a3* | b3**, b3**-CH2O, b2**, b2**-CH2O | y4**, y3**, y2**, y1**, y4**-CH2O, y3**-CH2O, y2**-CH2O, y1**-CH2O, y4**-2CH2O, y3**-CH2O | [#-H2O]+, [#-CH2O]+, [#-2CH2O]+, [#-3CH2O]+, [#-4CH2O]+, [#-H2O-CH2O]+, [#-H2O-2CH2O]+, [#-H2O-3CH2O]+, [#-2H2O-3CH2O]+ |
| [S5+2Na-H]+ | a4**-CH2O, a4**-2CH2O, a4**-3CH2O, a3**-CH2O | b2**, b4*, b4*-CH2O | y4**, y3**, y2**, y1**, y4**-CH2O, y3**-CH2O, y2**-CH2O, y1**-CH2O, y3**-2CH2O, y2**-2CH2O | [#-H2O]+, [#-CH2O]+, [#-2CH2O]+, [#- 3CH2O]+, [#-4CH2O]+, [#-5CH2O]+, [#-H2O- CH2O]+, [#-H2O- 2CH2O]+, [#-H2O- 3CH2O]+, [#-H2O- 4CH2O]+ |
| [S5+2K-H]+ | a4**-H2O | b3**-CH2O, b3**-2CH2O, b2**-CH2O | y4**, y3**, y2**, y1**, y3**-CH2O, y2**-CH2O, y1**-CH2O, y2**-2CH2O | K+, [#-H2O]+, [#-2H2O]+, [#-CH2O]+, [#-2CH2O]+, [#-3CH2O]+, [#-4CH2O]+, [#-H2O-CH2O]+, [#-H2O-2CH2O]+, [#-2H2O- 2CH2O]+, [#-H2O- 3CH2O]+, [#-2H2O- 3CH2O]+, [#-H2O- 4CH2O]+ |
| [S5+2Rb-H]+ | a4**, a4**-H2O | b3**, b1**, b3**-2H2O | y4**, y3**, y2**, y1** | Rb+, [#-H2O]+, [#-CH2O]+, [#-2CH2O]+, [#-3CH2O]+, [#-H2O- CH2O]+, [#-H2O-2CH2O]+ |
| [S5+2Cs-H]+ | a4**-H2O | b3** | y4**, y3**, y1**, y1**-CH2O | Cs+, [#-H2O]+, [#-CH2O]+, [#-2CH2O]+, [#-H2O-CH2O]+, [#-H2O-2CH2O]+, [#-3CH2O]+ |
Table 5 Main fragment ions of protonated and alkali metal cationized S5 complexes obtained with CID
| Precursor ion(#) | an/an*/an** | Other | ||
|---|---|---|---|---|
| [S5+H]+ | a4, a3, a2, a1, a4-H2O, a4-2H2O, a3-H2O, a3-2H2O, a2-H2O | b4, b3, b2, b4-H2O, b3-H2O, b2-H2O, b3-2H2O | y3, y2, y1 | [#-H2O]+, [#-2H2O]+, [#-3H2O]+, [#-4H2O]+ |
| [S5+Li]+ | a4*, a4*-CH2O, a4*-H2O, a3*, a3*-CH2O, a3*-H2O, a2*, a2*-CH2O, a2*-H2O, a1* | [b4+Li+OH]+, [b4+Li+OH- CH2O]+, b4*-H2O, b4*-CO2, [b3+Li+OH]+, b4*-H2O-CH2O, b3*-H2O, b4*, b3*-CO2, [b3+ Li+OH-CH2O]+, b3*, b2*, b3*-H2O-CH2O, b2*-H2O, b2*-H2O-CH2O | y2*, y2*-H2O, y1* | [#-H2O]+, [#-CH2O]+, [#-H2O-CH2O]+ , [#-H2O-CO2]+, [#-H2O-2CH2O]+, [#-2H2O-CH2O]+ |
| [S5+Na]+ | a4*, a4*-CH2O, a3*, a1* | [b4+Na+OH]+, [b4+Na+OH- CH2O]+, b4*, b4*-H2O, [b3+ Na+OH]+, b3*, b2*, [b3+Na+ OH-CH2O]+ | y2* | [#-H2O]+, [#-CH2O]+, [#-H2O-CH2O]+, [#-H2O-CO2]+ |
| [S5+K]+ | a4*, a4*-CH2O | [b4+K+OH]+, [b4+K+OH- CH2O]+, b4*, b4*-H2O, [b3+ K+OH]+, [b3+K+OH-CH2O]+, b3* | K+, [#-H2O]+, [#-CH2O]+, [#-2CH2O]+ | |
| [S5+Rb]+ | Rb+ | |||
| [S5+Cs]+ | Cs+ | |||
| [S5+2Li-H]+ | a4**, a3**, a4*, a3* | b3**, b3**-CH2O, b2**, b2**-CH2O | y4**, y3**, y2**, y1**, y4**-CH2O, y3**-CH2O, y2**-CH2O, y1**-CH2O, y4**-2CH2O, y3**-CH2O | [#-H2O]+, [#-CH2O]+, [#-2CH2O]+, [#-3CH2O]+, [#-4CH2O]+, [#-H2O-CH2O]+, [#-H2O-2CH2O]+, [#-H2O-3CH2O]+, [#-2H2O-3CH2O]+ |
| [S5+2Na-H]+ | a4**-CH2O, a4**-2CH2O, a4**-3CH2O, a3**-CH2O | b2**, b4*, b4*-CH2O | y4**, y3**, y2**, y1**, y4**-CH2O, y3**-CH2O, y2**-CH2O, y1**-CH2O, y3**-2CH2O, y2**-2CH2O | [#-H2O]+, [#-CH2O]+, [#-2CH2O]+, [#- 3CH2O]+, [#-4CH2O]+, [#-5CH2O]+, [#-H2O- CH2O]+, [#-H2O- 2CH2O]+, [#-H2O- 3CH2O]+, [#-H2O- 4CH2O]+ |
| [S5+2K-H]+ | a4**-H2O | b3**-CH2O, b3**-2CH2O, b2**-CH2O | y4**, y3**, y2**, y1**, y3**-CH2O, y2**-CH2O, y1**-CH2O, y2**-2CH2O | K+, [#-H2O]+, [#-2H2O]+, [#-CH2O]+, [#-2CH2O]+, [#-3CH2O]+, [#-4CH2O]+, [#-H2O-CH2O]+, [#-H2O-2CH2O]+, [#-2H2O- 2CH2O]+, [#-H2O- 3CH2O]+, [#-2H2O- 3CH2O]+, [#-H2O- 4CH2O]+ |
| [S5+2Rb-H]+ | a4**, a4**-H2O | b3**, b1**, b3**-2H2O | y4**, y3**, y2**, y1** | Rb+, [#-H2O]+, [#-CH2O]+, [#-2CH2O]+, [#-3CH2O]+, [#-H2O- CH2O]+, [#-H2O-2CH2O]+ |
| [S5+2Cs-H]+ | a4**-H2O | b3** | y4**, y3**, y1**, y1**-CH2O | Cs+, [#-H2O]+, [#-CH2O]+, [#-2CH2O]+, [#-H2O-CH2O]+, [#-H2O-2CH2O]+, [#-3CH2O]+ |
| Precursor ion(#) | an/ | Other | ||
|---|---|---|---|---|
| [L5+H]+ | a4, a2, a1, a4-NH3, a3-NH3, a4-CH2-CH-(CH3)2 | b3, b2 | y4, y3, y2 | [#-H2O]+ |
| [L5+Li]+ | a4*, a3*, a3*-(CH3)2, a4*- CH2-CH-(CH3)2, a2*, a2*-(CH3)2 | [b4+Li+OH]+, b4*-NH3, [b3+Li+OH]+, b3*-NH3, b4*, b3*, b2* | y3*, y2*, y1* | [#-H2O-NH3]+ |
| [L5+Na]+ | a4*, a3*, a1*, a3*-CH2-CH- (CH3)2, a2*-CH2-CH-(CH3)2 | [b4+Na+OH]+, b3*, [b3+Na+OH]+, b2* | y2*, y1* | |
| [L5+K]+ | a4*, a3* | [b4+K+OH]+, b4*, [b3+K+OH]+ | y2*, y2*-NH3, y4*-CO | K+, [#-CO]+, [#-CO2]+ |
| [L5+Rb]+ | [b4+Rb+OH]+ | Rb+ | ||
| [L5+Cs]+ | Cs+ | |||
| [L5+2Li-H]+ | a4**, a3**, a4*, a3*, a1*, a3*-CO2, a2*-CO2 | b2*, b4*-H2O-NH3, b3*-H2O-NH3 | y4**, y2**, y1**, y3*, y1* | [#-CO2]+, [#-H2O-NH3]+ |
| [L5+2Na-H]+ | a4**, a4*, a3*, a2*, a1*, a3*-H2O-NH3, a2*-H2O-NH3, a2*-CH2-CH-(CH3)2 | b3*, b3*-NH3 | y4**, y3**, y2**, y1**, y2**-CO2 | [#-CO2]+ |
| [L5+2K-H]+ | a4*, a3*, a2*, a1*-(CH3)2 | y4**, y3**, y2**, y1**, y1**-H2O-NH3 | K+ | |
| [L5+2Rb-H]+ | a3**-CH2-CH-(CH3)2, a2**-CH2-CH-(CH3)2 | y3**, y2**, y1**, y3**-NH3 | Rb+ | |
| [L5+2Cs-H]+ | a2**-CH2-CH-(CH3)2 | y3**, y2**, y1**, y4**-CH2-CH-(CH3)2 | Cs+ |
Table 6 Main product ions of protonated and alkali metal cationized L5 complexes obtained with CID
| Precursor ion(#) | an/ | Other | ||
|---|---|---|---|---|
| [L5+H]+ | a4, a2, a1, a4-NH3, a3-NH3, a4-CH2-CH-(CH3)2 | b3, b2 | y4, y3, y2 | [#-H2O]+ |
| [L5+Li]+ | a4*, a3*, a3*-(CH3)2, a4*- CH2-CH-(CH3)2, a2*, a2*-(CH3)2 | [b4+Li+OH]+, b4*-NH3, [b3+Li+OH]+, b3*-NH3, b4*, b3*, b2* | y3*, y2*, y1* | [#-H2O-NH3]+ |
| [L5+Na]+ | a4*, a3*, a1*, a3*-CH2-CH- (CH3)2, a2*-CH2-CH-(CH3)2 | [b4+Na+OH]+, b3*, [b3+Na+OH]+, b2* | y2*, y1* | |
| [L5+K]+ | a4*, a3* | [b4+K+OH]+, b4*, [b3+K+OH]+ | y2*, y2*-NH3, y4*-CO | K+, [#-CO]+, [#-CO2]+ |
| [L5+Rb]+ | [b4+Rb+OH]+ | Rb+ | ||
| [L5+Cs]+ | Cs+ | |||
| [L5+2Li-H]+ | a4**, a3**, a4*, a3*, a1*, a3*-CO2, a2*-CO2 | b2*, b4*-H2O-NH3, b3*-H2O-NH3 | y4**, y2**, y1**, y3*, y1* | [#-CO2]+, [#-H2O-NH3]+ |
| [L5+2Na-H]+ | a4**, a4*, a3*, a2*, a1*, a3*-H2O-NH3, a2*-H2O-NH3, a2*-CH2-CH-(CH3)2 | b3*, b3*-NH3 | y4**, y3**, y2**, y1**, y2**-CO2 | [#-CO2]+ |
| [L5+2K-H]+ | a4*, a3*, a2*, a1*-(CH3)2 | y4**, y3**, y2**, y1**, y1**-H2O-NH3 | K+ | |
| [L5+2Rb-H]+ | a3**-CH2-CH-(CH3)2, a2**-CH2-CH-(CH3)2 | y3**, y2**, y1**, y3**-NH3 | Rb+ | |
| [L5+2Cs-H]+ | a2**-CH2-CH-(CH3)2 | y3**, y2**, y1**, y4**-CH2-CH-(CH3)2 | Cs+ |
| Precursor ion(#) | an/ | Other | ||
|---|---|---|---|---|
| [K5+H]+ | a4-(CH2)4-NH2, a3-(CH2)4-NH2, a1, a1-NH3 | b4, b3, b2, b1, b4-H2O, b3-H2O, b2-H2O, b4-H2O-NH3, b4-H2O-2NH3, b3-H2O-NH3, b3-H2O-2NH3, b2-H2O-NH3, b2-H2O-2NH3 | y4, y3, y2, y1 | [#-H2O]+ |
| [K5+Li]+ | a1*, a2*- (CH2)4-NH2 | [b4+Li+OH]+, b4*, b3*, b2*, b1*, b4*-H2O, b4*-H2O-NH3, [b3+Li+OH]+, b3*-H2O, b3*-H2O-NH3, b2*-H2O, b2*-H2O-NH3 | y2*, y1* | [#-H2O]+ |
| [K5+Na]+ | a1*, a3*- (CH2)4-NH2 | [b4+Na+OH]+, b4*, b3*, b2*, b1*, b4*-H2O, b2*-H2O-NH3, [b3+Na+OH]+, b3*-H2O | y4*-CO2, y2*, y1* | [#-H2O]+ |
| [K5+K]+ | a2* | [b4+K+OH]+, b4*, b3*, b2*, b1*, b3*-H2O, b2*-H2O, [b3+K+OH]+ | y2*, y1*, y1*-(CH2)4 | K+, [#-H2O]+, [#-CO2]+ |
| [K5+Rb]+ | [b4+Rb+OH]+ | Rb+ | ||
| [K5+Cs]+ | [b4+Cs+OH]+ | Cs+ | ||
| [K5+2Li-H]+ | a4**, a3**, a2**, a2**-H2O-NH3, a1* | b3**, b2**, b4*, b3*, b1* | y4**, y3**, y2**, y1**, y4*, y1* | [#-CO2]+, [#-CO2-CH2-NH2]+ |
| [K5+2Na-H]+ | a4**, a4**-4CH2 | b4**, b4**-CO2, b4*, b3*, b2* | y4**, y3**, y2**, y1**, y3**-CO2, y2**-CO2, y4*, y3*, y2*, y1*, y1**-4CH2 | [#-CO2]+ |
| [K5+2K-H]+ | a2**, a2**- (CH2)4-NH2 | b4* | y4**, y3**, y2**, y1**, y4*, y3* | K+, [#-CO2]+ |
| [K5+2Rb-H]+ | b4* | y3**, y2**, y1**, y3* | Rb+ | |
| [K5+2Cs-H]+ | b4* | y3**, y2**, y1** | Cs+ |
Table 7 Main product ions of protonated and alkali metal cationized K5 complexes obtained with CID
| Precursor ion(#) | an/ | Other | ||
|---|---|---|---|---|
| [K5+H]+ | a4-(CH2)4-NH2, a3-(CH2)4-NH2, a1, a1-NH3 | b4, b3, b2, b1, b4-H2O, b3-H2O, b2-H2O, b4-H2O-NH3, b4-H2O-2NH3, b3-H2O-NH3, b3-H2O-2NH3, b2-H2O-NH3, b2-H2O-2NH3 | y4, y3, y2, y1 | [#-H2O]+ |
| [K5+Li]+ | a1*, a2*- (CH2)4-NH2 | [b4+Li+OH]+, b4*, b3*, b2*, b1*, b4*-H2O, b4*-H2O-NH3, [b3+Li+OH]+, b3*-H2O, b3*-H2O-NH3, b2*-H2O, b2*-H2O-NH3 | y2*, y1* | [#-H2O]+ |
| [K5+Na]+ | a1*, a3*- (CH2)4-NH2 | [b4+Na+OH]+, b4*, b3*, b2*, b1*, b4*-H2O, b2*-H2O-NH3, [b3+Na+OH]+, b3*-H2O | y4*-CO2, y2*, y1* | [#-H2O]+ |
| [K5+K]+ | a2* | [b4+K+OH]+, b4*, b3*, b2*, b1*, b3*-H2O, b2*-H2O, [b3+K+OH]+ | y2*, y1*, y1*-(CH2)4 | K+, [#-H2O]+, [#-CO2]+ |
| [K5+Rb]+ | [b4+Rb+OH]+ | Rb+ | ||
| [K5+Cs]+ | [b4+Cs+OH]+ | Cs+ | ||
| [K5+2Li-H]+ | a4**, a3**, a2**, a2**-H2O-NH3, a1* | b3**, b2**, b4*, b3*, b1* | y4**, y3**, y2**, y1**, y4*, y1* | [#-CO2]+, [#-CO2-CH2-NH2]+ |
| [K5+2Na-H]+ | a4**, a4**-4CH2 | b4**, b4**-CO2, b4*, b3*, b2* | y4**, y3**, y2**, y1**, y3**-CO2, y2**-CO2, y4*, y3*, y2*, y1*, y1**-4CH2 | [#-CO2]+ |
| [K5+2K-H]+ | a2**, a2**- (CH2)4-NH2 | b4* | y4**, y3**, y2**, y1**, y4*, y3* | K+, [#-CO2]+ |
| [K5+2Rb-H]+ | b4* | y3**, y2**, y1**, y3* | Rb+ | |
| [K5+2Cs-H]+ | b4* | y3**, y2**, y1** | Cs+ |
| [1] | Li Y., Lin H. Q., Deng C. H., Yang P. Y., Zhang X. M., Proteomics, 2008, 8(2), 238—345 |
| [2] | Xu Y. W., Zhang L. J., Lu H. J., Yang P. Y., Anal. Chem., 2008, 80(21), 8324—8328 |
| [3] | Zhou M., McDonald J. F., Fernandez F. M., J. Am. Soc. Mass Spectrom., 2010, 21(1), 68—75 |
| [4] | Chen C., Chu Y. Q., Dai X. H., Fang X., Ding C. F., Acta Phys. Chim. Sin., 2013, 29(6), 1336—1343 |
| (陈琛, 储艳秋, 戴新华, 方向, 丁传凡.物理化学学报, 2013,29(6), 1336—1343) | |
| [5] | Halim V. A., Muck A., Hartl M., Ibanez A. Z., Giri A., Proteomics, 2009 , 9(1) , 171—181 |
| [6] | Wilson J.J., Brodbelt J. S., Anal.Chem.,2007, 79,2067—2077 |
| [7] | Biemann K., Methods Enzymol., 1990, 193, 455—460 |
| [8] | Good D. M., Wenger C. D., Coon J. J., Proteomics, 2010, 10(1), 164—167 |
| [9] | Spengler B., J. Mass. Spectrom., 1997, 32, 1019—1036 |
| [10] | Qin Y. J., Wei S. G., Wang X. L., Yang F., Wang B., Guo X. H., Chem. J. Chinese Universities, 2011, 32(8), 2748—2756 |
| (秦玉娇, 魏士刚, 王晓录, 杨帆, 汪兵, 国新华.高等学校化学学报, 2011,32(8), 2748—2756) | |
| [11] | Wysocki V. H., Smith L. L., Breci L. A., J. Mass Spectrom., 2000, 35, 1399—1406 |
| [12] | Harrison A. G., Mass Spectrom. Rev., 2009, 28(4), 640—654 |
| [13] | Knapp-Mohammady M., Young A. B., Paizs B., Harrison A.G., J. Am. Soc. Mass Spectrom., 2009, 20(11), 2135—2143 |
| [14] | Paizs B., Suhai S., Mass Spectrom. Rev., 2005, 24(4), 508—548 |
| [15] | Bythell B. J., Somogyi A., Paizs B., J. Am. Soc. Mass Spectrom., 2009, 20(4), 618—624 |
| [16] | Cydzik M., Rudowska M., Stefanowicz P., Szewczuk Z., J. Am. Soc. Mass Spectrom., 2011, 22(12), 2013—2107 |
| [17] | Shields S. L., Bluhm B. K., Russell D. H., J. Am. Soc. Mass Spectrom., 2000, 11, 626—633 |
| [18] | Pingitore F., Wesdemiotis C., Anal. Chem., 2005, 77, 1796—1803 |
| [19] | Russell D. H., Mass Spectrom. Rev., 1986, 5, 167—189 |
| [20] | Tang X., Ens W., Standing K. G., Westmore J. B., Anal. Chem., 1988, 60, 1791—1799 |
| [21] | Teesch L. M., Adams J., J. Am. Chem. Soc., 1990, 112, 4110—4120 |
| [22] | Teesch L. M., Orlando R. C., Adams J., J. Am. Chem. Soc., 1991, 113, 3668—3675 |
| [23] | Crizer D. M., Xia Y., McLuckey S. A., J. Am. Soc. Mass Spectrom., 2009, 20(9), 1718—1722 |
| [24] | Zhang H. R., Chen G., Wang L., Ding L., Tian Y., Jin W. J., Zhang H. Q., Int. J. Mass Spectrom., 2006, 252(1), 1—10 |
| [25] | Chu Y. Q., Dai X. H., Jiang D., Fang X., Ding C. F., Rapid Commun. Mass Spectrom., 2010, 24, 2255—2262 |
| [26] | Guo C., Hu N., Jiang K. Z., Chen W. X., Wang X. X., Pan Y. J., Rapid Commun. Mass Spectrom., 2010, 24, 409—414 |
| [27] | Dunbar R. C., Polfer N. C., Berden G., Oomens J. , Int. J. Mass Spectrom., 2012, 330, 71—77 |
| [28] | Dunbar R. C., Steill J. D., Polfer N. C., Oomens J., J. Phys. Chem. A, 2013, 117(6), 1094—1101 |
| [29] | Pu D., Vincent J. B., Cassady C. J., J. Mass Spectrom., 2008, 43, 773—781 |
| [30] | Wang Q., Chu Y. Q., Zhang K., Dai X. H., Fang X., Ding C. F., Acta Phys. Chim. Sin., 2012, 28, 971—978 |
| (王青, 储艳秋, 张开, 戴新华, 方向, 丁传凡.物理化学学报, 2012, 28, 971—978) |
| [1] | 魏王慧, 储艳秋, 陈鹰, 高艳秋, 丁传凡. 质谱法定量测定高密度脂蛋白结合蛋白的糖基化水平[J]. 高等学校化学学报, 2019, 40(6): 1141. |
| [2] | 李文红, 王丹阳, 曹津津, 魏永巨. 黄豆黄素与黄豆黄苷吸收光谱和荧光光谱的比较研究[J]. 高等学校化学学报, 2019, 40(1): 47. |
| [3] | 吴芳玲, 储艳秋, 陈新, 魏王慧, 丁传凡. 电喷雾电离质谱法研究影响五肽间非共价作用的主要因素[J]. 高等学校化学学报, 2018, 39(9): 1927. |
| [4] | 黄彦东, 吴若菲, 储艳秋, 丁传凡. α-氨基酸及其酯化物侧链对其β-环糊精复合物稳定常数的影响[J]. 高等学校化学学报, 2017, 38(5): 743. |
| [5] | 吴若菲, 储艳秋, 许崇晟, 刘智攀, 丁传凡. 碱金属离子与三肽复合物的气相裂解反应[J]. 高等学校化学学报, 2016, 37(12): 2150. |
| [6] | 陈海燕, 丁兰, 刘密兰. 微波辅助合成分子印迹聚合物用于萃取蜂蜜中的氯霉素[J]. 高等学校化学学报, 2015, 36(1): 67. |
| [7] | 李雪, 方小伟, 李银萍, 陈焕文. 羟基多环芳烃的电喷雾电离离子阱串联质谱检测[J]. 高等学校化学学报, 2013, 34(8): 1840. |
| [8] | 赵志强, 邱辉华, 姚军, 陈永峰. 基于化学衍生和CID碎裂模式鉴定蛋白精氨酸-ADP-核糖基化的新方法[J]. 高等学校化学学报, 2013, 34(12): 2704. |
| [9] | 赖莺 陈和秀 林睿 王鸿辉 董清木 黄宗平. 气相色谱-质谱法同时测定氧化型染发剂中的17种染料[J]. 高等学校化学学报, 2011, 32(10): 2286. |
| [10] | 郭继芬, 张绍东, 孟繁华, 赵毅民. 液相色谱-串联质谱法测定大鼠脑透析液中CTN986及其脱糖产物的含量[J]. 高等学校化学学报, 2009, 30(8): 1528. |
| [11] | 张娥,祖莉莉,方维海,黄凌云,何大澄 . 精氨酸残基在质子化多肽RRMKWKK的气相碰撞诱导解离过程中的作用[J]. 高等学校化学学报, 2008, 29(6): 1185. |
| [12] | 胡斌,陈兰慧,郇延富,张燮, 李明,梁华正,陈焕文 . 甲基羟基铀酰离子与水的复分解反应[J]. 高等学校化学学报, 2008, 29(5): 912. |
| [13] | 史朝辉,王文亮,王渭娜,李春迎,吕剑 . CH3CH2S自由基H迁移异构化及裂解反应的理论研究[J]. 高等学校化学学报, 2008, 29(4): 812. |
| [14] | 刘淑清,孙明忠,赵宝昌 . 质谱法分析蛇毒蛋白翻译后修饰[J]. 高等学校化学学报, 2008, 29(11): 2194. |
| [15] | 周原,,,梅虎,,杨力,周鹏,杨善彬,,李志良,,. 虚拟原子探针随机采样法用于氨基酸结构描述及其构效关系研究[J]. 高等学校化学学报, 2007, 28(7): 1263. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||